Abstract
In this study, a novel photocatalyst, pentarylenebis(dicarboximide) dye: (1,6,13,18-tetra(4-(2,3,3-trimethylbut-2-yl)phenoxy)-N,N’-(2,6-diisopropylphenyl)-pentarylene-3,4,15,16-tetracarboxidiimide) (TTPDPT), was first used in metal-free photoinduced atom transfer radical polymerization (ATRP) of methyl methacrylates (MMA). The initiator was methyl α-bromoisobutyrate (MBI) and the light source was mild near-infrared (NIR) light irradiation (λmax ≈ 870 nm). The TTPDPT-mediated ATRP relies on in situ photoreduction of a MBI through an electron transfer process to generate the desired alkyl radical, which could induce polymerization of the monomer. The photoinduced metal-free ATRP of MMA shows typical characteristics of controlled free radical polymerization, showing the linear evolution of number-average molecular weight (Mn,GPC) with monomer conversion, where polymers with predetermined degree of polymerization have well-controlled molecular weights and narrow molecular weight distribution (Mw/Mn). The photoinduced metal-free ATRP of MMA can be carried out with just ppm level of TTPDPT. The polymerization initiation and propagation can be operated by the aid of pulsed light sequences while NIR light source was used to promote carbon–carbon bond formation and to produce poly(methyl methacrylate) (PMMA) with Mw/Mn as low as 1.5. The synthesized PMMA was characterized by 1H nuclear magnetic resonance (1H NMR). The resultant PMMA contained a bromide end group that can be employed to reinitiate styrene polymerization to produce block copolymers through chain extension experiments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atom transfer radical polymerization (ATRP), discovered in 1995, is an efficient way for living radical polymerization [1,2,3]. In ATRP, transition metal salts or complexes [4, 5] have been employed to maintain a dynamic equilibrium between active species and dormant species. One major shortcoming of classical ATRP was due to the use of relatively high catalyst concentration relative to monomer in contaminating the final polymer. Some efficient technologies have been developed to reduce the catalyst concentration [6,7,8], even ATRPs were performed without catalyst [9,10,11].
Recently, light-induced polymerization techniques have been a dramatic resurgence of interest in the field of photomediated ATRP due to its easy operation at ambient temperature and atmospheric pressure, and they offered spatial and temporal control over the polymerization [12,13,14]. In this system, photoinitiator played a key role in the photopolymerization. Many low-molecular weight photoinitiators and their derivatives, for example, benzophenone [15], phenothiazine [16], and phenoxazine [17], have been reported in photomediated ATRP. In addition, some semiconductor nanoparticles and transition metal-based photoredox catalysts, such as ZnO [18], Fe/ZnO [18], TiO2 [19], and Fe2O3 [20], have also been used in photomediated ATRP systems. However, the photomediated ATRP described above were carried out under UV, visible, and blue light [21]. There are some limits to use the short-wavelength light, for example, undesired side reactions [22]. Some dyes absorbed in the near-infrared (NIR) and red region have attracted more and more attention. Excellent efficiencies were reported using chlorophyll a [22,23,24] in living radical polymerization, perylene derivative in cationic photopolymerization [25], etc. In the case of bacteriochlorophyll a as photoinitiator, far-red (λ = 780 nm) and near-infrared light (λ = 850 nm) have been successfully employed for the activation of photoinduced electron transfer/reversible addition-fragmentation chain transfer (PET–RAFT) polymerization, respectively [22].
Recently, photoinduced metal-free ATRPs have been increasingly attracting attention [26,27,28]. Fluorescein (FL) [29] and 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) [30] have been also reported in photoinduced metal-free ATRP. Miyake et al. have reported perylene as a photoorganocatalyst for the metal-free ATRP of (meth)acrylates and styrene through direct reduction of alkyl bromides in its photo-excited state to induce the polymerization [31]. Our group reported that perylene derivatives 3,4,9,10-tetra-(12-alkoxycarbonyl)-perylene was used as photocatalyst in metal-free ATRP under blue light irradiation [32]. Some perylene derivatives have been reported to absorb in this region of the NIR spectrum. For example, pentarylenebis(dicarboximide) dye: (1,6,13,18-tetra(4-(2,3,3-trimethylbuty-2-yl)phenoxy)-N,N’-(2,6-diisopropylphenyl)-pentarylene-3,4,15,16-tetracarboxidiimide) (TTPDPT), which was first synthesized in 2006 [33], absorbed in near-infrared region.
In this work, we employed this photoinduced living polymerization technique for the controlled polymerization of methyl methacrylate (MMA) with TTPDPT as photocatalyst in the presence of prior deoxygenation processes under NIR light irradiation. The block copolymers were synthesized through successive chain extensions. Furthermore, the effect of light on this photoinduced metal-free ATRP was investigated in this article.
Experimental
Materials
The chemical structure of the studied TTPDPT is shown in Scheme 1. MMA and styrene (St) were purchased from Tianjin Bodi Chemical Co. (Tianjin, China) and purified by vacuum distillation before use. Methyl α-bromoisobutyrate (MBI) was purchased from Shanghai Aladdin Reagent Co. (Shanghai, China) and used as received. Other reagents were commercially purchased. The synthesis of the TTPDPT was conducted according to the literature procedures [33]. The detailed procedure is seen in Supplementary materials.
Photopolymerization
The photoinduced metal-free ATRP of MMA was subsequently adopted for the following photopolymerization reactions. A deoxygenated mixture containing methyl methylacrylate (MMA, 10 mmol, 1 g), dichloromethane (CH2Cl2, 20 mL), methyl α-bromoisobutyrate (MBI, 1 mmol, 0.1813 g) and TTPDPT (20 ppm) was introduced into 50-mL three-neck round-bottom flask equipped with a magnetic stirrer, which was subjected to NIR light irradiation (830–890 nm, 35 W/cm2) in a water bath at room temperature for a period of time. After the polymerization, the reaction mixture was diluted with tetrahydrofuran (THF) and then it was poured into methanol. The product was filtered from the solution and dried under vacuum for 24 h at 60 °C.
Light sources
Photomediated ATRP of MMA was carried out in the flask where the reaction mixtures were irradiated by near-infrared (NIR) laser irradiation (Shenzhen Xinkun Yang Science and Technology Co.). The distance of the flask to light source was 10 cm.
Chain extension polymerization
A PMMA macroinitiator was prepared with [MMA]0/[MBI]0 = 100/1 under NIR light irradiation. The concentration of TTPDPT is 20 ppm. Chain-end functionality of the PMMA macroinitiator was confirmed using 1H NMR and chain extension. The initial PMMA macroinitiator was successfully chain extended with St monomer to yield P(MMA-b-St).
Characterization
1H nuclear magnetic resonance (1H NMR) measurements were recorded in CDCl3 on a Bruker 400 MHz spectrometer (Bruker, Germany).
Molecular weight properties of the polymers were determined by gel permeation chromatography (GPC). The GPC instrument (Waters, America) has a Waters 515 pump and a Waters 2414 differential refractometer using Waters Styragel columns (HR-1, HR-2, and HR-3) with THF as eluent at 35 °C and a flow rate of 1 mL/min. Linear PMMA standards were used for calibration. The Mark–Houwink–Sakurada equation (MHS) was used to convert the PMMA molecular weight with MHS parameters: KPMMA = 12.8 × 10− 5 dL/g and αPMMA = 0.69 [34].
UV spectrum was collected using TU-1901 double beam UV–Vis spectrophotometer (Beijing Purkinje General Instrument Co., China).
Results and discussion
UV spectrum of TTPDPT
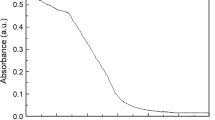
UV spectrum of TTPDPT in CH2Cl2 is shown in Fig. 1. As shown, a CH2Cl2 solution of TTPDPT (0.001 mmol/L) exhibits wide-range optical absorption in the NIR region with a strong absorption at λmax = 876 nm. Two relatively weak absorption peaks at λmax = 257 nm and 833 nm were detected [33], respectively.
Kinetics studies of photoinduced metal-free ATRP of MMA under NIR light irradiation
The UV spectrum of TTPDPT showed strong absorption at 876 nm (Fig. 1) which belonged to the NIR region of the solar spectrum. The photoinduced metal-free ATRP reduced the side reaction with wavelength of lower energy such as NIR light. Initial photoinduced metal-free ATRPs of MMA with TTPDPT were carried out with a molar ratio of [MMA]0/[MBI]0/[TTPDPT]0 of 150/1/0.0001 under NIR (λmax = 876 nm) LED irradiation. Kinetic analysis showed a linear increase of ln([M]0/[M]) vs. irradiation time (Fig. 2). Interestingly, we observed a typical induction period of around 2.4 h in the polymerization, indicating that the propagating radicals were almost unchanged throughout the polymerization process. The induction period was due to the generation of free radicals which initiated the polymerization rests with the electron transfer process from the photo-excited TTPDPT to MBI [17]. A similar phenomenon was observed in the other photoinduced ATRP [27, 35, 36]. It indicated that there needed some time to establish the dynamic equilibrium between the active propagating radicals and the dormant species, which resulted in the slow formation of the carbon-centered radicals [31]. As shown in Fig. 3, the molecular weight of Mn,GPC increased linearly with monomer consumption. The good correlation between the theoretical values (Mn,th) and the experimental values (Mn,GPC) further confirmed the controlled character of the polymerization. Furthermore, lower Mw/Mn values (< 1.5) were obtained. The corresponding GPC curves are shown in Fig. S1 (Supplementary Materials).
The effect of [TTPDPT]0 content
To investigate the effect of TTPDPT in the photoinduced metal-free ATRP reaction, a range of experiments was performed with the molar ratio of [MMA]0/[MBI]0 of 150:1 (Table 1). The photopolymerization was first carried out in the absence of TTPDPT, and no conversion was observed (Entry 1, Table 1) for 24 h under NIR irradiation. When TTPDPT was added, PMMA at 10.46% monomer conversion was obtained after 10 h irradiation (Entry 2, Table 1), indicating that the TTPDPT is essential in this system. Furthermore, the conversion increased with increasing the amount of TTPDPT (Entries 2, 3, 4, 5, and 6, Table 1). The reaction mixture was placed in dark conditions for 24 h where no polymers were yielded (Entry 7, Table 1). More importantly, Mn,GPC increased with monomer conversion and were in accordance with the values of Mn,th. In addition, the resulting PMMA displayed a well-controlled molecular weight distribution (Mw/Mn<1.50), demonstrating the feasibility of TTPDPT to conduct the photoinduced ATRP reaction.
Effect of light on photoinduced metal-free ATRP of MMA
To investigate the photo-regulating effect of TTPDPT for the photoinduced metal-free ATRP reaction, the light was switched “on” and “off” periodically during the photoinduced metal-free ATRP process (Fig. 4). The reaction mixture was first exposed to NIR light for 5 h, affording 13.9% conversion of monomer (0–5 h, Fig. 4). The polymerization ceased immediately when the light source was removed (5–6 h, Fig. 4), indicating that the amount of active radical was ignored under dark conditions. When the reaction mixture was re-exposed to NIR light source (6–7 h, Fig. 4), the polymerization continued with the same polymerization rate as observed in the former light-on process. Subsequently, such cycle with “on–off” light switch could be repeated multiple times. These experiments successfully verified that the growth of polymer chains can be well regulated by periodic light control process.
Analysis of chain end and chain extension of PMMA macroinitiator
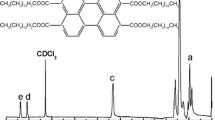
The chain end of PMMA-Br macroinitiator prepared by the photoinduced metal-free ATRP method was analyzed by 1H NMR (Fig. 5).
As shown in Fig. 5, the signal at 3.76 ppm (a in Fig. 5) corresponded to the protons of methyl groups (–CH3) next to the halogen chain end, which showed deviation from the chemical shift 3.61 ppm (b in Fig. 5) because of the electron-attracting function of ω-Cl atom. The chemical shifts at 0.51–1.27 ppm (d in Fig. 5) and 1.54–2.05 ppm were attributed to the methyl group (–CH3) and methylene group (–CH2–), respectively. The chain extension experiments were carried out with the prepared above PMMA-Br as macroinitiator (Fig. 6). As shown in Fig. 6, the Mn,GPC increased from 5600 to 18,600 g/mol. The Mw/Mn changed from 1.34 to 1.52. The chain extension experiments demonstrated the process in a living radical polymerization way. 1H NMR spectrum of P(MMA-b-St) block copolymer is shown in Fig. 7, which demonstrated further the formation of P(MMA-b-St) block copolymer.
Analysis of mechanism
The proposed mechanism for photoinduced metal-free ATRP of MMA is provided in Scheme 2. As shown in Scheme 1, TTPDPT (PCn) was first excited under NIR light irradiation to generate the excited TTPDPT (PCn*). Then, a MBI molecule was reduced by the electron transfer from a photo-excited PC (PCn*) and provided a radical (Pn-R•) and PCn+1X−. The Pn-R·radical can initiate MMA monomer to generate Pn-R-MMA. PCn+1X− can deactivate the propagating polymer chain Pn-R-MMA·to generate Pn+1-R-X and ground state PCn. The PCn can re-enter the catalytic cycle under NIR light irradiation.
Conclusion
Photoinduced metal-free ATRP of MMA was successfully carried out in CH2Cl2 at 25 °C with TTPDPT as photocatalyst and MBI as ATRP initiator under NIR light irradiation. The MBI can be reduced by the excited TTPDPT through electron transfer process, which generated radicals to initiate the polymerization. The ATRP of MMA followed good first-order kinetics throughout the reaction. The Mn,GPC values increased with monomer conversion and were well in agreement with the theoretical values (Mn,th). Lower Mw/Mn values were obtained. The polymerization rate increased with increasing the amount of TTPDPT. However, the polymerization stopped in the absence of TTPDPT and continued in the presence of TTPDPT under light irradiation. Furthermore, the polymerization can be easily governed by on/off switching light. It is noteworthy that NIR light source, which was easy, convenient, and inexpensive, can be used to initiate the polymerization reaction. A successful well-defined di-block copolymer was obtained through chain extension experiments.
References
Matyjaszewski K (2018) Advanced materials by atom transfer radical polymerization. Adv Mater 30:1706441
Matyjaszewski K (2012) Atom transfer radical polymerization: from mechanisms to applications. Isr J Chem 52:206–220
Pan X, Tasdelen MA, Laun J, Junkers T, Yagci Y, Matyjaszewski K (2016) Photomediated controlled radical polymerization. Prog Polym Sci 62:73–125
Boyer C, Corrigan NA, Jung K, Nguyen D, Nguyen T-K, Adnan NNM, Oliver S, Shanmugam S, Yeow J (2016) Copper-mediated living radical polymerization (atom transfer radical polymerization and copper(0) mediated polymerization): from fundamentals to bioapplications. Chem Rev 116:1803–1949
Tsarevsky NV, Matyjaszewski K (2007) “Green” atom transfer radical polymerization: from process design to preparation of well-defined environmentally friendly polymeric materials. Chem Rev 107:2270–2299
Cordero R, Jawaid A, Hsiao M-S, Lequeux Z, Vaia RA, Ober CK (2018) Mini monomer encapsulated emulsion polymerization of PMMA using aqueous ARGET ATRP. ACS Macro Lett 7:459–463
Lee H-C, Antonietti M, Schmidt BVKJ (2016) Cu(II) metal-organic framework as recyclable catalyst for ARGET ATRP. Polym Chem 7:7199–7203
Garcia-Valdez O, Champagne P, Cunningham MF (2018) Graft modification of natural polysaccharides via reversible deactivation radical polymerization. Prog Polym Sci 76:151–173
Treat NJ, Sprafke H, Kramer JW, Clark PG, Barton BE, Read de Alaniz J, Fors BP, Hawker CJ (2014) Metal-free atom transfer radical polymerization. J Am Chem Soc 136:16096–16101
Shanmugam S, Boyer C (2016) Organic photocatalysts for cleaner polymer synthesis. Science 352:1053–1054
Theriot JC, Lim C-H, Yang H, Ryan MD, Musgrave CB, Miyake GM (2016) Organocatalyzed atom transfer radical polymerization driven by visible light. Science 352:1082–1087
Fu Q, McKenzie TG, Ren JM, Tan S, Nam E, Qiao GG (2016) A novel solid state photocatalyst for living radical polymerization under UV irradiation. Sci Rep 6:20779
Ramsey BL, Pearson RM, Beck LR, Miyake GM (2017) Photoinduced organocatalyzed atom transfer radical polymerization using continuous flow. Macromolecules 50:2668–2674
Ciftci M, Yoshikawa Y, Yagci Y (2016) Living cationic polymerization of vinyl ethers through a photoinduced radical oxidation/addition/deactivation sequence. Angew Chem Int Ed 56:519–523
Allushi A, Kutahya C, Aydogan C, Kreutzer J, Yilmaz G, Yagci Y (2017) Conventional type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. Polym Chem 8:1972–1977
Dadashi-Silab S, Pan X, Matyjaszewski K (2017) Phenyl benzo[b]phenothiazine as a visible light photoredox catalyst for metal-free atom transfer radical polymerization. Chem A Eur J 23:5972–5977
Koyama D, Dale HJA, Orr-Ewing AJ (2018) Ultrafast observation of a photoredox reaction mechanism: photoinitiation in organocatalyzed atom-transfer radical polymerization. J Am Chem Soc 140:1285–1293
Dadashi-Silab S, Atilla TM, Mohamed AA, Bahadar KS, Yagci Y (2014) Photoinduced atom transfer radical polymerization using semiconductor nanoparticles. Macromol Rapid Commun 35:454–459
Wang G-X, Lu M, Hou Z-H, Yang C-A, Liang E-X, Liu L-C, Wu H, Li X-L, Xu Y-X (2015) Photo-induced atom transfer radical polymerization in ionic liquid. J Polym Res 22:60–65
Liu L-C, Lu M, Hou Z-H, Wang G-X, Yang C-A, Liang E-X, Wu H, Li X-L, Xu Y-X (2015) Photo-induced atom transfer radical polymerization with nanosized α-Fe2O3 as photoinitiator. J Appl Polym Sci 132(32):42398
Dadashi-Silab S, Pan X, Matyjaszewski K (2017) Photoinduced iron-catalyzed atom transfer radical polymerization with ppm levels of iron catalyst under blue light irradiation. Macromolecules 50:7967–7977
Shanmugam S, Xu J, Boyer C (2016) Light-regulated polymerization under near-infrared/far-red irradiation catalyzed by bacteriochlorophyll a. Angew Chem Int Ed 55:1036–1040
Wu C, Shanmugam S, Xu J, Zhu J, Boyer C (2017) Chlorophyll a crude extract: efficient photo-degradable photocatalyst for PET-RAFT polymerization. Chem Commun 53:12560–12563
Shanmugam S, Xu J, Boyer C (2015) Utilizing the electron transfer mechanism of chlorophyll a under light for controlled radical polymerization. Chem Sci 6:1341–1349
Xiao P, Dumur F, Graff B, Gigmes D, Fouassier JP, Lalevée J (2013) Red-light-induced cationic photopolymerization: perylene derivatives as efficient photoinitiators. Macromol Rapid Commun 34:1452–1458
Pan X, Fang C, Fantin M, Malhotra N, So WY, Peteanu LA, Isse AA, Gennaro A, Liu P, Matyjaszewski K (2016) Mechanism of photoinduced metal-free atom transfer radical polymerization: experimental and computational studies. J Am Chem Soc 138:2411–2425
Wang J, Yuan L, Wang Z, Rahman MA, Huang Y, Zhu T, Wang R, Cheng J, Wang C, Chu F, Tang C (2016) Photoinduced metal-free atom transfer radical polymerization of biomass-based monomers. Macromolecules 49:7709–7717
Kutahya C, Aykac FS, Yilmaz G, Yagci Y (2016) LED and visible light-induced metal free ATRP using reducible dyes in the presence of amines. Polym Chem 7:6094–6098
Liu X-D, Zhang L, Cheng Z-P, Zhu X (2016) Metal-free photoinduced electron transfer-atom transfer radical polymerization (PET-ATRP) via a visible light organic photocatalyst. Polym Chem 7:689–700
Huang Z, Gu Y, Liu X, Zhang L, Cheng Z, Zhu X (2016) Metal-free atom transfer radical polymerization of methyl methacrylate with ppm level of organic photocatalyst. Macromol Rapid Commun 38:1600461
Miyake GM, Theriot JC (2014) Perylene as an organic photocatalyst for the radical polymerization of functionalized vinyl monomers through oxidative quenching with alkyl bromides and visible light. Macromolecules 47:8255–8261
Wang G-X, Lu M, Zhou M-J, Liang E, He B (2018) Photo-induced ATRP of MMA under blue light irradiation in the presence of 3,4,9,10-tetra-(12-alkoxycarbonyl)-perylene as a photocatalyst. Iran Polym J 27:43–48
Pschirer NG, Kohl C, Nolde F, Qu J, Müllen K (2006) Pentarylene- and hexarylenebis(dicarboximide)s: near-infrared-absorbing polyaromatic dyes. Angew Chem Int Ed 45:1401–1404
Rudin A, Hoegy HLW (1972) Universal calibration in GPC. J Polym Sci Part A-1 Polym Chem 10:217–235
Mosnáček J, Ilčíková M (2012) Photochemically mediated atom transfer radical polymerization of methyl methacrylate using ppm amounts of catalyst. Macromolecules 45:5859–5865
Chen M, Zhong M, Johnson JA (2016) Light-controlled radical polymerization: mechanisms, methods, and applications. Chem Rev 116:10167–10211
Acknowledgements
We thank the National Natural Science Foundation of China (51674117, 51503063), Scientific Research Fund of Hunan Provincial Education Department (Grant Nos. 16K036, 15B101).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Le-Lin Zeng and Wan-Yun Xie are the first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, LL., Xie, WY., Yang, CX. et al. Photomediated atom transfer radical polymerization of MMA under long-wavelength light irradiation. Iran Polym J 27, 881–887 (2018). https://doi.org/10.1007/s13726-018-0661-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-018-0661-2