Abstract
A 76-year-old woman was admitted with progressive renal function decline. A kidney biopsy was performed because of myeloperoxidase anti-neutrophil cytoplasmic antibody (ANCA; 333 IU/mL), proteinuria (1.21 g/d), and urinary erythrocyte sediment (10–19/high-power field). Renal-limited ANCA-positive vasculitis with pauci-immune necrotizing crescentic glomerulonephritis (ANCA-associated vasculitis, AAV) was diagnosed. Glucocorticoid therapy was started, and the patient responded well. About 1 year later, avacopan treatment was started and glucocorticoid therapy was discontinued. Avacopan did not normalize ANCA levels and did not make urinary findings negative. However, further progression of renal function decline is prevented. Factors attributed to the development of AAV in this case were investigated; AAV developed after the second dose of the COVID-19 vaccine and ANCA levels re-elevated after the fifth dose. This suggests that the COVID-19 vaccine may have contributed to the development of AAV in this elderly patient. Avacopan monotherapy has been shown to be effective as maintenance therapy to control the progression of renal failure although not sufficient for complete remission of AAV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) has a high rate of renal involvement. Kidney biopsy has been reported to be useful in the diagnosis of vasculitis because it frequently shows characteristic signs of the disease, such as crescentic glomerulonephritis and small arteritis [1, 2]. In recent years, the cause of the AAV was unknown, but several triggering causes including the influenza and COVID-19 vaccines began to be reported [3,4,5,6]. Combination treatment with glucocorticoids and immunosuppressive agents, such as cyclophosphamide and rituximab, has improved the efficacy of AAV treatment, but the next challenge is to reduce the dose of glucocorticoids because they cause glucocorticoid-induced osteoporosis and infections [7]. In AAV, complement C5a primes neutrophils and increases ANCA antigen expression, leading to subsequent tissue damage [8]. Subsequently, a therapeutic drug that suppresses C5a was developed and is expected to become a therapeutic agent for AAV and to reduce glucocorticoid dosage [9,10,11,12].
Here, we present a case in which glucocorticoids could be discontinued after administration of Tavneos® (Amgen, Thousand Oaks, California, United States) (avacopan). We also discuss the limitations of avacopan.
Case presentation
A 75-year-old woman presented to our outpatient clinic due to rapid progression of renal function decline. Past laboratory findings confirmed that her renal function had not changed over the past 3 years with creatinine [Cre], 1.4 mg/dL. The patient had a history of hypertension and had been on an angiotensin II receptor blocker (valsartan 80 mg) for the past 10 years. Because she was obese (body mass index, 28; body weight, 60 kg), obesity-related glomerulonephritis was suspected as the cause of renal dysfunction.
On admission, the patient was 145.0 cm tall and weighed 64.0 kg. She originally weighed 60 kg but had increased to 64 kg at the time of admission. Her blood pressure was 151/80 mm Hg, and temperature, 36.8 °C. Heart and breath sounds were normal, and edema was present in the bilateral lower extremities.
Laboratory findings were as follows: red blood cells, 4.39 × 106/μL; hemoglobin, 13.9 g/dL; white blood cells, 8600/μL; platelets, 21.6 × 104/μL. Serological tests showed albumin, 3.5 g/dL; urea nitrogen, 60 mg/dL; Creatinine, 2.57 mg/dL; uric acid, 5.6 mg/dL, estimated glomerular filtration rate, 14.7 mL/min/1.73 m2; glucose, 91 mg/dL; HbA1c, 5.4%; C-reactive protein, 0.18 mg/dL; immunoglobulin (Ig) G, 1357 mg/dL; IgA, 277 mg/dL; IgM, 123 mg/dL; CH50, 53 U/mL (reference range, 30–46 U/mL); C3, 129 mg/dL (reference range, 86–160 mg/dL); C4, 28 mg/dL (reference range, 17–45 mg/dL); myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA), 333 IU/mL; PR3-ANCA, negative; glomerular basement membrane antibody, negative; antinuclear antibody, negative; and double-stranded DNA antibody, negative. Urinary protein excretion was 1.21 g/d, and the urinary sediment contained 10–19 erythrocytes per high-power field.
A kidney biopsy was performed the day after admission.
Kidney biopsy findings
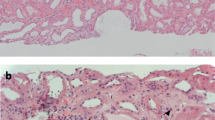
Light microscopy showed global sclerosis in 22 out of 30 glomeruli. Cellular crescent formation due to epithelial cell proliferation and glomerular basement membrane collapse was prominent in two glomeruli (Fig. 1a, b), and fibrin deposition was seen in the Bowman’s space of one glomerulus (Fig. 1c). About 70% of renal cortical areas had tubulointerstitial fibrosis and inflammatory cell infiltrates. Immunofluorescent analysis showed no significant positivity for IgG, IgA, IgM, C3, or C1q. Electron microscopy showed an enlarged capillary lumen space but no high electron density deposits (Fig. 1d).
Kidney biopsy. a and b: Cellular crescent formation due to epithelial cell proliferation (arrow) and prominent glomerular basement membrane collapse in two glomeruli a Periodic acid-Schiff (PAS) stain, b periodic acid-methenamine silver (PAM) stain (original magnification × 400) c Fibrin deposition (arrow head) in Bowman’s space of one glomerulus (PAS stain; original magnification × 400) d Electron microscopy image showing an enlarged capillary lumen but no high electron density deposits
The remaining glomeruli were large (220–250 μm), and one glomerulus showed focal segmental glomerulosclerosis. There was moderate arteriolar hyalinosis and moderate fibroelastosis of the interlobular arteries. These findings suggest the possibility of obesity-related glomerulopathy complicated by hypertension as a background renal disease.
Renal-limited MPO-ANCA-positive vasculitis with pauci-immune necrotizing crescentic glomerulonephritis due to MPA was diagnosed. There were no abnormal findings in the lungs, sinuses, or skin.
Clinical course
Combination treatment with glucocorticoids and immunosuppressive agents, such as cyclophosphamide and rituximab, is recommended as a common treatment for refractory AAV. However, in this case, the patient did not have extrarenal lesions, had renal limited AAV, and was elderly, so our treatment team chose glucocorticoid monotherapy.
Treatment was initiated with methylprednisolone 1000 mg/d for 3 days, followed by prednisolone (PSL) 30 mg/d. Three months later, the test for ANCA was negative; Cre temporarily decreased to 1.8 mg/dL but after 1 month stabilized at 2.0–2.2 mg/dL. The dose of PSL was gradually reduced; however, moon face phenomenon became prominent, so the patient requested discontinuation of PSL, and PSL was discontinued after 10 months. One month before the PSL was discontinued, avacopan was started at 30 mg and later increased to 60 mg. After 14 months of treatment, MPO-ANCA increased again to 51 IU/mL. However, the patient did not want to start PSL, so avacopan 60 mg was continued in monotherapy. ANCA then decreased and Cre did not increase. After 20 months of treatment with avacopan in monotherapy, MPO-ANCA had stabilized at 20–30 IU/mL and Cre, at 2.1–2.3 mg/dL although some hematuria was still present.
Relationship to COVID-19 vaccination
The patient’s aunt had a history of rheumatoid arthritis, and her eldest son was being treated for dermatomyositis. Recently, COVID-19 vaccination has been reported as a factor in the pathogenesis of AAV. Therefore, we also inquired about the patient’s vaccination history.
After the second Pfizer-BioNTech COVID-19 vaccination, the patient developed fever. One month later, blood began to appear in the urine, and the patient developed proteinuria, and 2 months later, renal function began to deteriorate, with Cre increasing from 1.5 mg/dL to 2.57 mg/dL over the course of 3 months. We determined that the re-increase in ANCA coincided with the patient’s fourth and fifth COVID-19 vaccinations (Fig. 2). Therefore, the patient was advised not to receive any additional COVID-19 vaccinations. Since being treated at our hospital, the AAV not exacerbated. Avacopan did not normalize ANCA levels and did not make urinary findings negative. However, further progression of renal function decline was prevented.
Clinical course. After the second Pfizer-BioNTech COVID-19 vaccination, the patient developed fever. One month later, the patient developed hematuria and proteinuria, and 2 months later, renal function began to deteriorate. The re-increase in serum ANCA level coincided with the patient’s fourth and fifth COVID-19 vaccinations. Vaccine is Pfizer-BioNTech COVID-19. MPO-ANCA myeloperoxidase anti-neutrophil cytoplasmic antibody, mPSL methyl prednisolone, PSL prednisolone
Considering the above, we hypothesized that COVID-19 vaccination not only induced AAV in this patient but was also involved in the reactivation of AAV on a familial genetic background such as autoimmune disease.
Discussion
We presented a case of AAV that developed after administration of the COVID-19 vaccine. Below, we discuss the possible mechanism by which COVID-19 vaccination causes AAV and also present the mechanism by which avacopan monotherapy is effective in AAV.
Prabhahar et al. [4] and Yang et al. [6] summarized reported cases of COVID-19 vaccine-induced AAV. They found that the majority of cases were caused by mRNA vaccines. The number of these AAV onsets after the second vaccination was large among vaccination-associated vasculitis, and in many cases, AAV was successfully treated but recurred after further administration of the COVID-19 vaccine [4,5,6]. The COVID-19 vaccines cause production of antiviral neutralizing immunoglobulins and stimulate a strong immune response by activating CD8 + and CD4 + T cells, resulting in antiviral activity [13]. These vaccines may stimulate the patient’s own innate and acquired immune systems, especially in a type of patients with a genetic or familial predisposition [4].
Xiao et al. suggest that stimulation of neutrophils by ANCA causes the release of factors that activate complement via alternative pathways, initiating an inflammatory amplification loop that mediates the severe necrotic inflammation of ANCA disease [14]. The mechanism of AAV is associated with the complement pathway, in which C5a plays an important role. Complement is essentially an integral part of the ecological defense mechanism against microorganisms and other foreign substances that enter the body from outside, and activation of leukocytes leads to the removal of foreign substances. However, when C5a is activated too strongly, neutrophils are altered and ANCA antigens, such as MPO and PR3, are transferred to the cell surface of neutrophils. In response, ANCA is mobilized and degrades neutrophils, causing ANCA antigens, such as MPO and PR3, to exit the cell and release neutrophil extracellular traps, resulting in endothelial cell injury and activation of the coagulation system and the alternative complement pathway. Thus, ANCA, neutrophils, and the complement system form an amplification loop that causes AAV. Avacopan is believed to inhibit the above-mentioned series of mechanisms by selectively blocking C5a receptors on the surface of neutrophils, thereby inhibiting the translocation of MPO and other molecules to the cell surface. It also suppresses ANCA production by reducing the amount of ANCA antigen released into the blood [8, 15].
The relationship between C5a and the neutrophil C5aR described by Xiao et al. [8] is important and will be added in the next paper. Schreiber et al. reported that C5a and the neutrophil C5aR may compose an amplification loop for ANCA-mediated neutrophil activation [16]. Hao et al. reported that activation of the p38 mitogen-activated protein kinase (p38MAPK), extracellular signal-regulated kinase (ERK) and phosphoinositol 3-kinase (PI3K) are important steps in the translocation of ANCA antigens and C5a-induced activation of neutrophils by ANCA. They reported the signaling pathways of C5a-mediated priming of human neutrophils for ANCA-induced neutrophil activation, and that C5a and the neutrophil C5a receptor play a central role in anti-neutrophil cytoplasmic antibody (ANCA)-mediated neutrophil recruitment and activation [17].
In conclusion, we experienced an elderly onset case of AAV after the second dose of COVID-19 vaccine. The patient went into remission after glucocorticoid therapy. In the process of glucocorticoid dose reduction, ANCA levels were again mildly elevated after the fifth vaccine administration. Subsequent treatment with avacopan monotherapy prevented progression of renal function although MPO-ANCA did not become completely negative and hematuria did not completely normalize. This clinical course suggests that mildly elevated MPO-ANCA can be suppressed by avacopan in monotherapy, but if MPO-ANCA levels are very high, avacopan alone may not be sufficient, and concomitant glucocorticoid administration may be necessary. Thus, continuous administration of avacopan does not appear to completely eliminate MPO-ANCA, but the drug may prevent elevation of MPO-ANCA levels.
Limitations
The following limitations were considered. Those were combination treatment with glucocorticoids and immunosuppressive agents, such as cyclophosphamide and rituximab, is recommended as a common treatment for AAV. Thus, information on the pros and cons of avacopan monotherapy is lacking. In fact, even in this case, complete remission of AAV was not achieved. It is noteworthy, however, that renal function did not progress in this patient. Avacopan-specific complications have not been observed. Older patients with AAV who have been treated with immunosuppressive agents, including glucocorticoid agents, have suffered from complications associated with these drugs. Therefore, although monotherapy with avacopan is not recommended, it may be considered as a treatment option.
References
Endo A, Hoshino J, Suwabe T, Sumida K, Mise K, Hiramatsu R, Hasegawa E, Yamanouchi M, Hayami N, Sawa N, Takaichi K, Ohashi K, Fujii T, Ubara Y. Significance of small renal artery lesions in patients with antineutrophil cytoplasmic antibody-associated glomerulonephritis. J Rheumatol. 2014;41(6):1140–6.
Hasegawa J, Hoshino J, Sekine A, Hayami N, Suwabe T, Sumida K, Mise K, Ueno T, Yamanouchi M, Hazue R, Sawa N, Ohashi K, Fujii T, Takaichi K, Ubara Y. Clinical and pathological features of anti-neutrophil cytoplasmic antibody-associated vasculitis in patients with minor urinary abnormalities. Nephrology (Carlton). 2018;23(11):1007–12.
Hara R, Hasegawa E, Inoue N, Sekine A, Tanaka K, Ikuma D, Mizuno H, Oba Y, Yamanouchi M, Suwabe T, Sawa N, Kono K, Kinowaki K, Ohashi K, Ubara Y, Hoshino J. Crescentic glomerulonephritis with fibrinoid vasculitis after administration of influenza vaccine. Intern Med. 2023;62(7):1077–80.
Prabhahar A, Naidu GSRSNK, Chauhan P, Sekar A, Sharma A, Sharma A, Kumar A, Nada R, Rathi M, Kohli HS, Ramachandran R. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol Int. 2022;42(4):749–58.
Suzuki M, Sekiguchi Y, Sasaki M, Inaba S, Oyama S, Inoue Y, Warabi M, Ohashi K, Inoshita S. Antineutrophil cytoplasmic antibody-associated vasculitis after COVID-19 vaccination with pfizer-BioNTech. Intern Med. 2022;61(19):2925–9.
Yang Y, Xiong Y, Xu G. New insights of antineutrophil cytoplasmic antibody-associated vasculitis from the perspective of COVID-19 vaccination. Clin Exp Immunol. 2023;213(3):301–9.
Hellmich B, Sanchez-Alamo B, Schirmer JH, Berti A, Blockmans D, Cid MC, Holle JU, Hollinger N, Karadag O, Kronbichler A, Little MA, Luqmani RA, Mahr A, Merkel PA, Mohammad AJ, Monti S, Mukhtyar CB, Musial J, Price-Kuehne F, Segelmark M, Teng YKO, Terrier B, Tomasson G, Vaglio A, Vassilopoulos D, Verhoeven P, Jayne D. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis. 2024;83(1):30–47.
Xiao H, Dairaghi DJ, Powers JP, Ertl LS, Baumgart T, Wang Y, Seitz LC, Penfold ME, Gan L, Hu P, Lu B, Gerard NP, Gerard C, Schall TJ, Jaen JC, Falk RJ, Jennette JC. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol. 2014;25(2):225–31.
Atrens AD, Hutton H, O’Sullivan E, Moore J, Madhan K. Avacopan as an additional therapeutic intervention to promote renal recovery in severe ANCA-associated vasculitis: a case report. Nephrology. 2024;29(2):105-107.
Gabilan C, Pfirmann P, Ribes D, Rigothier C, Chauveau D, Casemayou A, Huart A, Schanstra J, Colombat M, Faguer S, Belliere J. Avacopan as first-line treatment in antineutrophil cytoplasmic antibody-associated vasculitis: a steroid-sparing option. Kidney Int Rep. 2022;7(5):1115–8.
Monti S, Brandolino F, Milanesi A, Xoxi B, Delvino P, Montecucco C. Novel therapies for ANCA-associated vasculitis. Curr Rheumatol Rep. 2021;23:38.
Osman M, Cohen Tervaert JW, Pagnoux C. Avacopan for the treatment of ANCA-associated vasculitis. Expert Rev Clin Immunol. 2021;17:717–26.
Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D, Brachtendorf S, Lörks V, Sikorski J, Hilker R, Becker D, Eller AK, Grützner J, Boesler C, Rosenbaum C, Kühnle MC, Luxemburger U, Kemmer-Brück A, Langer D, Bexon M, Bolte S, Karikó K, Palanche T, Fischer B, Schultz A, Shi PY, Fontes-Garfias C, Perez JL, Swanson KA, Loschko J, Scully IL, Cutler M, Kalina W, Kyratsous CA, Cooper D, Dormitzer PR, Jansen KU, Türeci Ö. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–9.
Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170(1):52–64.
Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. 2021;384(7):599–609.
Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20:289–98.
Hao J, Meng LQ, Xu PC, Chen M, Zhao MH. p38MAPK, ERK and PI3K signaling pathways are involved in C5a-primed neutrophils for ANCA-mediated activation. PLoS ONE. 2012;7: e38317.
Acknowledgements
We thank Dr Setsuko Oyama for her accomplishment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests and no conflicts of interest.
Ethical approval
The present report was produced in conformity with the Declaration of Helsinki, and the patient gave her written informed consent for the case report to be published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ubara, Y., Oba, Y., Kurihara, S. et al. A case of renal limited myeloperoxidase anti-neutrophil cytoplasmic antibody-positive vasculitis treated with maintenance avacopan monotherapy. CEN Case Rep (2024). https://doi.org/10.1007/s13730-024-00910-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13730-024-00910-1