Abstract
Sarcoidosis affects multiple organs including lung, heart and kidney. Sarcoidosis causes hypercalcemia, hypergammaglobulinemia, and rarely, granulomatous interstitial nephritis, resulting in renal stromal damage. Granulomatous interstitial nephritis is characterized as interstitial nephritis with noncaseating epithelioid granulomas. Diagnosing granulomatous interstitial nephritis before patient’s death is challenging; hence, only few cases proven by renal biopsy have been reported till date. We present a case of acute kidney injury caused by granulomatous interstitial nephritis as a renal manifestation of sarcoidosis proven by renal biopsy, which can be confirmed by 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Glucocorticoid therapy was helpful for improving and maintaining her renal function over a 6-year period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is an idiopathic chronic systemic disease characterized by the formation of epithelioid cell granuloma in the involved organs. In patients with sarcoidosis, however, granulomatous infiltration in the kidney is rarely diagnosed pathohistologically, because renal examinations including laboratory data and imaging tests mostly do not detect any abnormalities. We herein report a case of acute kidney injury caused by granulomatous interstitial nephritis as a renal manifestation of sarcoidosis proven by renal biopsy, which was seen as intrarenal nodular lesions before diagnosis on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT).
Case report
A 62-year-old Japanese woman was referred to our hospital because of general fatigue, weight loss, and renal dysfunction. She had a 2-year history of uveitis. On admission, physical examination revealed no remarkable findings except cutaneous dryness without skin turgor reduction. Her body pressure and temperature were 130/70 mmHg and 36.5 °C, respectively. White blood cell count was 6500/mm3, with 7.0% eosinophils. Red blood cell count was 347 × 104/mm3 and haemoglobin level was 10.1 g/dL. Platelet count was 20.3 × 104/mm3. C-reactive protein level was 0.5 mg/dL. Blood urea nitrogen (BUN), serum creatinine (SCr), and uric acid levels were elevated at 74, 4.56, and 7.5 mg/dL, respectively. The BUN/SCr ratio was 16.2 (< 20). Levels of plasma calcium and serum 1,25-(OH)2D3 were elevated to 13.1 mg/dL and 122 pg/mL, respectively. Intact parathyroid hormone level was suppressed to 8.1 pg/mL. Lysozyme level was elevated to 53.5 µg/mL, while serum angiotensin-converting enzyme level was 19.0 IU/L. Soluble IL-2R level was elevated to 7760 U/mL. Urinalysis revealed proteinuria (1.4 g/g·Cr) and glycosuria (50 mg/dL), respectively. Urinary N-acetylglucosaminidase and β2-microgloburine levels were elevated to 22.1 U/g·Cr and 70,462 µg/L, respectively. Other laboratory examination data, including antinuclear antibody, rheumatoid factor, ANCA, liver function, and tumour markers, were all within normal limits. Electrocardiography showed no arrhythmia and block. Chest radiography and computed tomography (CT) showed multiple hilar and mediastinal lymphadenopathy without pulmonary infiltrates. Abdominal echography and CT revealed splenomegaly without any remarkable findings in the bilateral kidneys (Fig. 1a, b). Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) demonstrated increased FDG uptake within the multiple lymph nodes in the mediastinum, the multiple nodular lesions in bilateral kidneys, and spleen (Fig. 2a, c). Abdominal echography revealed that recovery rate of bronchoalveolar lavage fluid (BALF) was 60% (100/150 mL). BALF analysis revealed that total cell concentration was 2.5 × 105 cells/mL, with 72.0% macrophages, 16.0% eosinophils, and 12.0% lymphocytes (CD4 80.3%, CD8 15.6%, CD4/CD8 ratio 5.14 > 3.5), consistent with sarcoidosis. We strongly suspected sarcoidosis that caused renal failure. Therefore, we performed renal biopsy, which revealed marked interstitial fibrosis, severe mononuclear inflammatory cell infiltration, and noncaseating epithelioid granulomas (Fig. 3a–c) without any remarkable glomerular lesions (Supplementary Fig. 1) or any remarkable findings by immunofluorescence staining (Fig. 3d), findings consistent with granulomatous interstitial nephritis due to sarcoidosis. As shown in Fig. 4, we initiated treatment with 40 mg daily of furosemide, 80 units daily of elcatonin, and 5 mg daily of alendronate sodium hydrate. Subsequently, 30 mg daily of oral prednisolone (PSL) was started for treatment of sarcoidosis. The patient’s hypercalcaemia improved markedly on day 19. Thereafter, plasma calcium level was maintained in the normal range. Her symptoms and renal dysfunction and proteinuria improved (Fig. 4). After 2-month hospitalization, her SCr and plasma calcium levels improved to 1.5 and 8.8 mg/dL, respectively, and she was discharged without any symptoms. 1 year later, oral PSL dose was tapered to 5 mg daily without relapse, and steroid therapy was continued. At 6-year follow-up, the patient had no clinical, hematological, biochemical, or radiological abnormality, particularly on follow-up 18F-FDG-PET/CT (Fig. 2b, d).
18F-FDG PET/CT findings. a, c 18F-FDG PET/CT on admission showing 18F-FDG uptake within the multiple lymph nodes in the mediastinum (red arrows), the multiple nodular lesions in bilateral kidneys (yellow arrows), and spleen (green arrow). b, d Follow-up 18F-FDG PET/CT showing no remarkable 18F-FDG uptake
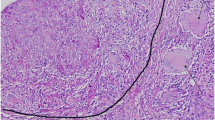
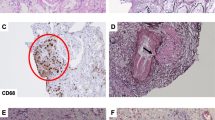
Pathohistological findings of renal biopsy. Renal biopsy specimen showing interstitial nephritis with noncaseating epithelioid granuloma (arrowheads), consistent with granulomatous interstitial nephritis. (a periodic acid-Schiff, × 400, b periodic acid methenamine silver, × 400, c Masson trichrome, × 400). Immunofluorescence staining showing no remarkable findings (d IgM)
Discussion
Renal involvement occurs in less than 10% of the cases with sarcoidosis and due to one or more of following three mechanisms: (1) granulomatous interstitial nephritis, (2) long-standing hypercalcemia or hypercalciuria with nephrocalcinosis, and (3) secondary to hypergammaglobulinemia [1]. Granulomatous interstitial nephritis is a rare histologic diagnosis that presents in less than 1% of native renal biopsies [2], and biopsy-proven granulomatous interstitial nephritis in patients with sarcoidosis is rarely reported because most cases of granulomatous interstitial nephritis do not present with any clinical signs [3].
A previous report demonstrated that patients with renal sarcoidosis also exhibit hypercalcaemia more frequently than those without renal involvement [4]. It is believed that the main mechanism of abnormal calcium metabolism in sarcoidosis involves activated monocytes/macrophages in granulomas, which results in the overproduction of calcitriol [5]. Previous investigators hypothesized that the high frequency of hypercalcaemia in renal sarcoidosis could be caused by a large amount of granulomas in the kidney [6], and that the low frequency of biopsy-proven granulomatous interstitial nephritis might be caused by sampling errors [6, 7]. In our case, hypercalcaemia was initially observed; therefore, we speculated that hypercalcaemia was involved in the aetiology of acute kidney injury. However, although hypercalcaemia was initially notable, renal dysfunction gradually improved after the initiation of steroid therapy. Furthermore, the 18F-FDG-PET/CT demonstrated that nearly all kidney lesions were resolved by treatment. Additionally, the hypercalcaemia did not recur. Therefore, we speculated that the granulomatous interstitial nephritis could be the primary cause of renal damage, and that secondary hypercalcaemia might be associated with progressive renal dysfunction.
18F-FDG-PET/CT is currently considered to be a useful tool for detecting several inflammatory diseases as well as malignant neoplasms. Inflammatory cells such as neutrophils, activated macrophages, and lymphocytes have increased 18F-FDG uptake, causing important tracer accumulation in inflammatory and infectious processes [8]. In sarcoidosis proven by biopsies, the sensitivity of 18F-FDG-PET/CT has been reported to be higher than that of 67Ga scintigraphy (thoracic disease: 100 vs 71%; sinonasal: 100 vs 71%; pharyngo-laryngeal: 80 vs 67%) [8]. Furthermore, although there is no study on renal lesions, 18F-FDG-PET/CT is a useful tool to detect not only pulmonary involvement but also extrapulmonary involvement [8, 9]. 18F-FDG-PET/CT has several advantages over 67Ga scintigraphy. Due to the favourable physical conditions of positron emitters, PET enables higher spatial resolution than conventional nuclear medicine imaging techniques [9]. Therefore, the high-quality images produced by 18F-FDG-PET/CT enable the detection of even small lesions [9]. In our case, as previously reported [10], steroid therapy is usually effective in granulomatous interstitial nephritis associated with sarcoidosis, resulting in resolution of renal dysfunction, while it is considered that delay in diagnosis and treatment could lead to poor prognosis. We speculate that 18F-FDG-PET/CT might also detect renal sarcoidosis with granulomatous involvement prior to renal biopsy for diagnosis and might be useful to determine the indication for renal biopsy. Furthermore, 18F-FDG uptake of lung in pulmonary sarcoidosis has been reported to be concordant with histopathologic activity of pulmonary sarcoidosis and was found to be decreased after steroid therapy [9, 11]. In our case, follow-up 18F-FDG-PET/CT revealed that 18F-FDG uptake in renal sarcoidosis was found to be decreased after steroid therapy. Therefore, we speculated that 18F-FDG-PET/CT could be useful for evaluating the therapeutic effect in renal sarcoidosis.
Our study has some limitations. First, this is a case report describing only one and its 18F-FDG-PET/CT findings. Therefore, it is considered necessary to accumulate similar cases. Second, repetitive 18F-FDG-PET/CT examination on sarcoidosis is also a heavy burden on medical economics. Therefore, in clinical practice, physicians should avoid 18F-FDG-PET/CT frequently and the necessity and timing of 18F-FDG-PET/CT examination should be thoroughly considered so that its efficacy over medical burden can be obtained.
In conclusion, this is a case of acute kidney injury caused by granulomatous interstitial nephritis associated with sarcoidosis which was proven by renal biopsy and detected as intrarenal nodular lesions by 18F-FDG-PET/CT. We propose that although the pathohistological findings of renal biopsy are necessary to diagnose granulomatous interstitial nephritis accurately, 18F-FDG-PET/CT might be very helpful in estimating renal involvement of sarcoidosis and in considering the adaptation of renal biopsy.
References
Mitwalli AH, Abu-Aisha H, Al-Wakeel J, Memon NA, Al-Mobeireek A, Huraib SO. Sarcoidosis with partial reversibility of renal failure: two case reports with review of literature. Saudi J Kidney Dis Transpl. 1999;9:451–6.
Joss N, Morris S, Young B, Geddes C. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol. 2007;2:222–30.
Ricker W, Clark M. Sarcoidosis: a clinicopathologic review of 300 cases including 33 autopsies. Am J Clin Pathol. 1959;19:725–49.
Semenzato G. ACCESS: a case control etiologic study of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22:83–6.
Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–60.
Mahévas M, Lescure FX, Boffa JJ, Delastour V, Belenfant X, Chapelon C, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore). 2009;88:98–106.
Shah R, Shidham G, Agarwal A, Albawardi A, Nadasdy T. Diagnostic utility of kidney biopsy in patients with sarcoidosis and acute kidney injury. Int J Nephrol Renovasc Dis. 2011;4:131–6.
Braun JJ, Kessler R, Constantinesco A, Imperiale A. 18F-FDG PET/CT in sarcoidosis management: review and report of 20 cases. Eur J Nucl Med Mol Imaging. 2008;35:1537–43.
Nishiyama Y, Yamamoto Y, Fukunaga K, Takinami H, Iwado Y, Satoh K, et al. Comparative evaluation of 18F-FDG PET and 67 Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006;47:1571–6.
Hilderson I, Van Laecke S, Wauters A, Donck J. Treatment of renal sarcoidosis: is there a guideline? Overview of the different treatment options. Nephrol Dial Transpl. 2014;29:1841–7.
Brudin LH, Valind SO, Rhodes CG, Pantin CF, Sweatman M, Jones T, et al. Fluorine-18 deoxyglucose uptake in sarcoidosis measured with positron emission tomography. Eur J Nucl Med. 1994;21:297–305.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13730_2017_287_MOESM1_ESM.ppt
Pathohistological findings of renal biopsy. The renal biopsy specimen showing interstitial nephritis with noncaseating epithelioid granuloma, which is consistent with granulomatous interstitial nephritis. No other remarkable glomerular lesions were noted. (A: periodic acid-Schiff, × 100) (PPT 2553 KB)
About this article
Cite this article
Horino, T., Matsumoto, T., Inoue, K. et al. A case of acute kidney injury caused by granulomatous interstitial nephritis associated with sarcoidosis. CEN Case Rep 7, 34–38 (2018). https://doi.org/10.1007/s13730-017-0287-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-017-0287-9