Abstract
Application of novel potassium salts (from mono- to penta-potassium) of 2,2,6,6-tetrakis(hydroxymethyl) cyclohexanol-activated 18C6 for propylene oxide polymerization is investigated. The novelty of the research concerns utilization of the cyclic-structured initiator with various substitution levels applied for anionic ROP of oxiranes. It results in the formation of bimodal macropentols (Scheme 1) with molar mass values in the range of Mn 2100–13200 g/mol and unsaturation, which depends on the initial concentration of monomer and alkoxide groups. The mechanism of the studied processes is discussed. The newly synthesized polyether-polyols (PEPOs) were used for the synthesis of new rigid polyurethanes (PURs) in one-step process. Thermogravimetric analysis (TGA) showed that the analyzed polyurethanes based on star-shaped polyols were characterized by two-stage thermal degradation and higher thermal stability in comparison to the linear polyols. The first stage of thermal decomposition of the tested PURs is related to the breaking of the urethane bonds in the rigid chains, while during the second stage, oligo-diol and oligo-pentol chains break down. A significant amount of solid degradation residue is advantageous in terms of the flame retardation of the obtained PUR, as this is usually associated with less products released during the degradation process. It was shown that the higher content of rigid segments in the PUR structure results in higher thermal resistance. The thermal behavior of the PURs was also investigated by differential scanning calorimetry (DSC).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyether-polyols (PEPOs) are the most important macromonomers for the synthesis of polyurethane elastomers, cross-linked foams and polyurethane coatings [1, 2]. Polyurethane foams with remarkable mechanical and thermal properties have been synthesized using polyols based on oleic acid, which is an important fatty acid [3]. Two hydrophilic poly(ethylene oxide) (PEO) blocks attached to both sides of a linear hydrophobic poly(propylene oxide) (PPO) block would form a PEO–PPO–PEO block copolymer. These are highly surface-active compounds such as Pluronics or Poloxamers [4], which properties are dependent on the mutual hydrophilic–lipophilic interaction correlated with utilized PEO to PPO mass ratio [5]. The usefulness of these copolymers results from their ability to create ordered moieties such as micelles, emulsions or liquid crystalline phases [6, 7]. They are usually obtained through anionic polymerization of oxiranes such as ethylene oxide (EO), propylene oxide (PO) or 1,2-butylene oxide (BO) using KOH (catalyst) and 1,2-propylene glycol or glycerol (starters) at high temperature (> 100 °C) and pressure. Molecular weights (Mn) of polyether diols and polyether triols prepared industrially from monomeric PO are 2000–4000 g/mol and 3000–6500 g/mol, respectively [1]. Application of several novel initiators for PO anionic polymerization, i.e., dipotassium salts of resorcinol, 4,4′-bisphenol or 4,4′-sulfonyldiphenol gave polyether diols with relatively low molecular weight of 1000–3000 g/mol [8].
Recently, we [9] used dipotassium salts of different glycols, for example, di- or tri-propylene glycol-activated 18-crown-6 (18C6) as initiator. It allowed to prepare bimodal PPOs containing a fraction with two terminal OK groups (Mn ~ 10 000 g/mol; yield ~ 30%) and other fraction with allyloxy and OK terminal groups (Mn ~ 30 000 g/mol; yield ~ 70%). Both kinds of terminal groups can be easily converted to OH groups [10]. Moreover, cyclic oligo(potassium glycidoxide)s with three- or six-OK group-activated 18C6 were applied as macro-initiators for the polymerization of PO, BO and styrene oxide (SO). Star-shaped polyether-polyols with Mn 1200–8000 g/mol were prepared in this way. These polymers can be used as substrates for the synthesis of new polyurethanes with enhanced mechanical properties [11].
The aim of the present work was the application of other initiating systems for the preparation of new star-shaped polyether-polyols. These are potassium hydroxyalkoxides obtained from the reaction of KH-activated 18C6 with 2,2,6,6-tetrakis(hydroxymethyl) cyclohexanol (THMC). PO was used as monomer for the synthesis of polymers. All syntheses were performed in mild conditions, i.e., tetrahydrofuran (THF) solution at room temperature. Characterization of polymers was made by use of 13C NMR, MALDI-TOF, FTIR-ATR and SEC techniques. Cross-linked polyurethanes (PURs) were prepared using two chosen polyols and polymeric methylene diphenyl diisocyanate (PMDI) as an isocyanate source [12,13,14] containing 31.5 wt% free isocyanate groups. The resultant PURs were preliminarily characterized by thermal analysis methods, including TGA, DTG and DSC.
Experimental
Materials
Propylene oxide (Aldrich, Poland) was dried over CaH2 and then distilled at 307 K (34 °C). Anhydrous tetrahydrofuran (THF) (Acros Organics, Poland) was kept over CaH2 and distilled at 339 K (66 °C). Potassium hydride (KH) was purified according to the procedure described by Brown [15]. A 35 wt% dispersion of KH in mineral oil (Aldrich/Poland) was mixed with n-pentane in a dry argon atmosphere and then decanted. This was repeated three times followed by a threefold washing with dry THF. Finally, THF was evaporated in a vacuum. Coronand 18C6 (1,4,7,10,13,16-hexaoxacyclooctadecane) (Merck, Poland), 2,2,6,6-tetrakis(hydroxymethyl)-cyclohexanol (THMC) (Aldrich, Poland) and dipropylene glycol (DPG) (Aldrich, Poland) were used for synthesis without purification. Polymeric methylene diphenyl diisocyanate (PMDI) was used without purification (Aldrich, Poland).
Polymerization
Mono-, di-, tri- tetra- and penta-potassium salts of 2,2,6,6-tetrakis(hydroxymethyl) cyclohexanol (nK-THMC)-activated 18C6 were prepared from the reaction of THMC with appropriate amount of KH in THF at 20 °C. The initial concentrations of the monomer were 2.0–6.0 mol/dm3 and the initial concentration of the initiator was 0.02 mol/dm3. For example, in the synthesis of 3K-THMC-activated 18C6, potassium hydride (0.047 g, 1.2 mmol), THF (4.0 cm3) and 18C6 (0.32 g, 1.2 mmol) were introduced into the reactor. Then, THMC (0.088 g, 0.4 mmol) was dissolved in boiling THF (10.0 cm3) and the obtained solution was dropped to the system at 20 °C (Scheme 1). After mixing for 1.5 h, hydrogen (26.8 cm3) was evolved. Finally, propylene oxide (5.6 cm3, 4.6 g, 80 mmol) was added and the reaction mixture was stirred for several days. After complete conversion of the monomer, the reaction mixture was treated with HCl/H2O system (0.01 mol/dm3, 70 cm3) and transferred to the separator containing chloroform (70 cm3). After shaking, within 5 min, two layers were formed, i.e., an inferior polyether layer and a superior layer containing water and the potassium salt. These layers were separated and the superior layer was removed. After threefold washing with distilled water, polyether was obtained by evaporating chloroform and water. The presence of monomer during polymerization was monitored by chromatographic method. In the studied systems, the final conversion was 97–99% after 1–3 weeks. Polymerizations initiated with K-DPG, K-THMC and 2K-THMC were homogeneous, while heterogeneity was observed in the systems containing 3K-4K and 5K-THMC. Scheme 2 represents exemplary synthesis of star-shaped PPO-pentol.
Monopotassium salt of dipropylene glycol (K-DPG) was obtained from the reaction of potassium hydride with an appropriate amount of glycol dissolved in THF at 20 °C (Scheme 3).
The initial concentration of propylene oxide was 4.0 mol/dm3 and the initial concentration of the initiator and 18C6 was 0.1 mol/dm3. Potassium hydride (0.08 g, 2.0 mmol), 18C6 (0.53 g, 2.0 mmol) and THF (8.5 cm3) were introduced into a 50-cm3 reactor equipped with magnetic stirrer and a Teflon valve enabling substrate delivery and sampling under argon atmosphere. Then, dipropylene glycol (2.0 mmol) was added to the THF solution (0.5 mol/dm3, 4.0 cm3) by use of a syringe. The reaction mixture was stirred for 1.5 h until all hydrogen (44.7 cm3) was evolved. It resulted in a solution of pure anhydrous monopotassium salt of dipropylene glycol activated by 18C6 in THF. That system was used as the initiator, when propylene oxide (5.6 cm3, 4.6 g, 80 mmol) was introduced into the reactor (Scheme 4). The reaction mixture was then stirred for several days. After complete conversion of the monomer, the system was neutralized with HCl/H2O (0.1 mol/dm3, 70 cm3) and transferred to the separator containing chloroform (70 cm3). After shaking for 5 min, two layers were formed, i.e., inferior polyether layer and superior layer containing water and the potassium salt. These layers were separated and the superior layer was removed. After three washings with distilled water, polyether was obtained by evaporating chloroform and water under vacuum. The presence of monomer during polymerization was monitored by chromatographic method and controlled by 1,4-dioxane method. The final monomer conversion was 99%.
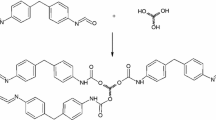
During the PUR production, the polyols and PMDI were mixed by a mechanical stirrer at 1000 rpm for 20 min. PMDI (Mn = 371.8 g/mol; functionality (f) = 2.7; LNCO = 30.5% mass; RNCO = 137.7 g/mol) was added to polyol to maintain the NCO/OH ratio at 2.0 for polydiol (Mn = 3200 g/mol; functionality (f) = 2; hydroxyl number–LOH = 33.66 mgKOH/g; equivalent of OH–ROH groups = 1667 g/mol) and at 5.0 for polypentol (Mn = 3200 g/mol; f = 5; LOH = 22.44 mgKOH/g; ROH = 2500 g/mol). n-Pentane was used as a physical blowing agent (foaming agent). The reaction (one-step polycondensation) took place at room temperature and a cooling system was applied to keep the temperature of the system at 40 °C. The relative humidity was kept below 30%. The proposed scheme of the reaction of the synthesized polyols and PMDI is presented in Scheme 5.
Measurements
100 MHz 13C NMR spectra were recorded in CDCl3 at 25 °C on a Bruker Avance 400 pulsed spectrometer equipped with a 5-mm broad-band probe and applying Waltz16 decoupling sequence. Chemical shifts were referenced to tetramethylsilane serving as an internal standard. To obtain good spectrum of the polymer main chain exhibiting its microstructural details, about 3000 scans were satisfactory, but to observe the signals of the polymer chain ends, more than 10,000 scans were necessary.
Molar masses and dispersities of polymers were obtained by means of size-exclusion chromatography (SEC) on a Shimadzu Prominence UFLC instrument at 40 °C on a Shodex 300 mm × 8-mm OHpac column using tetrahydrofuran as a solvent. Poly(propylene glycol)s were used as calibration standards.
Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) spectra were recorded on a Shimadzu AXIMA Performance instrument. Dithranol was used as a matrix. Infrared (IR) spectra of samples were recorded on a Shimadzu IR Prestige spectrometer with an attenuated total reflectance (ATR) accessory. The diamond ATR crystal was purified prior to each measurement with isopropanol. Data were analyzed using LabSolutions program. Each sample was scanned at a resolution of 2 cm− 1.
Thermogravimetric thermal stability analysis (TGA) was performed in an air atmosphere using Mettler Toledo TGA/SDTA 851e. The heating rate was 10 °C/min. Star 8.1 software was used to analyze the measurement data. Differential scanning calorimetry (DSC) was performed using the Mettler Toledo apparatus. Samples were heated, cooled and reheated with a speed of 10°C/min, in the temperature range − 100 to 120 °C. The DSC curves taken for the analysis were obtained from the second run. Temperature calibration was performed with indium (melting temperature = 156.6 °C, heat of fusion (∆Hf) = 28.5 J/g).
Results and discussion
Synthesis of star-shaped polypentols using potassium salts of 2,2,6,6-tetrakis(hydroxymethyl) cyclohexanol (THMC)-activated 18C6
In the series of experiments, the potassium salts (from mono- to penta-potassium) of THMC were prepared in the reaction with KH suspension in THF (Scheme 1). Solubility of the pristine pentol in THF is relatively low, i.e., 0.04 mol/dm3. In the presence of 18C6, it was possible to obtain mentioned salts of the pentol, which appeared to be active initiators of oxirane polymerization (Scheme 2). They are marked as K-THMC, 2K-THMC, 3K-THMC, 4K-THMC and 5K-THMC, respectively. Data concerning molar masses (Mn) and dispersities (Mw/Mn) of prepared star-shaped PEPOs are shown in Table 1.
Generally, two polymer fractions were formed with different yields and molar masses depending on the initial monomer and alkoxide group concentrations. Fractions (a) were obtained with 73–85% yield and Mn in the range 2100–13,200 g/mol, whereas for fractions (b), Mn values were in the range 900–5000 g/mol. The highest Mn values were detected for polymers obtained by the use of 2K- and 3K-THMC. Figure 1 shows exemplary SEC chromatogram of PPO (2).
Figure 2 presents a comparison of Mn values for PPOs synthesized with multiple substituted potassium salts of THMC initiator. Upon the results of the SEC study, one may indicate that the triple-substituted system seems to be most effective to obtain high-mass PEPOs. It may be caused by steric hindrance that occurs as the substitution level of initiator increases. In this case, growing of the macromolecules chains may also be handicapped by slower monomer diffusion and changes of the heterogeneity of the system.
MALDI-TOF technique was used for the determination of chemical structure of the prepared polymers. Figure 3 shows, as an example, MALDI-TOF spectrum of sample (4) obtained after quenching with CH3I. The spectrum of sample (4) reveals at broad range of m/z from 1000 to 7000 two series of signals (spectrum presented in 1300–2900 mass range). The signals of the main series, for example, at m/z 1488.9, 1836.9 and 2300.5 represent macromolecules with central part derived from the initiator as well as five PPO arms with three –OH and two –OCH3 end-groups. These macromolecules contain 21-, 27- and 35-mers of propylene oxide, respectively, and form adducts with one sodium ion (Mcalc = 1491.0, 1839.5 and 2304.1 g/mol, respectively). The second series of the signals, for example, at m/z 1357.1, 1936.8 and 2399.7 represents macromolecules with the five PO arms containing 19-, 29- and 37-mers of propylene oxide, respectively, as well as four –OH and one –OCH3 end-groups. They form adducts with sodium ion (Mcalc = 1360.8, 1940.6 and 2405.4 g/mol, respectively). The source of the sodium cation can be the glass vials used for preparing and storing of the polyol samples.
Unsaturation of polymers was examined by 13C NMR. Polymer samples prepared with K-THMC and 2K-THMC at [PO]o = 2.0–6.0 mol/dm3 revealed very low unsaturation (< 1%). Higher unsaturation was found for polymers synthesized using 3K-THMC, 4K-THMC (1–3%) and 5K-THMC (5%), especially at [PO]o = 6.0 mol/dm3. It is represented exclusively by starting allyloxy groups (Fig. 4) formed in the side chain transfer reaction with monomer. In general, the monol percentage in the final product was relatively low. 13C NMR analysis confirmed the presence of end-groups in five arms of star-shaped polymers, i.e., –CH(CH3)OH (at 65.6 ppm) and –OCH3 groups (56.5 ppm) after methylation.
Preparation of cross-linked polyurethanes
Synthesized star-shaped polyethers neutralized by HCl/H2O, after purification, i.e., removing of alkali metal cations and traces of water, were used for fabrication of polyurethanes. The reaction was performed with polydiisocyanate (PMDI) resulting in cross-linked polyurethanes. For the synthesis of cross-linked polyurethane (named as PUR_2), one polyether-pentol was chosen due to its high molecular weight (sample no. 8, Table 1). Moreover, in this case, THMC tripotassium salt was used for the synthesis, in which the two OH groups limited the formation of double bonds in the polymer. For comparison purposes, second PUR (named as PUR_1) was also obtained. As a hydroxylic component, polyether-diol, synthesized by the use of monopotassium salt of dipropylene glycol as initiator, was used (Scheme 4). In this case, the molecular weight of polyether-diol corresponded to the molecular weight of one arm in star-shaped polyether-pentol (PEPOs).
The syntheses produced rigid polyurethanes of a porous nature, with PUR_2 being more stiff than PUR_1, which was more flexible. One of the most important tools used to investigate the PUR chemical structure and phase separation process is infrared spectroscopy (IR) [16]. A qualitative description of the chemical structure of cross-linked PURs makes it easy to compare the characteristic absorption bands of the materials.
The FTIR spectra obtained for cross-linked polyurethanes based on polyether-diol (PUR_1) and polyether-pentol (PUR_2) are shown in Fig. 5. The FTIR spectrum of cross-linked polyurethane PUR_1 reveals peaks at 3340 cm− 1, which confirm the stretching vibration of N–H II-order groups of urethane bond [17]. Wide peak at 3550–3100 cm− 1 indicates the presence of hydrogen bonding between chains. Stretching band for free NH, not involved in hydrogen bonding, is located at 3420 cm− 1 [18] and is overlapped with shoulder band at 3250 cm− 1. It reflects stretching bands of hydrogen bonding for NH···O– couple below Tg of soft segments [19]. For PUR_2 (pentol based) sample, the band at 3200–3500 cm− 1 is more intense and complex, which can be attributed to more intense inter-molecular interaction of hydrogen bonding occurring in urethane bond –NH···O=C– [19, 20].
Furthermore, the results of the Fourier deconvolution of spectra for the carbonyl stretching region support the conclusion about hydrogen bond formation within the material’s structure. In this range (1780–1660 cm− 1), the bands at 1732 and 1739 cm− 1 represent the band of the free, unbounded carbonyl group C=O from urethane bond [19]. The shift of further peaks to lower wave number (bands below 1700 cm− 1) corresponds to the associated hydrogen bonding of a carbonyl group in the disordered (1700 cm− 1) or ordered (1695 cm− 1) phase [19,20,21,22]. For PUR_1, the carbonyl range region reveals four bands (Fig. 5a, insert) with dominant intensity coming from mid-range bands (located at 1711–1710 cm− 1). For PUR_2, the system is much more complex with eight bands (Fig. 5b, insert) reflecting greater variety of formed structures.
The transmittance of measured signals is more equally expanded with greater content of the bands located at the lowest wave number which indicates its higher content of hydrogen-bonded structures. At 2278 cm− 1, there is a band corresponding to absorption of isocyanate group representing unreacted NCO group. Their presence in the final PUR material reflects side reactions concurring with polyaddition main reaction leading to consumption of hydroxyl groups. Additionally, the steric hindrance can be responsible for leaving these groups unreacted. For PUR_2 (pentol based), the peak of –NCO group is less intense in comparison to that for PUR_1 (diol based), which reflects lower amounts of free, unreacted isocyanate groups in the material’s structure. Further bands at 2855 and 2930 cm− 1 reflect the symmetric and non-symmetric vibrations of C–H and CH2. More intense signal of CH2 at 2930 cm− 1 for PUR_2 proves the incorporation of cyclohexane ring into this structure. The presence of ether moiety is confirmed by peak at 1100 cm− 1.
For perspective application of the obtained material, PUR thermal resistance is important and TGA technique allows its evaluation. Figure 6 shows the TG and DTG curves of the analyzed polyurethanes: (a) PUR_1 (polyurethane based on polyether-diol) and (b) PUR_2 (polyurethane based on polyether-pentol).
Based on the literature, it is known that the thermal degradation of polyurethanes can take two or three stages [23, 24]. The first step is the decomposition of the rigid segments by the disintegration of urethane bonds in the polymer resulting in the formation of isocyanate, alcohol, I- and II-order amine, olefins and carbon dioxide. The rate of the first degradation step decreases as the content of the flexible segments increases. The second and third stages correspond to the thermal decomposition of the flexible segments which is slower and depends on their chemical structure. At the third stage, the contained solid residue and the compounds are oxidized [25]. The TGA results show that the analyzed polyurethanes are characterized by two-stage thermal degradation. The temperature of 5% of weight loss is considered as the onset of degradation for polyurethane. It was determined as 255 °C for PUR_1 and about 310 °C for PUR_2 (Table 2). Sample weights did not change up to 225 °C for PUR_1 and up to 240 °C for PUR_2. The 50% weight loss was recorded at approximately 390 °C (for PUR_1) and 392 °C (for PUR_2), while the solid residue was 9 and 13% (PUR_1 and PUR_2), respectively (Fig. 6a, b). The maximum weight loss rate was recorded at approximately 390 °C for both samples. To determine the thermal stability of the PURs, the onset temperatures for degradation process (Tonset) (Table 2) were determined. The calculated values are comparable to the ones derived for polyurethanes obtained in the polyethylene glycol synthesis with MDI by the Liang-Siong group [26]. Also, the maximum mass loss rate (TDTGmax) and the amount of solid residue (Table 2) were denoted. The first stage of thermal decomposition of the tested PURs is in the temperature range 350–360 °C and is related to the breaking of the urethane bonds in the rigid polyurethane chains. During the second stage of degradation, starting at around 400 °C, oligo-diol and oligo-pentol chains break down.
From the shape of DTG curve of PUR_2 it is clearly visible that the intensity of lower temperature peak is much higher. According to the study of Ryszkowska et al. [27], this indicates on higher phase separation in this system. This conclusion is supported by results of the FTIR analysis described previously, where we noted large diversity in the PUR_2’s structure, which can be responsible for more intense phase separation tendency. A significant amount of solid degradation residue (9 and 13%) may be advantageous in terms of the flame retardation of the obtained PUR, as this is usually associated with less degradation products released during the degradation process, which burns rapidly causing fire to propagate [28, 29].
Another technique for analyzing thermal behavior of polyurethanes is differential scanning calorimetry (DSC). It enables to describe the PUR structure by determining the temperature and effects associated with physical changes in these materials [30,31,32]. Figure 7 shows the thermograms of the obtained polyurethanes.
The increase in the glass transition temperature in PUR_2 (− 50.09 °C) is due to the limitation of mobility of the flexible segments in comparison to PUR_1 (− 60.21 °C). For PUR_2 flexible segments, higher energy is required to move the chains. This suggests the presence of some additional interaction of intra-/inter-molecular nature. We think that it could be the effect of hydrogen bonding interaction as well as π–π interaction of aromatic rings in the isocyanate segments. For star-shaped PUR_2, the structure favors hydrogen bond formation, which in turn, enables the mutual π–π interaction. In this case, the possibility of close interaction between aromatic rings is higher, and as a result higher Tg is observed for PUR_2. For PUR_1, aromatic ring interaction seems to be less plausible as linear structure imparts more flexibility for the segments of the macromolecules.
The presence of aromatic rings in the isocyanate structure also affects the stiffening of the foams by increasing their thermal stability. The obtained rigid polyurethane foams were characterized by increased thermal resistance compared to the resistance of conventional rigid polyurethane foams (90–200 °C – 5% weight loss) [11] and with other rigid foams obtained with oligoether-triols (190–215 °C − 5% weight loss) [33] or polyether-diol (130 °C − 15% weight loss) [34].
Conclusion
Mono-, di-, tri- tetra- and penta-potassium salts of 2,2,6,6-tetrakis(hydroxymethyl) cyclohexanol (THMC)-activated 18-crown-6 are effective initiators of the polymerization of propylene oxide. The processes were carried out at mild conditions, i.e., in THF solution at room temperature. The main characteristic features of the studied processes were
-
Application of K-, 2K-, 3K- 4K- and 5K-THMC-activated 18C6 gave bimodal PPO-pentols with Mn = 2100–13,200 g/mol.
-
Unsaturation of PPO-pentols was dependent on the initial concentration of monomer and alkoxide groups; the lowest unsaturation was observed for K-THMC and [PO]o = 2.0 mol/dm3.
-
The results of the SEC study indicated that triple-substituted system was the most effective to obtain high-mass PEPOs.
-
Polyether-polyols prepared in this work could be used for the synthesis of new polyurethanes.
-
Rigid polyurethane prepared with new polyether-polyols was porous in nature being stiffer than the one produced with linear one polyether-diol.
-
TGA analysis showed that the analyzed polyurethanes were characterized by two-stage thermal degradation, with the temperature of 5% weight loss higher for the newly synthesized PUR_2 (310 °C) in comparison to the linear PUR_1 (255 °C).
References
Wegener G, Brandt M, Duda L, Hofmann J, Klesczewski B, Koch D, Kumpf RJ, Orzesek H, Pirkl HG, Six CH, Steinlein CH, Weisback M (2001) Trends in industrial catalysis in the polyurethane industry. Appl Catal A General 221:303–335
Fuensanta M, Martin-Martinez JM (2018) Thermoplastic polyurethane coatings made with mixtures of polyethers of different molecular weights with pressure sensitive adhesion property. Prog Org Coat 118:148–156
Jing L, Jianchun J, Junming X, Haihong X, Peng L (2016) Branched polyols based on oleic acid for production of polyurethane foams reinforced with bamboo fiber. Iran Polym J 25:811–822
Singh V, Khullar P, Dave PN, Kaur N (2013) Micelles, mixed micelles, and applications of polyoxypropylene (PPO)-polyoxyethylene (PEO)-polyoxypropylene (PPO) triblock polymers. Int J Ind Chem 4:12–30
Alexandridis P, Olsson U, Lindmann B (1998) A record nine different phases (four cubic, two hexagonal, and one lamellar lyotropic liquid crystalline and two micellar solutions) in a ternary isothermal system of an amphiphilic block copolymer and selective solvents (water and oil). Langmuir 14:2627–2638
Groot RD, Madden TJ (1998) Dynamic simulation of diblock copolymer microphase separation. J Chem Phys 108:8713–8724
Yang S, Zhang Z, Wang F, Feng L, Wang T (2013) Surface properties and aggregation behaviors of amphiphilic highly-branched block polyethers in aqueous solution. J Polym Res 20:205
Cendejas G, Arreguin F, Flores C, Villalobos I, Flores E, Vázquez F (2008) Novel initiators for the synthesis of propylene oxide oligomers by anionic ring opening polymerization. Catal Today 130:486–491
Grobelny Z, Matlengiewicz M, Golba S, Jurek-Suliga J, Swinarew AS, Skrzeczyna K, Michalak M, Swinarew B (2015) Application of dipotassium glycoxides—activated 18-Crown-6 for the synthesis of poly(propylene oxide) with increased molar mass. Int J Polym Anal Charact 20:206–222
Ionescu M (2005) Chemistry and technology of polyols for polyurethanes. Rapra Technology Limited, Shawbury
Morejko B, Stolarzewicz A, Grobelny Z, Piekarnik B, Niedziela T, Trzebicka B (2007) New kind of star-shaped polyethers prepared with cyclic oligo (potassium glycidoxide) as a macroinitiator. React Funct Polym 67:669–674
Bassyouni M, Sherif SA, Sadek MA, Ashour FH (2012) Synthesis and characterization of polyurethane—treated waste milled light bulbs composites. Composites Part B 43:1439–1444
Kuranska M, Prociak A (2016) The influence of rapeseed oil-based polyols on the foaming process of rigid polyurethane foams. Ind Crops Prod 89:182–187
Adly Rahandi Lubis M, Park BD, Lee SM (2017) Modification of urea-formaldehyde resin adhesives with blocked isocyanates using sodium bisulfate. Int J Adhes Adhes 73:118–124
Brown CA (1974) Saline hydrides and superbases in organic reactions. VII. potassium hydride, highly active new hydride reagent. reactivity, applications, and techniques in organic and organometallic reactions. J Org Chem 39:3913–3918
Wang Y, Li T, Wang X, Ma P, Bai H, Dong W, Xie Y, Chen M (2016) Superior performance of polyurethane based on natural melanin nanoparticles. Biomacromolecules 17:3782–3789
Madhavan K, Reddy BSR (2006) Synthesis and characterization of poly(dimethylsiloxane-urethane) elastomers: effect of hard segments of polyurethane on morphological and mechanical properties. J Polym Sci A 44:2980–2989
Schuur M, Noordover B, Gaymans RJ (2006) Polyurethane elastomers with amide chain extenders of uniform length. Polymer 47:1091–1100
Ayres E, Oréfice RL, Yoshida MI (2007) Phase morphology of hydrolysable polyurethanes derived from aqueous dispersions. Eur Polym J 43:3510–3521
Seymour RW, Estes GM, Cooper SL (1970) Infrared studies of segmented polyurethane elastomers. I. hydrogen bonding. Macromolecules 3:579–583
Coleman MM, Skrovanek DJ, Hu J, Painter PC (1988) Hydrogen bonding in polymer blends. 1. FTIR studies of urethane-ether blends. Macromolecules 21:59–65
Pretsch T, Jakob I, Müller W (2009) Hydrolytic degradation and functional stability of a segmented shape memory poly(ester urethane). Polym Degrad Stab 94:61–73
Petrović Z, Zavargo Z, Flynn J, Macknight W (1994) Thermal degradation of segmented polyurethanes. J Appl Polym Sci 51:1087–1095
Janowski B, Pielichowski K (2008) Thermo(oxidative) stability of novel polyurethane/POSS nanohybrid elastomers. Thermochim Acta 478:51–53
Chattopadhyay D, Webster D (2009) Thermal stability and flame retardancy of polyurethanes. Prog Polym Sci 34:1068–1133
Liang-Siong T, Chuh-Yung C, Jen-Feng K (1997) Fourier transform infrared spectroscopy study on effects of temperature on hydrogen bonding in amine-containing polyurethanes and poly(urethane—urea)s. Macromolecules 30:1793–1799
Prociak A, Rokicki G, Ryszkowska J (2014) Materiały poliuretanowe. Wydawnictwo Naukowe PWN, Warszawa
US patent No. 6 362 279 (2002)
McKenna ST, Hull TR (2016) The fire toxicity of polyurethane foams. Fire Sci Rev 5:3–29
Michałowski S, Hebda E, Pielichowski K (2017) Thermal stability and flammability of polyurethane foams chemically reinforced with POSS. J Therm Anal Calorim 130:155–163
Wang LF (2005) Effect of soft segment length on the thermal behaviors of fluorinated polyurethanes. Eur Polym J 41:293–301
Koberstein JT, Galambos AF, Leung LM (1992) Compression-molded polyurethane block copolymers. 1. Microdomain morphology and thermomechanical properties. Macromolecules 25:6195–6204
Lubczak J, Łukasiewicz B (2012) Oligoeterole i pianki poliuretanowe z pierścieniem 1,3,5-triazynowym i atomami boru. Polimery 57:819–829
Kim DH, Kwon OJ, Yang SR, Park JS, Chun BC (2007) Structural, thermal, and mechanical properties of polyurethane foams prepared with starch as the main component of polyols. Fiber Polym 8:155–162
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no known conflict of interest concerning the given work.
Supporting Information
Supporting Information
Supporting Information is available from the author.
Rights and permissions
About this article
Cite this article
Grobelny, Z., Golba, S. & Jurek-Suliga, J. A new cyclic initiator system for the synthesis of novel star-shaped polyether-polyols (PEPOs) for fabrication of rigid cross-linked polyurethanes. Iran Polym J 27, 745–754 (2018). https://doi.org/10.1007/s13726-018-0653-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-018-0653-2