Abstract
There are no clinical guidelines for performing nephrectomy in patients with autosomal recessive polycystic kidney disease (ARPKD). Few reports have described the clinical course of ARPKD diagnosed in the neonatal period in detail. Here, we report seven patients diagnosed with ARPKD and treated at our center during the neonatal period. Two died within 48 h of life due to pulmonary hypoplasia. Of the remaining five patients, three had anuria and required for kidney replacement therapy (KRT) within one week after birth, whereas two with a milder phenotype survived without KRT. All three patients who received KRT underwent unilateral nephrectomy and peritoneal dialysis (PD) catheter placement. To prevent fluid leakage, PD was initiated 7–14 days after catheter placement. However, peritoneal leakage occurred in two patients, resulting in peritonitis and discontinuation of PD; one who required long-term hemodialysis contracted a catheter-related bloodstream infection as well as developed subdural and epidural hematomas. Meanwhile, two patients underwent a second nephrectomy within 6 weeks after birth; one developed severe persistent hypotension and neurological complications, while the other died of bacteremia that may have resulted from cholangitis diagnosed on day 67 of life. A severe clinical course, life-threatening adverse events, and severe neurological sequalae may occur in patients with ARPKD who receive KRT in neonatal period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Autosomal recessive polycystic kidney disease (ARPKD) is a rare hereditary disorder with an estimated incidence of 1 in 20,000 live births [1]. The clinical course of the disease is variable, and it has a broad spectrum [2, 3]. Patients with ARPKD and oligohydramnios are often diagnosed prenatally or in the neonatal period, and they may have a poor prognosis due to respiratory failure with pulmonary hypoplasia, severely impaired kidney function, and cholangitis early in life [2, 4]. Additionally, enlarged bilateral kidneys may lead to respiratory distress or difficulties with peritoneal dialysis (PD) because of the lack of space for catheterization and fluid accumulation. Early nephrectomy has been suggested for these cases; however, several studies have reported the occurrence of post-nephrectomy hypotension [5, 6]. A recent study reported that very early bilateral nephrectomy was independent risk factor for severe neurological complications [7].
Currently, there are no clinical guidelines for performing nephrectomy and initiating kidney replacement therapy (KRT) in patients with ARPKD, especially in neonates. Moreover, few reports have described in detail the clinical course of ARPKD diagnosed in the neonatal period.
Here, we report the cases of patients diagnosed with ARPKD due to massive bilateral nephromegaly and kidney disfunction in the neonatal period between January 1, 2007 and April 1, 2022 and treated during the neonatal period in the National Center for Child Health and Development in Tokyo.
Case report
Seven neonates were identified (Table 1); two died within 48 h of life due to pulmonary hypoplasia (patients 6 and 7). All neonates, except for patient 7, received mechanical ventilator support soon after birth because of pulmonary hypoplasia and pulmonary hypertension. All met the diagnostic criteria for ARPKD [8]; fetal MRI of patients 6 and 7 showed enlarged kidney with numerous small cysts and typical liver imaging. Two of the five neonates who survived beyond 48 h after birth had sufficient urine output 90 and 48 h after birth, respectively (patients 4 and 5). Their Cr- eGFR at last follow-up was 23.8 and 32.0 mL/min/1.73m2 at 6 and 7 years of age, respectively (patients 4 and 5). The other three neonates who survived beyond 48 h after birth were required for KRT in the neonatal period (patients 1–3). Five patients received genetic test after informed consent was obtained from the parents. The clinical course and complications in the three patients are shown in Figs. 1, 2 and 3. The clinical course of the three patients who required KRT in the neonatal period are as follows.
Case 1
A male neonate weighing 2483 g who was antenatally suspected of having ARPKD was born at 37 weeks of gestation. Abdominal ultrasound showed massive bilateral nephromegaly, a typical pepper-salt pattern for ARPKD, and inhomogeneous liver parenchyma; his older sister was also diagnosed with ARPKD. As he met the diagnostic criteria for ARPKD [8], he did not undergo genetic testing. The patient presented with anuria after birth; he was transferred to our facility on the first 8 days of life to initiate HD for anuria and hypertension. In addition to PD catheter placement, unilateral nephrectomy was performed on day 14 to create space for catheterization. PD was initiated on postoperative day 7. Peritoneal leakage and bacterial peritonitis occurred soon after the initiation of PD, prompting its discontinuation. His body weight when peritoneal leakage occurred was 3036 g, which was 500 g higher than birth weight. Although PD was re-initiated 14 days after peritoneal leakage, continuing PD was difficult because of poor ultrafiltration and poor drainage condition. As massive unilateral enlarged kidney seemed to be a contributing factor for poor ultrafiltration and poor drainage condition, a second nephrectomy was performed on day 38. Severe hypotension (systolic blood pressure < 40 mmHg) occurred just after the second nephrectomy, and hypotension persisted for more than two months. PD catheter replacement was performed because poor drainage continued after the second nephrectomy, and HD had to be re-initiated. One month after the second nephrectomy, clinical seizure occurred during HD, and the patient was diagnosed with global cerebral hypoxia–ischemia (Fig. 4a). On day 80, the patient suffered from bacterial peritonitis again, which was treated with antibiotics for three weeks.
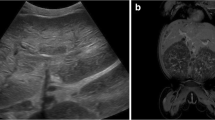
a Brain MRI of case 1 obtained two days after onset of convulsion with multiple axial T2WI images; temporal and occipital lobes (a), basal ganglia (b), lateral ventricles (c), and centrum semiovale (d), showing diffuse areas of edema. b Brain MRI of case 2 obtained one month after onset of convulsion with multiple axial T2WI images; temporal and occipital lobes (a), basal ganglia (b), lateral ventricles (c), and centrum semiovale (d), showing diffuse high signal area which is consistent with multicystic encephalomalacia due to hypoxic-ischemic encephalopathy. A high signal area in the right occipital lobe is observed, which is consistent with an old subdural hematoma
He was discharged at the age of 8 months. He suffered from sepsis shock, which seemed to be associated with cholangitis, at 4 years old. Commencing approximately at the age of five, there was a reduction in the quantum of platelets, reaching a nadir of 150,000 or lower. Concurrently, there was a high levels of collagen type 7 and M2BPGi, indicating progression of hepatic fibrosis. The patient underwent deceased-donor kidney transplantation at four years old. However, antibody-mediated rejection which could be associated with past transfusion resulted in kidney graft loss at 6 years old. Currently, he is seven years old, on PD, and is bedridden with a feeding tube due to global cerebral hypoxia–ischemia. We previously reported the clinical course of this patient as a case of severe hypotension after a second nephrectomy [5].
Case 2
A male neonate weighing 2778 g suspected of having ARPKD was delivered at 35 weeks of gestation because of premature rupture of membranes. Abdominal ultrasound showed smooth and enlarged bilateral kidneys with poor corticomedullary differentiation, inhomogeneous liver parenchyma and dilated intrahepatic biliary ducts. He met the diagnostic criteria for ARPKD [8], and genetic analysis of the PKHD1 gene revealed novel compound heterozygous mutations: a paternally inherited c.11G > A, (p.Trp4Ter) and a maternally inherited c.977-3C > G. As the patient had anuria, KRT was initiated early in life. In addition to PD catheter insertion, unilateral nephrectomy was performed on day 4. PD was initiated on postoperative day 14; however, peritoneal leakage and fungal peritonitis occurred soon after. His body weight when peritoneal leakage occurred was 3059 g, which was about 300 g heavier than birth weight. PD was discontinued, and the catheter was removed to treat fungal peritonitis. A second nephrectomy was performed on day 25 because his skin and subcutaneous tissues were stretched by the enlarged second kidney, which prevented the PD catheter cuff from adhering to the peritoneum. HD was performed for more than three weeks until the PD catheter was replaced. A tracheostomy was also performed because he was not able to be extubated by day 48. During the second HD period, the patient contracted a catheter-related bloodstream infection. Moreover, on day 59, acute anemia and clinical seizure occurred, and he developed subdural and epidural hematomas. After initiation of PD, the patient contracted bacteremia, which may have resulted from cholangitis that was diagnosed on day 77. More than 50 days after the second nephrectomy, episodic hypotension (systolic blood pressure < 45 mmHg) was observed for more than two weeks especially while sleeping. On day 89, clinical seizure occurred, and he was diagnosed with global cerebral hypoxia–ischemia (Fig. 4b).
Currently, he is now 11 months old and on PD. Despite the initiation of immunoglobulin G replacement therapy, sepsis, which seemed to be associated with cholangitis, occurred four times after birth. Biomarkers of liver fibrosis exhibited heightened levels, yet no collateral circulation was detected on computed tomography (CT). The patient is dependent on ventilatory support due to severe pulmonary hypoplasia and is bedridden with a feeding tube due to severe neurological complications.
Case 3
A male neonate weighing 3559 g was born at 36 weeks of gestation. Abdominal ultrasound showed smooth and enlarged bilateral kidneys with poor corticomedullary differentiation and the central dot sign, which is defined as a dot of strong contrast enhancement within the dilated intrahepatic ducts; thus, he met the diagnostic criteria for ARPKD [8]. Genetic analysis of the PKHD1 gene revealed compound heterozygous mutations: a paternally inherited c.7867delT, (p.Tyr2623Thrfs44) and a maternally inherited exon 50 deletion of the PKHD1 revealed with multiple ligation probe amplification (MLPA) analysis. Anuria persisted after birth, and HD was initiated on day 4. PD catheter insertion and unilateral nephrectomy were performed to create space for catheterization on day 9. PD was initiated on postoperative day 14 and continued without peritoneal leakage or poor drainage condition; a second nephrectomy was not necessary. However, peritonitis occurred on day 33. Additionally, bacteremia occurred due to Pseudomonas aeruginosa infection, which may have resulted from cholangitis that was diagnosed on day 67; he died of septic shock 10 h after symptom onset.
Discussion
We report seven patients who were diagnosed with ARPKD in the neonatal period. Two neonates died soon after birth, three required KRT, and two did not require KRT in the first six and seven years of life, respectively. Three patients who received KRT in the neonatal period had a severe clinical course; two developed severe neurological complications, and the other died of infection at 2 months old.
Although KRT early in life should be considered when a patient has insufficient urine output after birth, improvement in kidney function may be observed during the first month of life [9]. In the present study, sufficient urine output was observed in two patients 90 and 48 h after birth, respectively. Additionally, the timing of nephrectomy to create space for PD catheter insertion and PD fluid accumulation should be carefully considered because even unilateral nephrectomy may result in the loss of kidney function. However, managing anuria for an extended period after birth without KRT causes severe edema, which could lead to peritoneal leakage after PD catheter placement in neonates. Hence, temporary HD to improve edema before PD catheter placement and nephrectomy also should be considered.
In the present study, all three patients who received KRT underwent unilateral nephrectomy and initial PD catheter placement, and the interval from catheter placement to PD was 7–14 days to prevent fluid leakage. Additionally, all three patients developed several complications from PD and HD in the first three months after birth. Of the three patients, all contracted bacterial or fungal peritonitis and two had peritoneal leakage, resulting in peritonitis and discontinuation of PD. Meanwhile, one patient on long-term HD contracted a catheter-related bloodstream infection and developed subdural and epidural hematomas.
Possible reasons why these three patients developed several complications are susceptibility for PD fluid leakage due to fragility of the neonatal peritoneum and enlarged kidneys, complications due to long-term hemodialysis after leakage, hypotension due to nephrectomy, and cholangitis attributed to ARPKD. Peritoneal leakage frequently occurs in neonates because of the fragile skin and decreased amounts of subcutaneous tissues [10]. Additionally, anuria after birth causes severe edema of the subcutaneous tissues, which could also interfere with wound healing. In Case 2, a severely enlarged kidney stretched the skin and seemed to prevent the PD catheter cuff from adhering to the peritoneum.
Once peritoneal leakage occurred, long-term temporary HD was necessary. Temporary continuous hemodialysis may be a risk factor for not only catheter-related bloodstream infections but also hemorrhage and hypovolemia [11]. In case 2, temporary continuous hemodialysis had been performed for more than eight weeks, which might have engendered augmented intricacy in the regulation of hemostatic elements, subsequently leading to subdural and epidural hematomas. Hypertension not related to hypervolemia was also observed in a patient with ARPKD [2], which could cause severe hypotension due to aggressive ultrafiltration during continuous hemodialysis, especially post-nephrectomy [2, 5, 12]. Our two patients (case 1 and 2) also developed persistent hypotension after the second nephrectomy, which could lead to global cerebral hypoxia–ischemia during the HD period.
Although unilateral or bilateral nephrectomy was suggested to improve pulmonary distress, nutrition, and blood control as well as creates space for performing PD in patients with severe ARPKD, there is no consensus on who should undergo unilateral or bilateral nephrectomy [2, 12,13,14,15]. However, a recent study on patients with ARPKD showed that 12/19 (63%) patients who underwent bilateral nephrectomy within the first three months of life developed severe neurological complications, which was significantly higher than patients who underwent nephrectomy more than three months after birth [7]. The study also showed that bilateral nephrectomy within three months after birth was an independent risk factor for severe neurological complications. These results were consistent with our results wherein two patients who underwent bilateral nephrectomy in the neonatal period developed severe neurological complications; however, a second nephrectomy was the only option to improve the status of these patients. Our experience may help explain the difficulty in managing neonates with ARPKD who present with anuria.
Several cohort studies about genotype–phenotype correlations reported that missense mutations are associated with neonatal survival [16, 17]. Recent study showed that patients who had two missense variants which affect fibrocystin amino acids 709–1837 or one missense variant in this region with a null variant less frequently developed end-stage kidney disease [18]. In the present study, cases 4 and 5 had a missense variant which did not affect amino acids 709–1837 and their renal function were gradually worsened, which could be in accordance with previous report.
A previous comparative cohort study of the International Pediatric Peritoneal Dialysis Network registry compared PD between pediatric patients with ARPKD and patients with congenital anomalies of the kidney and the urinary tract or congenital nephrotic syndrome who were matched for age and time on dialysis [19]. The study revealed no difference in the incidence of infections or rate of access revisions between the groups. However, the median baseline age of the cohort in that study was 2.4 years. Meanwhile, few studies reported the complication rate of PD in neonates with ARPKD who received KRT due to anuria at birth; this was not assessed in our study due to the small sample size. Regardless, the management of neonates with ARPKD who receive KRT due to anuria at birth is challenging.
In conclusion, neonates diagnosed with ARPKD, especially those who require KRT within one week after birth, may show severe clinical course, life-threatening adverse events and severe neurological sequalae.
References
Zerres K, Mücher G, Becker J, Steinkamm C, Rudnik-Schöneborn S, Heikkilä P, Rapola J, Salonen R, Germino GG, Onuchic L, Somlo S, Avner ED, Harman LA, Stockwin JM, Guay-Woodford LM. Prenatal diagnosis of autosomal recessive polycystic kidney disease (ARPKD): molecular genetics, clinical experience, and fetal morphology. Am J Med Genet. 1998;76:137–44.
Liebau MC. Early clinical management of autosomal recessive polycystic kidney disease. Pediatr Nephrol. 2021;36:3561–70.
Sweeney WE Jr, Avner ED. Diagnosis and management of childhood polycystic kidney disease. Pediatr Nephrol. 2011;26:675–92.
Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics. 2003;111(5 Pt 1):1072–80.
Nishi K, Kamei K, Ogura M, Sato M, Ishiwa S, Shioda Y, Kiyotani C, Matsumoto K, Nozu K, Ishikura K, Ito S. Risk factors for post-nephrectomy hypotension in pediatric patients. Pediatr Nephrol. 2021;36:3699–709.
van Lieburg AF, Monnens LA. Persistent arterial hypotension after bilateral nephrectomy in a 4-month-old infant. Pediatr Nephrol. 2001;16:604–5.
Burgmaier K, Ariceta G, Bald M, Buescher AK, Burgmaier M, Erger F, Gessner M, Gokce I, Konig J, Kowalewska C, Massella L, Mastrangelo A, Mekahli D, Pape L, Patzer L, Potemkina A, Schalk G, Schild R, Shroff R, Szczepanska M, Taranta-Janusz K, Tkaczyk M, Weber LT, Wühl E, Wurm D, Wygoda S, Zagozdzon I, Dötsch J, Oh J, Schaefer F, Liebau MC, ARegPKD Consortium. Severe neurological outcomes after very early bilateral nephrectomies in patients with autosomal recessive polycystic kidney disease (ARPKD). Sci Rep. 2020;10:16025.
Sweeney WE, Gunay-Aygun M, Patil A, Avner ED. Childhood polycystic kidney disease. In: Avner ED, Harmon WE, Niaudet P, editors. Pediatric nephrology. 7th ed. Berlin: Springer; 2016. p. 1103–53.
Cole BR, Conley SB, Stapleton FB. Polycystic kidney disease in the first year of life. J Pediatr. 1987;111:693–9.
Imani PD, Carpenter JL, Bell CS, Brandt ML, Braun MC, Swartz SJ. Peritoneal dialysis catheter outcomes in infants initiating peritoneal dialysis for end-stage renal disease. BMC Nephrol. 2018;19:231.
Gautam SC, Lim J, Jaar BG. Complications associated with continuous RRT. Kidney360. 2022;3:1980–90.
Vidal E, Parolin M, Gamba P. Unilateral or bilateral early nephrectomy in infants with autosomal recessive polycystic kidney disease? Weighing risks and benefits. J Pediatr Surg. 2021;56:435–6.
Overman RE, Criss CN, Modi ZJ, Gadepalli SK. Early nephrectomy in neonates with symptomatic autosomal recessive polycystic kidney disease. J Pediatr Surg. 2021;56:328–31.
Mallett TM, O’Hagan E, McKeever KG. Early bilateral nephrectomy in infantile autosomal recessive polycystic kidney disease. BMJ Case Rep. 2015. https://doi.org/10.1136/bcr-2015-211106.
Shukla AR, Kiddoo DA, Canning DA. Unilateral nephrectomy as palliative therapy in an infant with autosomal recessive polycystic kidney disease. J Urol. 2004;172(5 Pt 1):2000–1.
Abdul Majeed N, Font-Montgomery E, Lukose L, Bryant J, Veppumthara P, Choyke PL, Turkbey IB, Heller T, Gahl WA, Gunay-Aygun M. Prospective evaluation of kidney and liver disease in autosomal recessive polycystic kidney disease-congenital hepatic fibrosis. Mol Genet Metab. 2020;131:267–76.
Rossetti S, Harris PC. Genotype-phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2007;18:1374–80.
Burgmaier K, Brinker L, Erger F, Beck BB, Benz MR, Bergmann C, Boyer O, Collard L, Dafinger C, Fila M, Kowalewska C, Lange-Sperandio B, Massella L, Mastrangelo A, Mekahli D, Miklaszewska M, Ortiz-Bruechle N, Patzer L, Prikhodina L, Ranchin B, Ranguelov N, Schild R, Seeman T, Sever L, Sikora P, Szczepanska M, Teixeira A, Thumfart J, Uetz B, Weber LT, Wühl E, Zerres K, Dötsch J, Schaefer F, Liebau MC. Refining genotype-phenotype correlations in 304 patients with autosomal recessive polycystic kidney disease and PKHD1 gene variants. Kidney int. 2021;100:650–9.
Akarkach A, Burgmaier K, Sander A, Hooman N, Sever L, Cano F, Zambrano P, Bilge I, Flynn JT, Yavascan O, Vallés PG, Munarriz RL, Patel HP, Serdaroglu E, Koch VH, Suarez ADC, Galanti M, Celedon CG, Rébori A, Kari JA, Wong CJ, Elenberg E, Rojas LF, Warady BA, Liebau MC, Schaefer F, IPPN Registry. Maintenance peritoneal dialysis in children with autosomal recessive polycystic kidney disease: a comparative cohort study of the international pediatric peritoneal dialysis network registry. Am J Kidney Dis. 2020;75:460–4.
Acknowledgements
The authors would like to thank Drs. Kandai Nozu, Naoya Morisada, and Hiroki Kurahashi for genetic testing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Koichi Kamei has received research funding from the Public Foundation of Vaccination Research Center, and the Taiju Life Social Welfare Foundation; donations from Chugai Pharmaceutical Co. Ltd., Teijin Pharma Ltd., Kyowa Kirin Co. Ltd., Shionogi & Co. Ltd., Daiichi Sankyo Co. Ltd., Mitsubishi Tanabe Pharma Co. Ltd, and Otsuka Pharmaceutical Co. Ltd.; and lecture fees from Terumo Co. Ltd., Baxter Ltd., and Zenyaku Kogyo Co., Ltd. Other authors have no potential conflicts of interests to disclose.
Ethical approval
The study was conducted according to the Helsinki Declaration’s principle and the Japanese Ministry of Health, Labor, and Welfare’s ethical guidelines. The study was approved by the Ethics Committee of the National Center for Child Health and Development (approval number: 2022-196).
Informed consent
Informed consent was obtained from guardians of all patients in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Inoki, Y., Nishi, K., Osaka, K. et al. Complications and prognosis of patients diagnosed with autosomal recessive polycystic kidney disease in neonatal period. CEN Case Rep 13, 181–187 (2024). https://doi.org/10.1007/s13730-023-00827-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-023-00827-1