Abstract
Ingestion of toxic alcohols (TA) typically presents with a high anion gap (AG) metabolic acidosis, and elevated osmolar gap (OG). Hemodialysis (HD) has not been recommended in early phases of intoxication with high OG and normal AG metabolic acidosis. We describe the case of a 40-year-old male who was brought to our emergency department for reported paint thinner ingestion. He was unable to protect his airway and required intubation. Blood gas showed respiratory acidosis, an initial AG, corrected by albumin of 12.75, lactic acid 5.26 mmol/L, and an OG of 170. Patient was treated with bicarbonate drip, fomepizole and emergent HD, which improved his neurologic status. Days after his admission, alcohol levels came positive for a co-ingestion of ethylene glycol, diethylene glycol, and methanol. Most of the TA are metabolized into their toxic byproducts by the enzyme alcohol dehydrogenase (ADH). The kinetics of these alcohols will be altered when there is co-ingestion of multiple substances. Moreover, early ingestions will translate in a high OG without a high AG. False elevation of lactate can occur with the ingestion of ethylene glycol due to a cross-reaction with l-lactate oxidase in the analyzer. In our case, the administration of fomepizole followed by an early HD given the poor clinical improvement, was followed by a fast recovery of the neurological status and potentially prevented renal failure. A high index of suspicion for TA ingestion should be raised when encountering an individual with lactic acidosis, high OG, and normal AG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Combined toxic alcohol ingestion is relatively rare, especially when three or more substances are involved. Although it is well known that in early stages of alcohol ingestion there will be a minor elevation of the Anion Gap (AG) compared to the brisk increase in the Osmolar Gap (OG), the most common cause of normal or mildly high AG with high OG is isopropyl alcohol ingestion [1, 2]. Most of the toxic alcohols (TA) are metabolized into their toxic byproducts by the enzyme alcohol dehydrogenase (ADH). The half-life of these substances can be prolonged with the co-ingestion of multiple alcohols competing for the same enzyme [3].
Moreover, false elevation of lactate can occur with the ingestion of ethylene glycol due to its metabolites cross-reacting with l-lactate oxidase in the blood gas analyzer [1]. In our case, a slightly elevated AG with a high OG and lactate elevation presented as a scenario of co-ingestion. The patient was given supportive treatment and fomepizole based on the recommendations. However, in the absence of serum toxic alcohol levels, co-ingestion was suspected, specially given his significant neurological compromise without improvement. Therefore, early hemodialysis (HD) was performed, despite this being a controversial decision. This was followed by a fast recovery of the neurological status of the patient and potentially prevented renal failure. A particularly high index of suspicion for combined TA ingestion should be raised when encountering an individual with lactic acid elevation, high OG, and normal AG. In these settings, early HD should be considered in cases of co-ingestion and absence of available serum alcohol levels.
Case report
40-year-old male was brought to our emergency department by his family members for reported paint thinner ingestion. His medical history included recent ischemic stroke, hypertension, diabetes, bipolar disorder, and schizophrenia. His initial vital signs were Temperature 98.4° F, pulse 76 bpm, blood pressure 87/56 mmHg, respiratory rate 16/min, and oxygen saturation of 97% on room air. The patient was intubated for airway protection, given his obtunded mental status. Prior to the intubation, arterial blood gas showed pH 7.22, PaCO2 54 mmHg, PaO2 75 mmHg. Complete blood count was unremarkable. His electrolytes included a sodium 134 mmol/L, potassium 4 mmol/L, chloride 97 mmol/L, bicarbonate 26 mmol/L, blood urea nitrogen 19 mg/dL, creatinine 1.27 mg/dL, blood glucose 158 mg/dL, albumin 3.3 g/dL. Serum measured lactic acid was 5.26 mmol/L. Six panel drug screen was negative. Serum lithium, salicylate, and ethanol levels were undetected. Serum valproate level was 30.0 µm/mL. Measured serum osmolality was 454 mosm/kg, and calculated serum osmolality was 284 mosm/kg with osmolar gap of 170 mosm/kg. Serum acetone and urine ketones were not detected. Urine microscopy did not reveal any evidence of crystals or stones. The initial measured AG was 11 and corrected by an albumin of 3.3 was 12.75, which was slightly elevated. Methanol, ethylene glycol and propylene glycol serum levels were obtained upon admission, but were processed outside of our facility. Therefore, results were available later on during the hospital course. The absence of ketonuria argued against isopropyl ingestion.
The patient was initially started on bicarbonate drip. Discussion with our nephrologist and poison control took place and decision was made to administer fomepizole. The onset of action of fomepizole is 1.5–2 h. Hence, the osmolar and anion gaps were followed every 4 h for 12 h, with a measured serum osmolality improving from 454 to 414 mosm/kg and a calculated serum osmolality increasing from 280 to 305 mosm/kg at 12 h. As noticed, the osmolar gap improved from 170 to 109 mosm/kg after the administration of fomepizole. However, given the lack of recovery in his mental status, suspected co-ingestion and unknown serologic alcohol levels, it was decided to initiate emergent hemodialysis 24 h after his admission. He was dialyzed without ultrafiltration for 3 h. Measured serum osmolality, osmolar gap and serum creatinine after dialysis was 327 mosm/kg, 32 mosm/kg and 0.71 mg/dL, respectively. The patient was extubated the day after and had an uneventful hospital stay afterwards. He was transferred to a psychiatric unit. The results of alcohol levels came back after the patient was treated. To reiterate, the serum alcohol levels were obtained upon admission, but they were reported days after, since these were send outs. They included: 86,500 mg/dL (288,333 mmol/L) for serum methanol, 2 days into admission and 351 mg/dL (2340 mmol/L) for ethylene glycol, 6 days into admission. These levels met criteria for dialysis but were not available initially. Serum ethanol, acetone, and isopropyl were not detected. We later found out from patient’s family that he actually ingested three solutions: paint thinner (turpentine), antifreeze coolant (ethylene glycol plus diethylene glycol), and windshield washer fluid (methyl alcohol).
Discussion
An early presentation after the ingestion will not give enough time for these toxic alcohols to be metabolized, translating in a high osmolar gap without a high anion gap. Isopropyl alcohol ingestion traditionally presents with a high osmolar gap without anion gap metabolic acidosis [1]. However, this should not remain the sole differential diagnosis when there is suspected or documented ingestion of other alcohols. In addition, false elevation of the lactate can occur with ingestion of ethylene glycol due to its metabolite products cross-reacting with l-lactate oxidase in blood gas analyzer [1].
Treatments for the ingestion of toxic alcohols include gastrointestinal decontamination if they are ingested within 30–60 min. Administration of ethanol or fomepizole, ADH inhibitors, can attenuate the metabolism of these toxic alcohols into organic acids, which carry most of the toxicity. Ethanol has 10–20 times greater affinity for ADH than the other alcohols, but is not approved by the FDA as a therapeutic intervention. Fomepizole has an affinity for ADH of 500–1000 times greater than ethanol [2]. Anecdotally, the threshold for initiating ADH inhibitors is a serum level of toxic alcohols of 20 mg/dL (3 mmol/L of ethylene glycol or 6 mmol/L of methanol) [3]. Nonetheless, other specific criteria have been proposed as well: toxic alcohol levels < than 10 mmol/L (62 mg/dL of ethylene glycol or 32 mg/dL of methanol) with clinical symptoms; suspected history of ingestion with an osmolal gap > than 25 mosm/kg; or any suspected ingestion with two or more serologic findings (arterial pH < than 7.3, serum bicarbonate < than 20 mmol/L, Osmolal gap > than 25 mosm/kg, urinary oxalate crystals or visual disturbances) [3]. Administration of bicarbonate can be considered in the case of ethylene glycol poisoning because it increases renal excretion of formate and glycolate. Hemodialysis can remove both, the alcohols and their organic acid anions. According to the American Academy of Clinical Toxicology, hemodialysis is recommended in the presence of severe metabolic acidosis unresponsive to conventional therapy, presence of severe symptoms, renal failure, or alcohol levels > 50 mg/dL [4, 5].
Acute intoxication with substances such as methanol, ethylene glycol and propylene glycol classically presents with a high anion gap metabolic acidosis, elevated osmolar gap and end organ damage including neurological toxicity and renal failure [2]. Toxic alcohol levels are measured by gas chromatography (GC), a technique not commonly performed on-site in hospital clinical laboratories. Therefore, in the absence of this information, clinical suspicion and classic metabolic derangements guide therapy. Most patients are treated with supportive measures and fomepizole, alcohol dehydrogenase inhibitor, when the toxic alcohol is unknown. Hemodialysis (HD) has been used to remove alcohols and its metabolites in specific cases [3]. However, early HD has not been recommended in early phases of intoxication with high osmolar gap and normal anion gap metabolic acidosis.
In our case, the administration of fomepizole followed by an early hemodialysis given the poor clinical improvement with supportive treatment was followed by a fast recovery of the neurological status of the patient and potentially prevented renal failure.
After his complete recovery, the patient was transferred to an inpatient psychiatric unit and then discharged home.
The latest report from the National Poison Data System (NPDS) in 2015 shows that less than 1% of human exposure cases involved 4 or more substances. Alcohols represented a 1.13% of the substance categories more frequently involved in human exposures. Regarding treatment, a total of 1850 patients received fomepizole and 2663 received hemodialysis. These are absolute numbers that were not divided in subgroups based on the ingested substance. Of 29% of patients treated in a Healthcare facility, only 4.9% were admitted to a critical care unit. The overall number of patients receiving fomepizole and/or hemodialysis is relatively low [4].
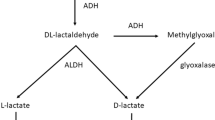
These toxic alcohols are known for their osmotic property. Once absorbed, they increase the measured serum osmolality above the calculated serum osmolality, producing an osmolar gap. Once in the blood, most of these alcohols are first metabolized into their toxic byproducts by the enzyme alcohol dehydrogenase which is found in the liver. This is the rate limiting step for the metabolism of toxic alcohols [2, 5]. Table 1 illustrates the half- lifes of these substances alone and in the presence of other ADH competitors. Each alcohol has its own kinetics and the ingestion of multiple elements can compete or saturate the enzyme faster. In the absence of competitor, the half-life of methanol is 14–30 h, while ethylene glycol is 3–8 h [2]. The production of organic acids occurs after the first step in the metabolism. Methanol is first metabolized to formaldehyde then to formic acid. Ethylene glycol is first metabolized to glycoaldehyde then to glycolic acid. These organic acids are responsible for creating anion gap metabolic acidosis [5].
Ethylene glycol is an organic compound used in industrial production of antifreeze formulations such as automobile coolants, convective heat transfer fluids, and road deicers. Methanol on the other hand, is a substance used in windshield wiper fluid, antifreeze, household cleaning products, and model airplane fuel. Both methanol and ethylene glycol can be absorbed through the skin but most intoxication of these alcohols occurs after oral ingestion [2, 3].
Traditionally, serum alcohol levels and both the osmolal and the anion gap guide treatment. However, clinical suspicion should prevail over falsely normal anion gap or in the setting of unknown values.
The osmolal gap is the difference between the measured serum osmolality minus the estimated osmolality. There are multiple formulas, but none of them has been identified as the most accurate. Currently, the most commonly used one in clinical practice is Posm (mOsm/kg) = 2 × Na+ (mEq/L) + glucose(mg/dl)/18 +BUN (mg/dl)/ 2,8. Not only has the formula been questioned, but also the wide range of the reference value, which can go from − 10 to + 19 mOsm/L [6, 7]. This is particularly relevant when a toxic alcohol level, which would constitute an indication for ADH antidotes, such as ethylene glycol (20 mg/dL) will correspond to an osmolal shift of only 3 mOsm approximately [5]. Moreover, toxic alcohols are not the only cause of an increased osmolal gap, which can also be seen in ketoacidosis, renal failure and severe lactic acidosis, amongst others [8].
The differential diagnosis of a high osmolar gap with a normal anion gap is not restricted to isopropyl alcohol ingestion, but could constitute an early presentation of a case of co-ingestion of combined toxic alcohols. This scenario should be suspected when encountering an individual with lactic acid elevation, high osmolar gap, and a normal anion gap. Likewise, a fast intervention with ADH inhibitors and early hemodialysis should be considered if there is poor clinical improvement.
References
Pernet P, Bénéteau-Burnat B, Vaubourdolle M, Maury E, Offenstadt G. False elevation of blood lactate reveals ethylene glycol poisoning. Am J Emerg Med. 2009;27(1):132.e1–.e2.
Kraut JA, Kurtz I. Toxic alcohol ingestions: clinical features, diagnosis, and management. Clin J Am Soc Nephrol. 2008;3(1):208–25.
McMartin K, Jacobsen D, Hovda KE. Antidotes for poisoning by alcohols that form toxic metabolites. Br J Clin Pharmacol. 2016;81(3):505–15.
Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. 2015 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd annual report. Clin Toxicol (Phila). 2016;54(10):924–1109.
Koppaka V, Thompson DC, Chen Y, Ellermann M, Nicolaou KC, Juvonen RO, et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol Rev. 2012;64(3):520–39.
Liamis G, Filippatos TD, Liontos A, Elisaf MS. Serum osmolal gap in clinical practice: usefulness and limitations. Postgrad Med. 2017;129(4):456–9.
Aabakken L, Johansen KS, Rydningen EB, Bredesen JE, Ovrebø S, Jacobsen D. Osmolal and anion gaps in patients admitted to an emergency medical department. Hum Exp Toxicol. 1994;13(2):131–4.
Hoffman RS, Smilkstein MJ, Howland MA, Goldfrank LR. Osmol gaps revisited: normal values and limitations. J Toxicol Clin Toxicol. 1993;31(1):81–93.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declared that no conflict of interest exists.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Cervantes, C.E., Chu, A., Heller, D. et al. Early dialysis in a rare case of combined toxic alcohols ingestion. CEN Case Rep 9, 11–14 (2020). https://doi.org/10.1007/s13730-019-00417-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-019-00417-0