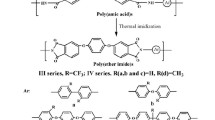
Abstract
A series of fluorinated aromatic poly(ether imide)s (PEIs)(IV) containing bulky fluorene bis(ether anhydride) [(9,9-bis[4-(3,4-dicarboxyphenoxy)-phenyl]fluorene, (I)] with various fluorinated diamines (II) were prepared through polyaddition and chemical, or, thermal imidization two-stage process. The resulting PEIs have inherent viscosities of 0.50–0.58 dL/g and are readily soluble in various organic solvents. Both chemically and thermally imidized PEI films are colorless and highly transparent with cut-off wavelengths below 375 nm and yellowness index ranging from 5.4 to 13.4. They also exhibited excellent thermal stability with glass transition temperatures (Tgs) up to 297 °C and 10% weight loss temperatures (Tds) up to 583 °C in nitrogen atmosphere. Furthermore, the films have tensile strengths of 90–147 MPa and initial modulus of 2.0–2.6 GPa. Meanwhile, all the obtained fluorinated PEIs exhibited low dielectric constant of 2.69–3.19 at 1 MHz and low water uptake of 0.17–0.34%. Finally, the non-fluorinated counterparts derived from similar bis(ether anhydride) (I) with various bis(ether amine)s were prepared and compared. The results demonstrate that the fluorinated PEIs are promising candidates for high-performance films due to the bulky fluorene bis(ether anhydride) and trifluoromethyl substituted along the polymer repeat unit chain.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic polyimides (PIs) are important high-performance polymers widely used in the fields of optical [1], microelectronic [2], aerospace and separation [3] due to their excellent thermal, mechanical and electrical properties as well as good chemical resistance [4, 5]. Despite the advantages mentioned above, the main drawback of this kind of polymers is poor solubility together with high softening/melting temperatures that restricts their processability [6]. Furthermore, the strong absorption in the visible region came from the strong intermolecular charge transfer complex (CTC) effect limits their applications in optoelectronic fields [7, 8]. To enhance the processability and optical properties of PIs, various techniques have been recently developed by researchers to overcome these critical issues, such as introducing (i) a fluorine-containing group [9], (ii) a bulky substituent [10], (iii) an asymmetric or twisted structure [11], (iv) a flexible linkage [12], and (v) an aliphatic unit [13] into the diamines or dianhydrides monomers, thus the novel PIs could be obtained from the above modified monomers.
An alternative polymer structure, named as poly(ether imide)s (PEIs), which contains flexible ether linkage in its dianhydride monomer, has been designed and adopted in industry due to its enhanced solubility and optical properties. An important PEI polymer, Ultem 1000, derived from 4,4’bisphenol-A dianhydride (BPADA) and m-phenylene diamine offered reasonable thermal stability, good mechanical property and acceptable optical property [14]. Since then, several types of modified bis(ether anhydride)s and their related PEIs have been reported [15,16,17,18]. For example: hydroquinone [15], adamantly [16], 2,2 ‘-dimethyl-4,4 ‘-biphenyl [17], 3,3’,5,5’-tetramethylbiphenyl [18] and fluorinated substituents [19, 20] as a core for preparing the corresponding bis(ether anhydride)s. With changing the core of the bis(ether anhydride), the related PEI properties could be changed significantly. To further improve the properties of PEIs, a pendent trifluoromethyl-substituted diamine [21,22,23] had been considered as a sufficient method to achieve excellent organo solubility, optical properties and dielectric properties. In summary, it has been found that fluorinated PEI has better properties than other polyamides, but its properties still need to be optimized.
The contribution of the fluorene moiety in its polymer structure has been significantly increased due to the advantages of the bulky and rigid fluorene unit for high thermal stability and good solubility in organic solvents, so as to obtain high-performance PIs [24,25,26]. Furthermore, incorporation of the fluorene moiety is also preferred to the lower transparency and dielectric constant of the PIs, because of the low packing density of the bulky cardo aromatic structure [27, 28]. In a previous study, Hsiao et al. (1999) reported the synthesis and characterization of a series of PEIs derived from a bulky fluorene dianhydride (9,9-bis[4-(3,4-dicarboxy-phenoxy)phenyl]fluorene (I) with various aromatic diamines [24]. Although most of them exhibited good film formability and high thermal stability, the organic solubility still needed to be improved. Furthermore, their optical properties were not investigated in detail. In this study, we thus designed and synthesized a series of fluorinated PEIs (IV) containing fluorene dianhydride (I) and various fluorinated diamines (II). In addition, reference non-fluorinated PEIs (V) were prepared and compared. The organic solubility, thermal, optical, mechanical and dielectric properties of all the prepared PEIs were assessed.
Experimental
Monomer synthesis
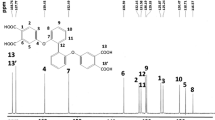
9,9-Bis(4-hydroxyphenyl)fluorene and 4-nitrophthalodinitrile were purchased from Acros, and were used as received. The target 9,9-bis[4-(3,4-dicarboxyphenoxy)-phenyl]fluorene dianhydride (I) was synthesized according to the three reported steps [23] and the synthetic routes are illustrated in Scheme 1. The corresponding characters of the precursors I’, I’’ and I are shown below: (a) compound I’: mp 269–270 °C ([24] 260–262 oC). IR (KBr): 2233 (C ≡ N), 1253 cm−1 (C-O). 1H NMR (500 MHz, DMSO-d6, δ, ppm): 8.03 (d, J = 8.7 Hz, 2H, Hb), 7.93 (d, J = 7.4 Hz, 2H, Hi), 7.74 (s, 2H, Ha), 7.49 (d, J = 7.6 Hz, 2H, Hf), 7.41 (t, J = 7.4 Hz, 2H, Hh), 7.35 (t, J = 7.4 Hz, 2H, Hg), 7.33 (dd, J = 8.7, 2.3 Hz, 2H, Hc), 7.24 (d, J = 8.4 Hz, 4H, He), 7.07 (d, J = 8.4 Hz, 4H, Hd). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 160.8 (C3), 152.6 (C7), 150.2 (C12), 142.8 (C17), 139.5 (C10), 136.3 (C5), 129.8 (C9), 128.2 (C14), 128.0 (C16), 126.1 (C13), 122.8 (C15), 122.1 (C4), 120.7 (C2), 120.1 (C8), 116.7 (C1), 115.9, 115.4 (C18,18’), 108.3 (C6), 64.1(C11); (b) compound II’: IR (KBr): 2400–3400 (O–H), 1704 (C = O), 1222 cm−1 (C–O–C). 1H NMR (500 MHz, DMSO-d6, δ, ppm): 7.93 (d, J = 7.6 Hz, 2H, Hi), 7.90 (d, J = 8.6 Hz, 2H, Hb), 7.49 (d, J = 7.5 Hz, 2H, Hf), 7.41 (t, J = 7.5 Hz, 2H, Hh), 7.34 (t, J = 7.5 Hz, 2H, Hg), 7.31 (s, 2H, Ha), 7.20 (d, J = 8.6 Hz, 4H, He), 7.09 (dd, J = 8.6, 2.5 Hz, 2H, Hc), 7.02 (d, J = 8.6 Hz, 4H, Hd). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 168.2 (C3), 167.5 (C18,18’), 158.6 (C7), 154.2 (C12), 150.6 (C13), 141.6 (C10), 139.6 (C17), 137.0 (C16), 129.6 (C5,9), 128.2 (C14), 128.0 (C15), 126.1 (C6), 120.7 (C4), 119.5 (C8), 119.3 (C2), 64.1 (C11); and (c) compound I: mp 244–245 oC ([24]) 239–241 oC). IR(KBr): 1851 (asym. C = O str.), 1770 (sym. C = O str.), 1264 cm−1 (C–O–C). 1H NMR (500 MHz, DMSO-d6, δ, ppm): 7.93 (d, J = 8.4 Hz, 2H, Hb), 7.83 (d, J = 7.5 Hz, 2H, Hi), 7.48 (d, J = 7.6 Hz, 2H, Hf), 7.45 (d, J = 8.4 Hz, 2H, Hc), 7.43 (t, J = 7.5 Hz, 2H, Hh), 7.42 (s, 2H, Ha), 7.37 (t, J = 7.6 Hz, 2H, Hg), 7.35 (d, J = 8.6 Hz, 4H, He), 7.01 (d, J = 8.6 Hz, 4H, Hd). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 164.8 (C3), 162.5, 162.0 (C18,18’), 152.9 (C7), 150.4 (C12), 143.5 (C13), 140.0 (C10), 133.9 (C1), 130.2 (C9), 128.1 (C5), 128.0 (C17), 127.6 (C16), 126.0 (C14), 125.0 (C15), 124.3 (C6), 120.5 (C4), 120.4 (C8), 112.5 (C2), 64.5 (C11).
The CF3-substituted bis(ether amine)s (IIa-IIg) used in this study were synthesized according to pervious literatures (IIa [29], IIb [30], IIc [31], IId [32], IIe [33], IIf [34], IIg [35]) through chloro-displacement reactions and reduction reactions. In addition, the analogous non-fluorinated bis(ether amine)s, II’c [36], II’d [37] and II’g [38] were prepared from p-chloronitrobenzene and the corresponding aromatic diols through similar synthetic procedures mentioned above. Other commercial non-fluorinated bis(ether amine)s, 1,4-bis-(4-aminophenoxy)benzene (II’a), 4,4’-bis(4-aminophenoxy)biphenyl (II’b), 2,2-bis[4-(4-amino-phenoxy)phenyl]propane (II’e) and 2,2-bis[4-(4-aminophenoxy)phenyl]-hexafluoropropane (II’f) were purchased from TCI and Chriskev, respectively. N,N-Dimethylacetamide (DMAc, Fluka) and N,N-dimethylformamide (DMF, Fluka) were purified by stirring with calcium hydride followed by distillation under vacuum pressure.
PEI synthesis
Thermal imidization
Take IVa (H) as an example. An amount of 0.257 g (0.6 mmol) of fluorinated bis(ether amine) IIa and 5.0 mL of dried DMAc were added ina flask equipped with a mechanical stirrer. After the diamine was dissolved completely, an equimolar of bis(ether anhydride) I (0.386 g, 0.6 mmol) was added in one portion. The mixture was stirred at room temperature for 12 h to obtain a poly(amic acid) (PAA) solution. Followed, the solution was poured into a glass culture dish, which was placed into a 100 °C oven for 1 h to remove the casting solvent. Finally, the thermal imidization was carried out by keeping this sample in a temperature-programmable oven by sequential heating from 100 °C to 250 °C at a heating rate of 2 °C/min and hold at 250 °C for another 30 min. The fully imidized flexible film IVa (H) was self-stripped from the glass surface by immersion in water.
Chemical imidization
Take IVa (C) as an example. Acetic anhydride (0.6 mL) and pyridine (0.3 mL) (2:1 v/v) were added to the PAA solution that prepared in the same manner as mentioned above, and the mixture was stirred for 1 h at a temperature of 80 °C to achieve a complete chemical imidization. The viscous solution was poured into a glass culture dish, which was placed in a 100 °C oven for 1 h to evaporate the solvent and form a thin film. Finally, the PEI film was obtained by keeping the samples in a temperature-programmable oven by sequential heating from 100 to 200 °C at a heating rate of 2 °C/min and hold at 200 °C for another 1 h. A flexible film was obtained through self-stripping from the glass surface by immersion in water.
(ηinh of IVa (H) = 0.54 dL/g). IR (PAA film): 2400–3600 (O–H), 1716, 1670 (C = O), 1489 cm−1 (arom. C = C). IR (PEI film): 1780 (asymmetric imide C = O stretch), 1728 (symmetric imide C = O stretch), 1500 (arom. C = C), 1379 (C-N stretch), 1243 (C-O), 1052, 746 cm−1 (imide ring deformation). 1H NMR (500 MHz, DMSO-d6, δ, ppm): 7.88 (d, J = 8.3 Hz, 2H, Hb), 7.81 (d, J = 7.5 Hz, 2H, Hi), 7.76 (d, J = 2.2 Hz, 2H, Hj), 7.52 (dd, J = 8.9, 2.2 Hz, 2H, Hk), 7.47 (d, J = 7.6 Hz, 2H, Hf), 7.45 (d, J = 2.0 Hz, 2H, Ha), 7.42 (t, J = 7.5 Hz, 2H, Hh), 7.36 (t, 2H, Hg), 7.34 (dd, 2H, Hc), 7.32 (d, J = 8.7 Hz, 4H, He), 7.14 (s, 4H, Hm), 7.03 (d, J = 8.9 Hz, 2H, Hl), 6.99 (d, J = 8.7 Hz, 2H, Hd). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 166.4, 166.3 (C27,27’), 163.7 (C3), 155.1 (C7), 153.6(C24), 152.3(C21), 150.6 (C12), 142.9 (C13), 140.0 (C10), 134.0 (C1), 131.3 (C18), 130.1 (C9), 128.0 (C5), 127.9 (C17), 126.2 (C6), 126.0 (C16), 125.9 (C14), 125.6 (C19), 124.9 (C15), 123.3 (C23), 122.8 (C26, quartet, 1JC-F = 272 Hz), 121.6 (C8), 121.5 (C20, quartet, 2JC-F = 32 Hz), 120.4 (C4), 120.1 (C25), 118.7 (C22), 112.2 (C2), 64.5 (C11).
The preparation of other PEIs was in the same manner as mentioned above. The thickness of these films was about 30–60 μm.
Measurements
The measurement details are presented in Supplementary Materials.
Results and discussion
Synthesis
The fluorene-based bis(ether anhydride) (I) compound was synthesized through a well-developed three-step synthetic procedures [23] as shown in Scheme 1. Although the structure has been presented in the literature[24], its structural identification is not fully performed, but only the representative IR spectra. In 1H NMR spectra (Fig. 1), an upfield shift of the protons on the outer benzene ring was observed after the cyano group of I’ was converted into the carboxyl group and anhydride group. In 13C NMR spectra (Fig. 2), the significant resonance carbon peaks of the cyano, carboxyl acid precursors to anhydride target compound shifted from 115–116 ppm, to 167–169 and 162–163 ppm, respectively. Hence, the 1H and 13C NMR spectra and the chemical shift peak assignments are in good agreement with the spectra of the synthesized ones.
Then, a series of fluorinated PEIs (IVa-g) and their analogous non-fluorinated PEIs (Va-g) that contain fluorene-based bis(ether anhydride) (I) and various fluorinated (IIa-g) or non-fluorinated (II’a-g) diamines were prepared (Scheme 2). The two-step procedures include ring-opening polyaddition between bis(ether anhydride) and various diamines to form PAA precursors and thermal (slow heating to 250 °C) or chemical (treatment with a mixture of acetic anhydride and pyridine) imidization to obtain the corresponding flexible and tough PEI films. Table 1 shows the inherent viscosities and GPC results of the thermally imidized fluorinated PEIs (IV series). The inherent viscosities are in the range of 0.50–0.58 dL/g. In addition, the number-average molecular weights (Mns) are in the range of 12,500–19,500, and the weight-average molecular weights (Mws) are in the range of 29,900–36,900, accordingly with the PDI (polydispersity index) values of 1.89–2.39.
The chemical compositions of the PEIs were also determined through elemental analysis (EA), IR and NMR spectroscopy techniques. Table 1 displays the EA results of the PEIs. All the found values are in good agreement with the calculated values of the proposed structures. Figure 3 illustrates the selected IR spectra of the completely imidized PEI (IVa) and its PAA precursor (IIIa). The characteristic absorption peaks of PAA appeared at 3600–2400 (O–H and N–H stretching), 1716, and 1670 cm−1 (C = O stretching of carboxyl and amide groups). After imidization, the above-mentioned amide and carboxyl bands disappeared, and the characteristic absorptions bands of imide group at 1780 and 1728 (imide carbonyl asymmetrical and symmetrical stretch), 1379 (C − N stretch), and 1052 and 746 cm−1 (imide ring deformation) appeared that indicates a virtually complete conversion of the PAA precursor into PEI. The 1H and 13C NMR spectra of the representative PEI IVa are also confirmed and illustrated in Fig. 4. Assignments of whole protons and carbons are present in the figure as well. 1H and 13C NMR spectra are generally well assigned to the repeat polymer backbone. Thus, the fluorinated PEIs were successfully synthesized.
Solubility
The solubility of the synthesized PEIs [IV(H)] was tested in various organic solvents, and the results are given in Table 2. All fluorinated PEIs, in addition to showing good solubility in DMSO and acetone, may dissolve in high-boiling point solvents such as NMP, DMAc, DMF, and also in low-boiling point solvents such as Py, THF, CH2Cl2, and CHCl3. The favorable organic solubility in different organic solvents makes it possible to prepare flexible and tough films with the help of the solution casting process. As compared to the non-fluorinated counterparts [V(H)], the IV(H) displayed an enhanced organic solubility because the presence of bulky pendent CF3 groups can efficiently lessen chains packing and intermolecular interactions. In addition, the bis(ether amine)s containing bulky di-tert-butyl (IVd), hexafluoropropane (IVf) and fluorine (IVg) moiety in their structures exhibited the best solubility among all.
Optical properties
UV–Vis absorption of PEIs was performed and the obtained absorption edge (cut-off wavelength, λ0) data are comparatively listed in Table 3. The chemically [IV(C)] and thermally [IV(H)]-imidized fluorinated PEIs showed the λ0 values in the range of 361–370 and 362–372 nm, respectively. The chemically-imidized films IV(C) showed higher optical transparency than did the thermally imidized films. This is due to close chain packing of thermally imidized films or oxidation of terminal amino groups during thermal curing. In contrast, the V(H) series exhibited the λ0 values ranging from 371 to 399 nm, indicating that fluorinated PEIs have better optical properties. To realize the effect of fluorene-based bis(ether anhydride), the polyimides (PIs) derived from bis(ether amine) (II’a) and various commercial dianhydrides are selected and compared with Va(H). All the PIs have significant lager λ0 values (387–440 nm) than Va(H) (λ0 = 375 nm). The results suggested that the introduction of fluorene-based bis(ether anhydride) is better than the introduction of fluorinated bis(ether amine) and the imidization method. That is, the bis(ether anhydride), containing electron-donating ether linkages, has decreased the overall electron affinity of the phthalimide units more sufficiently, and thus lowered the intermolecular charge transfer complexing (CTC) interaction. In addition, the CF3-substitution and chemical imidization process could also slightly reduce the presence of the CTC effect.
The color index of all the polymer films was elucidated from the yellowness (b*), redness (a*) and lightness (L*) values that are also summarized in Table 3. The chemically and thermally imidized fluorinated PEIs exhibited b* values ranged from 4.9 to 9.3 and 9.4 to 13.3, respectively. Again, the b* values of the chemically-imidized PEIs are lower than those of the corresponding thermally cured films. The IV series have revealed lower b* values than their respective CF3-free counterparts (Vseries) (b* = 10.9–30.6). Furthermore, the PIs derived from bis(ether amine) (II’a) with various commercial dianhydrides appeared with a deep yellow color and showed relatively the highest b* values (30.4–87.8). All the results are consisted with the cut-off wavelength conducted through UV–Vis spectroscopy (as above).
Mechanical and thermal properties
Except for Vg, all PEIs could afford good quality tough films. The mechanical properties of PEIs obtained through thermally-cured process are summarized in Table 4. The PEIs of the IV series showed strength-at-break of 90–147 MPa, elongation-at-break of 6–12%, and an initial modulus of 2.0–2.6 GPa. In addition, the V series exhibited the mechanical properties of 95–117 MPa, 8–15% and 1.5–2.1 GPa, respectively. The IVg film containing two bulky fluorene units in the repeat unit of the polymer chain showed the highest tensile strength and elongation properties. In addition, bulky CF3 substituents of the IV series seem to have insignificant impact on the mechanical properties compared to the analogs V series. However, it is difficult to determine the structure–property relationship based on different bi(amine ether) and fluorinated substituents because the test samples were not optimal.
Thermal stability values of the thermally imidized PEIs were measured by differential scanning calorimetry (DSC) and thermogravimetry (TGA) at a heating rate of 15 and 20 °C/min, respectively (Table 4). DSC experiments were carried out at a heating rate of 15 °C/min under nitrogen atmosphere. Rapid cooling from 400 °C to room temperature produced predominantly amorphous samples, therefore the obvious DSC baseline shift (glass transition temperature, Tg) for all the PEIs could be easily read in subsequent heating traces. The Tg values of the fluorinated PEIs [IV(H)] ranged from 234 to 297 °C, depending on various diamine structures. As expected, the presence of rigid tetramethylbiphenyl, tert-butyl and bulky fluorene moiety in its diamine compound caused relatively higher Tg values because of the increased barrier against chain rotation and movement. All the PEIs exhibited generally high Tg values because of the introduction of bulky fluorene unit in the dianhydride structure. For example, the IV series showed higher Tg values than the fluorinated PEIs derived from 4,4'-bisphenol-A dianhydride (BPADA) [40]. Furthermore, the non-fluorinated PEIs (V) were recorded in the range of 239 to 288 °C. Besides, the fluorinated PEIs [IVc(H) and IVd (H)] exhibited slightly higher Tg values than the corresponded V series, and most V series showed slightly higher Tg values due to the CF3-substituted-free characteristics that decreased the electronic interactions and dense packing. The effect of increased fractional free volume caused by the incorporation of bulky CF3 groups might be compensated by the restricted rotation within the diamine residues.
The TGA data for these polymers are also shown in Table 4. Thermal decomposition temperatures (Td) at a 10% weight loss for the whole fluorinated PEIs in nitrogen and air atmospheres were found over a range between 501–583 and 501–577 °C, respectively. In addition, most non-fluorinated PEIs showed lower Td values than the counterparts ranging from 483 to 562 and 496–550 °C. They also left 53–69% char yield for IV series and 46–65% for V series. Due to the presence of tetramethyl- or tert-butyl-substituted groups in IVc and IVd (or Vc and Vd), they began to decompose at relatively lower temperatures comparing to other polymers. Furthermore, IVa, IVb and IVg contain a relatively higher phenyl ratio in their polymer chain, so they exhibited higher Td values.
Electrical and water absorption properties
We also evaluated the electrical properties and water absorption ratios of the thermally imidized PEI films, as summarized in Table 5. The commercial Kapton film was also selected for comparison. The dielectric constants of the whole polymer films were conducted at room temperature over the frequency range from 1 kHz to 40 MHz. The IV and V series exhibited dielectric constants at 1 MHz ranging from 2.69 to 3.19 and 3.16 to 3.54, respectively. In addition, the reference Kapton film has a dielectric constant of 3.80. The fluorinated PEIs of IV series have lower dielectric constants than the non-fluorinated analogs because of the incorporation of bulky CF3 groups in their structure. These results suggested that the CF3 groups can inhibit chain packing and increase the free volumes. Furthermore, the low polarizability of C-F bonds, which is caused by the strong electronegativity of the fluorine atom, contributes to decrease the dielectric constant of fluorinated PEIs to some extent. Thus, IVf that contains hexafluoroisopropylidene and trifluoromethyl unit exhibited the lowest dielectric constant among all. In addition, water absorption rates of the IV and V series at room temperature were in the range of 0.17–0.34% and 0.28–0.68%, respectively. As expected, the hydrophobicity of fluorine unit displayed a lower water uptake. The lower water absorption ratios ensure these polymers have stable electric properties because they are usually utilized in the conditions of higher humidity.
Conclusion
A series of fluorene-based fluorinated PEIs (namely IVa-g) were successfully synthesized through a two-step thermal or chemical polycondensation reaction. Based on the systematical investigation, the thermal, organo solubility, optical properties and so on related to various fluorinated diamine would be fully understood. Meanwhile, the non-fluorinated PEIs were also prepared for comparison. These fluorene-based fluorinated PEIs exhibited high optical transparency, excellent solubility, thermal stability and good electrical properties. All of the obtained characterization data demonstrated that these PEI films are potential candidates for microelectronic or optical devices. In addition, similar structures of fluorinated PEIs and their properties are also summarized below for readers' reference.

Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Liu Y, Wang J, Guo J, Qi D, Li W, Shen K (2020) Novel fluorinated long linear segment hyperbranched polyimides bearing various pendant substituents for applications as optical materials. Polymer 190:122216
Chen CK, Lin YC, Miyane S, Ando S, Ueda M, Chen WC (2020) Thermally and mechanically stable polyimides as flexible substrates for organic field-effect transistors. ACS Appl Polym Mater 2:3422–3432
Deng G, Luo J, Liu S, Wang Y, Zong X, Xue S (2020) Molecular design and characterization of new polyimides based on binaphthyl-ether diamines for gas separation. Sep Pur Technol 235:116218
Ghosh MK, Mittak KL (2018) Polyimides: Fundamentals and Applications. CRC Press
Li Y, Sun G, Zhou Y, Liu G, Wang J, Han S (2022) Progress in low dielectric polyimide film-a review. Prog OrgCoat 172:107103
Orlova AM, Alentiev AY, Kolenikov TI, Tsegelskaya AY, Monakhova KZ, Chirkov SV, Nikiforov RY, Abramov IG, Kuznetsov AA (2022) Novel organo-soluble poly(ether imide)s based on diethyltoluenediamine: synthesis, characterization and gas transport properties. Polymer 256:125258
Ni HJ, Liu JG, Wang ZH, Yang SY (2015) A review on colorless and optically transparent polyimide films: chemistry, process and engineering applications. J Ind Eng Chem 28:16–27
Tapaswi PK, Ha CS (2019) Recent trends on transparent colorless polyimides with balanced thermal and optical properties: design and synthesis. Macromol Chem Phys 220:1800313
Wozniak AI, Yegorov AS, Ivanov VS, Igumnov SM, Tcarkova KV (2015) Recent progress in synthesis of fluorine containing monomers for polyimides. J Fluorine Chem 180:45–54
Hsiao SH, Liao WK, Liou GS (2017) Synthesis and electrochromism of highly organosoluble polyamides and polyimides with bulky trityl-substituted triphenylamine units. Polymers 9:511
Yang X, Dai F, Ke Z, Yan K, Chen C, Qian G, Li H (2022) Synthesis of colorless polyimides with high Tg from asymmetric twisted benzimidazole diamines. Eur Polym J 164:110975
Kaya I, Kamaci M (2018) Synthesis, optical, and thermal properties of polyimides containing flexible ether linkage. J Appl Polym Sci 135:46573
Zhuang Y, Seong JG, Lee YM (2019) Polyimides containing aliphatic/alicyclic segments in the main chains. Pro Polym Sci 92:35–88
Cao K, Xu Z, Guo D, Liu G (2021) Poly(ether imide)s with tailored end groups. J Polym Sci 59:2365–2377
Liu Y, Xing Y, Zhang Y, Guan S, Zhang H, Wang Y, Wang Y, Jiang Z (2010) Novel soluble fluorinated poly(ether imide)s with different pendant groups: synthesis, thermal, dielectric, and optical properties. J Polym Sci Part A Polym Chem 48:3281–3289
Bera D, Bandyopadhyay P, Ghosh S, Banerjee S (2014) Gas transport properties of aromatic polyamides containing adamantyl moiety. J Membr Sci 453:175–191
Liaw DJ, Huang CC, Chen WH (2006) Color lightness and highly organosoluble fluorinated polyamides, polyimides and poly(amide-imide)s based on noncoplanar 2,2’-dimethyl-4,4’-biphenylene units. Polymer 47:2337–2348
Chen YC, Shaio SH (2018) Optically transparent and organosoluble poly(ether imide)s based on a bis(ether anhydride) with bulky 3,3’,5,5’-tetramethylbiphenyl moiety and various fluorinated bis(ether amine)s. High Perform Polym 30:47–57
Li F, Liu J, Liu X, Wang Y, Gao X, Meng X, Tu G (2018) High performance soluble polyimides from ladder-type fluorinated dianhydride with polymorphism. Polymers 10:546
Liu Y, Gao C, Xiao L, Xie Y, Wang Y, Li W (2016) Novel soluble poly(ether imide)s with trifluoromethyl and chloride pendant groups for optical materials. RSC Adv 6:16404–16412
Chen YC, Su YY, Hsiao SH (2019) Colorless and organosoluble fluorinated poly(ether imide)s containing a asymmetry, bulky featured 4-tert-butylcatechol bis(ether anhydride) and trifluoromethyl substituents aromatic bis(ether amine)s. Fibers Polym 20:1755–1765
Zhang M, Miao J, Xu Y, Wang Z, Yan J (2022) Colorless polyimides from fluorinated ladder diamines containing norbornyl benzocyclobutene segments. Macromolecules 55:7992–8001
Chen YC, Su YY, Hsiao SH (2019) Colorless and organosoluble fluorinated poly(ether imide)s containing a asymmetry, bulky featured 4-tert-butylcatechol bis(ether anhydride) and trifluoromethyl substituents aromatic bis(ether amine)s. Fiber Polym 20:1755–1765
Hsiao SH, Li CT (1999) Synthesis and characterization of new fluorene-based poly (ether imide)s. J Polym Sci Part A Polym Chem 37:1403–1412
Yi L, Li C, Huang W, Yan D (2016) Soluble and transparent polyimides with high Tg from a new diamine containing tert-butyl and fluorene units. J Polym Sci Part A Polym Chem 54:976–984
Liu YW, Tang LS, Qu LJ, Liu SW, Chi ZG, Zhang Y, Xu JR (2019) Synthesis and properties of high performance functional polyimides containing rigid nonplanar conjugated fluorene moieties. Chin J Polym Sci 37:416–427
Zhang J, Lu Y, Xiao G, Hou M, Li L, Wang T (2021) Enhanced gas separation and mechanical properties of fluorene-based thermal rearrangement copolymers. RSC Adv 11:13164–13174
Hu M, Chen H, Wang M, Liu G, Chen C, Qian G, Yu Y (2021) Novel low-dielectric constant and soluble polyimides from diamines containing fluorene and pyridine unit. J Polym Sci 59:329–339
Xie K, Zhang SY, Liu JG, He MH, Yang SY (2001) Synthesis and characterization of soluble fluorine-containing polyimides based on 1, 4-bis (4-amino-2-trifluoromethylphenoxy) benzene. J Polym Sci Part A Polym Chem 39:2581–2590
Yang CP, Hsiao SH, Hsu MH (2002) Organosoluble and light-colored fluorinated polyimides from 4,4’-bis(4-amino-2-trifluoromethylphenoxy) biphenyl and aromatic dianhydrides. J Polym Sci Part A Polym Chem 40:524–534
Yang CP, Hsiao SH, Chen KH (2002) Organosoluble and optically transparent fluorine-containing polyimides based on 4,4’-bis(4-amino-2-trifluoromethylphenoxy)-3,3’,5,5’-tetramethylbiphenyl. Polymer 43:5095–5104
Yang CP, Hsiao FZ (2004) Synthesis and properties of fluorinated polyimides based on 1,4-bis(4-amino-2-trifluoromethylphenoxy)-2,5-di-tert-butylbenzene and various aromatic dianhydrides. J Polym Sci Part A Polym Chem 42:2272–2284
Yang CP, Chen RS, Chen KH (2005) Organosoluble and light-colored fluorinated polyimides based on 2,2-bis[4-(4-amino-2-trifluoromethylphenoxy)phenyl]propane and aromatic dianhydrides. J Appl Polym Sci 95:922–935
Yang CP, Chen RS, Chen KH (2003) Effects of diamines and their fluorinated groups on the color lightness and preparation of organosoluble aromatic polyimides from 2,2-bis[4-(4-amino-2-trifluoromethylphenoxy)phenyl]-hexafluoropropane. J Polym Sci Part A Polym Chem 41:922–938
Yang CP, Chiang HC (2004) Organosoluble and light-colored fluorinated polyimides based on 9,9-bis[4-(4-amino-2-trifluoromethylphenoxy) phenyl] fluorene and aromatic dianhydrides. Colloid Polym Sci 282:1347–1358
Hsiao SH, Yang CP, Lin CK (1995) Syntheses and properties of polyimides based on bis (p-aminophenoxy) biphenyls. J Polym Res 2:1–12
Liaw DJ, Liaw BY (1997) Synthesis and properties of polyimides derived from 1,4-bis(4-aminophenoxy)2,5-di-tert-butylbenzene. J Polym Sci Part A Polym Chem 35:1527–1534
YangCP LJH (1993) Syntheses and properties of aromatic polyamides and polyimides derived from 9,9-bis[4-(p-aminophenoxy)phenyl]fluorene. J Polym Sci Part A Polym Chem 31:2153–2163
Chen YC, Hsiao SH, Su YY (2017) Organosoluble and colorless fluorinated poly(ether imide)s derived from a highly contorted biphenyl-2,2’-diol bis(ether anhydride) and aromatic bis(ether amine)s with trifluoromethyl substituents. J Polym Res 24:87
Yang CP, Su YY, Chen YC (2005) Colorless poly(ether-imide)s deriving from 2,2-bis[4-(3,4-dicarboxyphenoxy)phenyl]propane dianhydride(BPADA) and aromatic bis(ether amine)s bearing pendent trifluoromethyl groups. Eur Polym J 42:721–732
Yang CP, Chen YC, Hsiao SH, Guo W, Wang HM (2010) Optically transparent and colorless poly(ether-imide)s derived from a phenylhydroquinone bis(ether anhydride) and various trifluoromethyl-substituted bis(ether amine)s. J Polym Res 17:779–788
Acknowledgements
Financial support from the National Science and Technology Council, Taiwan is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, YC., Su, YY. & Lin, SC. Organosoluble and colorless fluorinated poly(ether imide)s containing a bulky fluorene bis(ether anhydride) and various trifluoromethyl-substituted aromatic bis(ether amine)s: synthesis and characterization. Iran Polym J 32, 499–511 (2023). https://doi.org/10.1007/s13726-023-01140-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-023-01140-5