Abstract
Since ZnO nanoparticles increase the electrical conductivity of the polypyrrole (PPy) coatings, an investigation was carried out to evaluate the effect of ZnO nanoparticles loading on the corrosion protection performance of PPy coatings on AA2024 Al alloy in 3.5% NaCl solution. At first, some measurements were carried out to find the best experimental conditions containing the electrodeposition method, electrosynthesis solvent composition, and ZnO nanoparticles’ concentration for preparing the optimum PPy coating on Al alloy2024. Three different methods of electrodeposition, namely: cyclic voltammetry, galvanostatic, and potentiostatic techniques were analyzed. The anti-corrosion performance of the PPy coatings was evaluated by electrochemical impedance spectroscopy and Tafel polarization methods. The PPy prepared by potentiostatic method exhibited the best performance against corrosion of Al alloy2024 in 3.5% NaCl solution. Then, different mixtures of H2O/ethanol were tested as electrosynthesis solvents for preparation of PPy coatings on the alloy by optimized electrodeposition mode (i.e., potentiostatic). In evaluation of the prepared coatings, the pure water was introduced as the optimum solvent in electrodeposition of PPy. The investigation of different ZnO nanoparticles’ concentrations proved that the PPy coating containing 0.025% ZnO nanoparticles was the optimum coating against the corrosion of Al alloy in NaCl solution. Finally, the long-term evaluation of the corrosion protection performance of the coatings revealed that the optimum coating provided suitable protection against corrosion up to 14 days after immersion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminum alloys such as AA2024 are widely used in aircraft industry because of their superior mechanical properties and low weight. Al alloys contain considerable amount of intermetallic impurities. These impurities make this alloy vulnerable to galvanic corrosion. Until recently, corrosion protection of Al alloys was based on hexavalent chromium (Cr6+) coatings. However, these types of coatings are health and environmental hazard, and have to be replaced. Conducting polymers such as polypyrrole and polyaniline are effectively tested as corrosion inhibitors on non-ferrous metals and serve as reasonable alternative to Cr6+ technology [1,2,3].
Conducting polymers have unique properties which make them suitable for many applications in both industrial and academic fields such as anti-corrosion coatings, sensors, supercapacitors, and batteries [4,5,6]. Polypyrrole (PPy) as one of the highly used conducting polymers has great advantages such as environmental and thermal stability, low cost, and relatively easy polymerization [7,8,9]. Polypyrrole is an attractive organic coating for a large number of biological and biomedical applications due to its biocompatibility with the human body tissues [10, 11].
The properties of PPy coatings for corrosion protection of alloy surface strongly depend on the synthesis parameters such as electrodeposition method, solvent composition, and nano-size additives. Cyclic voltammetry (CV), galvanostatic (GS), and potentiostatic (PS) methods are some of the most important techniques for electrosynthesis of PPy coatings reported in the literature [9, 12, 13]. The side reactions cannot occur at the alloy surface in the PS mode due to the application of constant potential. The GS mode applies a constant current that is the preferred mode for preparing PPy coatings on the large size electrodes [12]. The CV technique employs a triangular potential waveform to record the resulting current. However, different modes of electrodeposition lead to manipulating the corrosion protection performance of PPy coatings.
The composition of the solvent required for electrosynthesis of PPy coatings can affect the corrosion protection performance of the coatings. However, this field has been rarely reported in published papers [14, 15].
Corrosion protection mechanism of conducting polymers can be explained as follows:
When conducting coatings are used, electrical contacts can be established between the intermetallic compound on Al alloy2024 substrate and the coating. Consequently, the electrons generated at any defective region will not be localized at the coating–metal interfaces as in the case of insulating coatings. Localization of electrons at the interface favors interfacial oxygen reduction and thus coating delamination [16]. In that case, debonding will be accelerated by the OH− resulting from the reduction of oxygen. Electrons generated at the defect will migrate both into the coating and to the interface. This spreading of electrons into the coating will drop the rate of oxygen reduction at the metal-coating interface and thereby reduces the coating delamination.
The performance of PPy coatings can be improved by doping metal-oxide nanoparticles [3]. Among the transition metal oxides, zinc oxide (ZnO) nanoparticles have high thermal stability, ultraviolet protection, good transparency, and high electron mobility [17]. It was previously shown that the electrical conductivity of PPy coatings increases dramatically with the increase in ZnO content [18]. Therefore, it seems that the corrosion protection of PPy coatings can be modified by loading ZnO nanoparticles. However, the nanoparticles’ concentration is a critical parameter in corrosion protection of PPy coating [3, 19].
The aim of the present work is to explore the corrosion protection performance of the PPy coatings containing ZnO nanoparticles. For this purpose, the optimum experimental conditions for preparing the optimum PPy coating for corrosion protection of Al alloy2024 including electrosynthesis method, solvent composition, and ZnO nanoparticle concentration were investigated.
The long-term corrosion protection performance of the optimum PPy coating was characterized after immersion in 3.5% NaCl solution by electrochemical impedance spectroscopy (EIS) and Tafel polarization technique.
Experimental
All chemicals and solvents were procured from Merck. Purification of pyrrole was performed by distillation under vacuum and it was stored in the dark below 5 °C. Solutions were prepared using distilled water.
A potentiostat/galvanostat, Autolab PGSTAT-302N made in The Netherlands was used to perform the electrochemical tests. X-ray diffraction (XRD) patterns were obtained by a Rigaku D-max C III, X-ray diffractometer using Ni-filtered CuKα radiation (Japan). Scanning electron microscopy (SEM) images were recorded by a KYKY-EM3200 SEM (China). The optical microscope images were recorded by a digital JM300 microscope made in China.
Synthesis modes of PPy-coated Al alloy2024
The polypyrrole was synthesized by electropolymerization. Briefly, a solution arising from mixing 0.1 M of pyrrole and 0.3 M oxalic acid in de-ionized water was used for the synthesis of polypyrrole. The electrosynthesis of PPy was performed by the following techniques: cyclic voltammetry method by potential scanning between − 1.0 and + 2.5 V (vs. Ag/AgCl) at 100 mV/s for 20 cycles, galvanostatic technique at 5 mA/cm2 for 5 min, and potentiostatic mode at 2.5 V for 3 min. A cell containing three electrodes was used for the electrosynthesis of PPy which consisted of a disk of Al alloy2024 (100 mm2) as working electrode, Ag/AgCl as the reference electrode, and a platinum rod as the counter electrode. Before the coating process, the Al alloy surface was polished with abrasive papers (600-2500 grades) and washed with ethanol. After rinsing with distilled water, the PPy-coated Al alloy2024 electrodes were exposed to 3.5% NaCl solution.
Synthesis media of PPy-coated Al alloy2024
Four volume ratios of water/ethanol were employed (1:3, 1:1, 3:1, and 1:0) for electrosynthesis of PPy on the working electrodes. These PPy electrodes were denoted as PPy-1:3, PPy-1:1, PPy-3:1, and PPy-1:0 which were prepared from 1:3, 1:1, 3:1, and 1:0 volume ratios, respectively.
Corrosion tests
For the EIS measurements, a sinusoidal potential signal of 10 mV amplitude was employed in the frequency range of 100 kHz–10 mHz. A conventional three-electrode cell containing a Pt rod as the counter electrode, a saturated Ag/AgCl electrode as the reference electrode and the coated AA2024 as the working electrode was used to measure the EIS data. The software of Nova 1.9 was employed for fitting the experimental EIS plots to the equivalent circuit.
Tafel plots were recorded for coated samples using a conventional three-electrode electrochemical cell with platinum rod as counter electrode and an Ag/AgCl as reference electrode. The working electrode was the coated AA2024 sample. The NaCl (3.5%, w/w) solution was used as the corrosive environment.
Results and discussion
Preparation of ZnO nanoparticles
ZnO nanoparticles were synthesized on the basis of the procedure which has been reported earlier [20]. The solutions of 0.45 M Zn(NO3)2·4H2O and 0.9 M NaOH were prepared in distilled water. Then, the sodium hydroxide solution was heated to 55 °C. The heated NaOH solution under high-speed stirring was mixed with the dropwise addition (for about 40 min) of zinc nitrate solution. After sealing the beaker at this condition for 2 h, the precipitates of ZnO nanoparticles were washed with both distilled water and ethanol and dried in the air at about 60 °C. The final product was a white powder.
Figure 1 shows the SEM image of ZnO nanoparticles. Figure 2 presents the XRD pattern of ZnO nanoparticles. All the peaks correspond to the reflections of ZnO nanoparticles (peak list) which are in good accordance with the standard diffraction data of ZnO (JCPDS card no. 80-0075).
Effect of electropolymerization mode
The electrosynthesis of PPy coatings on Al alloy2024 was performed by modes under cyclic voltammetry, galvanostatic, and potentiostatic. In the cyclic voltammetry during the application of potential sweep, the resulting current was recorded. The formation of PPy layer on the Al alloy electrode through the oxidation of monomers into the radical cations corresponds to an increase in the current at + 0.7 V (vs. Ag/AgCl). As a characteristic feature of cyclic voltammetry, the break in the process of deposition between each cycle leads to a discontinuous growth, while the potential scan of the growing PPy layer produces uniform PPy microstructures [9, 12]. From the cyclic voltammetry data, it is possible to determine the optimum current density (5 mA/cm2) and electrodeposition potential (0.8 V vs. Ag/AgCl) for PPy electrosynthesis by galvanostatic and potentiostatic methods, respectively.
In the galvanostatic technique, a constant current is applied to the working electrode and the potential change is recorded. The electrodeposition of PPy coating was performed at the constant current density of 5 mA/cm2. For oxidation of monomers into oligomers, not only a high potential is required at initial times, but also the constant potential at the following should be high enough to complete the polymerization of pyrrole [9, 12].
The electrodeposition of PPy coatings on Al alloy2024 was performed through potentiostatic method at the constant potential of 2.5 V (vs. Ag/AgCl). The initial increase in the current density is due to the formation of the double layer at the alloy/electrolyte interface. Afterward, the current decreases with decreasing the concentration of pyrrole due to the deposition of PPy on the Al alloy electrode.
EIS measurements are usually used to evaluate the corrosion protection performance of coated samples [21,22,23]. Figure 3 presents the impedance plots of different coating samples obtained at 2 h after immersion in 3.5% NaCl solution.
In the literature, most of the EIS data arising from polymer-coated samples exposed to corrosive media have been analyzed by the equivalent circuit, as shown in Fig. 4 [24]. The equivalent circuit consists of solution resistance, Rs, charge transfer resistance, Rct, associated with the corrosion process on the alloy surface, pore resistance, Rpo, the double layer capacitance, Qdl, and the coating capacitance, Qc. Because of the non-ideal capacitive behavior of the coatings, a constant phase element is used instead of a pure capacitance. Rct is used to measure the electron transfer across the metal surface. High value of Rct indicates low corrosion rate and good protection ability. Qc is often used to characterize the water absorption of coating. Water permeation can result in the swelling or blistering of the coating which will easily break through these places. Electrolyte solution will pass through these defects and arrive at metal surface, leading to metal corrosion. Thus, the coating will lose its protection.
Qdl usually reflects the delamination of the coating from metal substrate surface, because its value may increase with water spreading at the interface between metal surface and the coating.
The impedance function of CPE is given by
where Y0 is the magnitude of CPE constant (F cm−2 sn−1 or sn Ω−1 cm−2), ω is the angular frequency, and n is the CPE exponent. When n = 1, the CPE represents a pure capacitor and Y0 = C. For n = 0, the CPE represents an ideal resistor. For n = −1, the CPE is equivalent with an inductance. Using a CPE instead of a capacitor provides the deviation from ideal capacitive behavior [25].
Rpo is often used to describe the ionic transfer through the coating pores which reflects the anti-penetrating ability of the coating. The protection ability of coating becomes weak with decreasing Rpo. Table 1 shows the fitted parameters for different coating samples. The higher value of pore resistance was obtained for PPy-PS; it was larger than those of PPy-GS and PPy-CV samples (Fig. 3). Therefore, the PPy-PS sample provided the highest corrosion protection of Al alloy2024 in NaCl solution.
Effect of synthesis solvent
To improve the corrosion protective behavior of the PPy coatings, various binary mixtures were employed as the solvent for electrosynthesis of PPy films. The electrochemical impedance spectroscopy was utilized to investigate the corrosion protective behavior of the PPy coatings prepared in different binary mixtures. The impedance plots of different PPy-coating samples after immersion in 3.5% NaCl solution are shown in Fig. 5.
The equivalent circuit represented in Fig. 4 has been used to fit the impedance plots of the PPy-coated samples. The circuit parameters are tabulated in Table 2. The larger pore resistance (Rpo) value for the PPy-1:0 coating compared to those of the other PPy-coated samples implies that the water is the suitable solvent for preparing the best protective PPy coating through potentiostatic electrosynthesis.
Effect of nanoparticle concentration
For preparation of coatings containing various concentrations of ZnO nanoparticles, the different amounts of ZnO nanoparticle powder (0, 0.0125, 0.025, 0.05, and 0.1 g) were each introduced to 100 mL solution of 0.1 M pyrrole and 0.3 M oxalic acid, and then, the electropolymerization of PPy was carried out by potentiostatic mode at 2.5 V for 3 min. Various PPy coatings containing 0, 0.0125, 0.025, 0.05, and 0.1 wt% of ZnO nanoparticles were prepared. The coating without ZnO nanoparticles (0%) was used as the blank.
With increasing the concentration of ZnO nanoparticles in the electrolyte solution, the current decreased. Therefore, the addition of ZnO nanoparticles reduced the current of the monomer oxidation process at the same applied potentials. In addition, ZnO nanoparticles might act as a barrier and reduce the direct interaction between pyrrole monomers and the surface of Al alloy2024.
Figure 6 shows the electrochemical impedance spectra of the PPy coatings without/with various concentrations of ZnO nanoparticles after 2 h from immersion in 3.5% NaCl solution.
The Nyquist plots of the PPy-coating samples containing 0.1% and 0.05% ZnO nanoparticles showed two semi-circles (Fig. 6). These Nyquist plots were fitted to the equivalent circuit, as given in Fig. 4. This pattern, typical for two Randles circuits connected in parallel, confirmed the presence of two different phases in the coating containing nanoparticles as filler.
In general, the EIS response in high-frequency region illustrates the property of the prepared coating and the process occurred at coating–metal interface is reflected by the low-frequency region [26]. High-frequency semi-circle at the left-hand side of a curvature is usually related to the PPy phase of the coating regardless of the amount of nanoparticle filler with pore resistance within the range from 65 to 115 Ω. Low-frequency semi-circle on the right-hand side of a curvature corresponds to the metal response during the corrosion process [27]. This phase changed its charge transfer resistance from 884 to 656 Ω when ZnO nanoparticles contents increased from 0.05 to 0.1% (Table 3).
On the other hand, the Nyquist plots of the coating samples containing 0.025% and 0.0125% ZnO nanoparticles showed only one relaxation time [1]. This semi-circle is related to the protective properties of the PPy coatings.
Table 3 lists the impedance parameters for different PPy-coating samples. The Rpo value of 0.025% coating sample was higher than those of the other coating samples after 2 days of immersion time. It is clear that the pore resistance increased with the increase in concentration of nanoparticles within 0–0.025% range, and then, it decreased with increasing nanoparticles’ concentration to 0.1%. In general, with increasing the nanoparticle percent in the coated samples, the coatings’ surface roughness increases and the aesthetic properties of the coating decrease. It can be clearly seen from Fig. 7 that the surface roughness increases with increasing the concentration of ZnO nanoparticles. It can be concluded that the optimum coating is the one containing 0.025% ZnO nanoparticle.
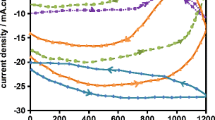
Figure 8 shows the Tafel polarization curves of coating samples containing different nanoparticle concentrations obtained at 2 h after immersion in 3.5% NaCl solution. The relevant parameters are listed in Table 4. The value of corrosion current for the coating containing 0.025% nanoparticle was lower than that of the other samples. Therefore, the PPy coating containing 0.025% nanoparticle was the best protective PPy coating on Al alloy2024 surface.
To gain further insight into corrosion protection properties, the Nyquist plots of the PPy coating containing 0.025% ZnO nanoparticle recorded after 2 h, 7 days, and 14 days of immersion times in aqueous 3% NaCl are shown in Fig. 9. These plots were analyzed in terms of the equivalent circuit, as shown in Fig. 4. The impedance parameters are shown in Table 5. The Rpo value decreases during the immersion time. The decrease in the value of the Rpo is attributed to the entry of the electrolyte into the coating micropores. After 14 days of immersion, the Rct value is observed to decrease, which may be due to the dissolution of the passive oxide layer on the alloy surface.
Figure 10 shows the Tafel plots of the PPy coating containing 0.025% ZnO nanoparticle at different immersion times in 3.5% NaCl solution. Tafel parameters are listed in Table 6. Therefore, the EIS and Tafel results have proved the suitable corrosion protection of PPy coating containing 0.025% ZnO nanoparticle up to 14 days after immersion time.
Conclusion
Due to an increase in the electrical conductivity of the PPy coatings by loading ZnO nanoparticles, it seems valuable to investigate the effect of loading ZnO nanoparticles on the corrosion protection performance of the PPy coatings on Al alloy2024. To find out the best experimental conditions for preparing the optimum PPy coating on Al alloy2024, some measurements were carried out. Three different electrodeposition methods for polypyrrole (PPy) coatings’ synthesis including: cyclic voltammetry, galvanostatic, and potentiostatic modes were employed to introduce the optimum PPy coating for corrosion protection of Al alloy2024 alloy in 3.5% NaCl solution. The electrochemical properties of PPy coatings were investigated by electrochemical impedance spectroscopy (EIS) and Tafel polarization techniques. Among three different PPy electrosynthesized, the PPy electrosynthesized by the PS mode provided the optimum protection of Al alloy against corrosion. In addition, different binary mixtures of H2O/ethanol were investigated as electrosynthesis solvents for the preparation of PPy coatings on Al alloy2024 by the potentiostatic mode. The results showed that the pure water was the optimum solvent for the PPy electrodeposition. The investigation of ZnO nanoparticles’ concentration effect proved that the synthesis solution containing 0.025% ZnO nanoparticles can lead to producing the optimum coating against corrosion protection of Al alloy2024 in NaCl solution. The long-term corrosion protection performance of the coatings revealed that the optimum coating provided suitable protection against corrosion by maximum 14 days after immersion.
References
Tallman DE, Levine KL, Siripirom C, Gelling VG, Bierwagen GP, Croll SG (2008) Nanocomposite of polypyrrole and alumina nanoparticles as a coating filler for the corrosion protection of aluminium alloy 2024-T3. Appl Surf Sci 254:5452–5459
Jadhav N, Jensen MB, Gelling V (2015) Tungstate and vanadate-doped polypyrrole/aluminum flake composite coatings for the corrosion protection of aluminum 2024-T3. J Coat Technol Res 12:259–276
Fekri F, Shahidi M, Foroughi MM, Kazemipour M (2019) Investigation of polypyrrole coatings containing nanosized metal oxides for corrosion protection of AA2024 Al alloy. J Electrochem Sci Technol 10:148–158
Lei Y, Sheng N, Hyono A, Ueda M, Ohtsuka T (2014) Influence of pH on the synthesis and properties of polypyrrole on copper from phytic acid solution for corrosion protection. Prog Org Coat 77:774–784
Venugopal V, Venkatesh V, Northcutt RG, Maddox J, Sundaresan VB (2017) Nanoscale polypyrrole sensors for near-field electrochemical measurements. Sens Actuat B 242:1193–1200
Shayeh JS, Siadat SOR, Sadeghnia M, Niknam K, Rezaei M, Aghamohammadi N (2016) Advanced studies of coupled conductive polymer/metal oxide nano wire composite as an efficient supercapacitor by common and fast fourier electrochemical methods. J Mol Liq 220:489–494
Qi K, Qiu Y, Chen Z, Guo X (2015) Corrosion of conductive polypyrrole: galvanic interactions between polypyrrole and metal substrates. Corros Sci 91:272–280
Arabzadeh H, Shahidi M, Foroughi MM (2017) Electrodeposited polypyrrole coatings on mild steel: modeling the EIS data with a new equivalent circuit and the influence of scan rate and cycle number on the corrosion protection. J Electroanal Chem 807:162–173
Sadki S, Schottland P, Brodie N, Sabouraud G (2000) The mechanisms of pyrrole electropolymerization. Chem Soc Rev 29:283–293
Flamini DO, Valle MI, Sandoval MJ, Massheimer VL, Saidman SB (2018) Electrodeposition study of polypyrrole-heparin and polypyrrole-salicylate coatings on Nitinol. Mater Chem Phys 209:76–85
Ershad-Langroudi A, Fadaei H, Ahmadi K (2019) Application of polymer coatings and nanoparticles in consolidation and hydrophobic treatment of stone monuments. Iran Ploym J 28:1–19
Wolfart F, Dubal DP, Vidotti M, Holze R, Gómez-Romero P (2016) Electrochemical supercapacitive properties of polypyrrole thin films: influence of the electropolymerization methods. J Solid State Electrochem 20:901–910
Ates M, Kalender O, Topkaya E, Kamer L (2015) Polyaniline and polypyrrole/TiO2 nanocomposite coatings on Al1050: electrosynthesis, characterization and their corrosion protection ability in saltwater media. Iran Ploym J 24:607–619
Aravindan N, Sangaranarayanan MV (2016) Influence of solvent composition on the anti-corrosion performance of copper–polypyrrole (Cu–PPy) coated 304 stainless steel. Prog Org Coat 95:38–45
Zhu RL, Li GX, Zheng JH, Jiang JW, Zeng HB (2009) Influence of electrosynthesis potential on corrosion performance of polypyrrole coated stainless steel and its mechanism research. Surf Eng 25:156–162
Foyet A, Wu TH, Kodentsov A, Ven LGJ, With G, Benthem R (2013) Corrosion protection and delamination mechanism of epoxy/carbon black nanocomposite coating on AA2024-T3. J Electrochem Soc 160:C159–C167
Wei XQ, Zhang Z, Yu YX, Man BY (2009) Comparative study on structural and optical properties of ZnO thin films prepared by PLD using ZnO powder target and ceramic target. Opt Laser Technol 41:530–534
Batool A, Kanwal F, Imran M, Jamil T, Siddiqi SA (2012) Synthesis of polypyrrole/zinc oxide composites and study of their structural, thermal and electrical properties. Synth Metals 161:2753–2758
Kumar AM, Rajendran N (2013) Electrochemical aspects and in vitro biocompatibility of polypyrrole/TiO2 ceramic nanocomposite coatings on 316L SS for orthopedic implants. Ceram Int 39:5639–5650
Moghaddam AB, Nazari T, Badraghi J, Kazemzad M (2009) Synthesis of ZnO nanoparticles and electrodeposition of polypyrrole/ZnO nanocomposite film. Int J Electrochem Sci 4:247–257
Kim YH, Kwon YS, Shon MY, Moon MJ (2018) Corrosion protection performance of PVDF/PMMA-blended coatings by electrochemical impedance method. J Electrochem Sci Technol 9:1–8
Macedo MCSS, Margarit-Mattos ICP, Fragata FL, Jorcin J-B, Pébère N, Mattos OR (2009) Contribution to a better understanding of different behaviour patterns observed with organic coatings evaluated by electrochemical impedance spectroscopy. Corros Sci 51:1322–1327
Sababi M, Pan J, Augustsson P-E, Sundell P-E, Claesson PM (2014) Influence of polyaniline and ceria nanoparticle additives on corrosion protection of a UV-cure coating on carbon steel. Corros Sci 84:189–197
Mansfeld F (1995) Use of electrochemical impedance spectroscopy for the study of corrosion protection by polymer coatings. J Appl Electrochem 25:187–202
Hao Y, Sani LA, Ge T, Fang Q (2017) Phytic acid doped polyaniline containing epoxy coatings for corrosion protection of Q235 carbon steel. Appl Surf Sci 419:826–837
Jiang L, Syed JA, Lu H, Meng X (2019) In-situ electrodeposition of conductive polypyrrole–graphene oxide composite coating for corrosion protection of 304SS bipolar plates. J Alloys Compd 770:35–47
Sanchez-Amaya JM, Osuna RM, Bethencourt M, Botana FJ (2007) Monitoring the degradation of a high solids epoxy coating by means of EIS and EN. Prog Org Coat 60:248–254
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fekri, F., Shahidi Zandi, M. & Foroughi, M.M. Polypyrrole coatings for corrosion protection of Al alloy2024: influence of electrodeposition methods, solvents, and ZnO nanoparticle concentrations. Iran Polym J 28, 577–585 (2019). https://doi.org/10.1007/s13726-019-00726-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-019-00726-2