Abstract
To obtain modified cyanate ester (CE) with good comprehensive properties and low cost, a novel phenolic resin containing diphenyl oxide (MPF) with high molecular weight was synthesized from diphenyl ether, formaldehyde, methanol and phenol by a two-step process which differed from polyphenylene oxide (PPO) in structure. The curing reaction and properties of the modified 2,2-bis(4-cyanatophenyl) propane (bisphenol-A-based cyanate ester, BADCy) by MPF were investigated. It was found that the curing temperature of the modified CE was lower than that of the unmodified CE. When the ratio of MPF and BADCy was 3:7, the cured resin exhibited low dielectric constant (3.00), low dielectric loss (0.0062) and high impact strength (12.5 kJ/m2), and its T d5% was 371 °C, being superior to CE in the comprehensive properties. When the content of MPF was above 30 %, MPF/BADCy had poor comprehensive properties. In order to improve MPF/BADCy with high content of MPF, epoxy resin (E51) was added. When the ratio of MPF, BADCy and E51 was 50:50:67, the cured resin exhibited low dielectric constant (2.96), dielectric loss (0.0078) and high impact strength (11.84 kJ/m2), and its T d5% was 365 °C. Small content of MPF or the combination of E51 and MPF were good for BADCy to improve its comprehensive properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanate ester resin (CE) has drawn much attention as one of the high-performance thermoset polymers. Cyanate esters undergo self-cyclotrimerization to form a three-dimensional network structure of polycyanurate containing triazine ring groups [1–3]. Polycyanurate possesses several superior properties including high heat resistance, low moisture absorption, low dielectric constant, and low shrinkage. To widen its applications, it is necessary to overcome its disadvantages and produce modified CE with good comprehensive properties, especially improved brittleness and reduced cost.
For the improvement of brittleness, rubber is a good modifier, which was investigated by Liu [4]. Thermoplastic with high glass transition temperature (T g) is appropriate to improve impact strength and fracture toughness of CE [5]. Cyanate ester resin (CE) has also been modified by poly(ether imide) (PEI) [6–8], poly(ether sulfone) (PES) [9–11] and poly(ether ketone) (PEK) [12, 13]. Some thermoset resins such as epoxies and bismaleimides are effective to improve CE properties. Wang et al. have studied the properties of CE/epoxy (EP) and they found it was tougher than CE [14]. Bismaleimide/CE hybrid polymer networks are characterized with good thermal, mechanical and dielectric properties [15–17]. On top of that, Zhou et al. has synthesized a number of epoxidized polysiloxane (HBPSiEP) to modify CE [18]. By blending glycidyl polyhedral oligomeric silsesquioxane (G-POSS) and phenol-A cyanate ester (CE), using triethylamine as the curing agent, the modified CE was very tough [19].
Although the above methods have been effective to improve toughness of CE, other properties such as dielectric properties were sacrificed. PPO is an amorphous thermoplastic polymer with excellent dielectric properties (extremely low dielectric constant and loss), outstanding moisture and thermal resistance, and desirable toughness [20–22] which can make CE tough and keep its dielectric properties unaffected. Hyperbranched PPO [23, 24] and epoxy-functionalized hyperbranched PPO [25] can avoid the phase separation between CE and PPO. Lin et al. [26] has reported cyanate ester modified by appropriate PPO-EPMS, a kind of PPO-epoxy polymer microsphere with reactive hydroxyl which showed good thermal and dielectric properties.
Based on the above studies, a new modifier of CE including diphenyl oxide segments and phenolic hydroxyl with high molecular weight was designed. Diphenyl oxide segments, similar to phenyl oxide bonds in PPO, have excellent dielectric properties. Phenolic hydroxyl can react with –OCN functional groups of CE to avoid phase separation. Molecular weight of the novel modifier of CE is close to that of PPO.
To achieve the goal, diphenyl ether, formaldehyde, methanol and phenol were chosen as experimental materials, whose preparation process is shown in Scheme 1. The novel modifier of CE was named by MPF, which was different from PPO in structure, and cheaper than PPO.
In this study, we intend to explore the possibility of introducing MPF into BADCy to achieve a tough CE with good dielectric properties and low cost which will have many applications, especially in printed circuit board (PCB).
Experimental
Materials
All materials were commercially available and were used as received. Phenol, methanol, formaldehyde (37 %), toluene, sulfuric acid (98 %) and p-toluenesulfonic acid (PTSA) were purchased from Shanghai LINGFENG Chemical Reagent Co. Ltd (China). The BADCy monomer (Scheme 2.) was supplied by Shanghai HUIFENG Technical & Business Co. Ltd (China) whose molecular weight is 278 g/mol. Bisphenol A diglycidyl ether type resin (E51, liquid) was purchased commercially (China) whose epoxy equivalent value and viscosity were 185–208 g/mol and <2500 mPa s, respectively. Diphenyl ether, other reagents and solvents were purchased commercially (China).
Synthesis of phenolic containing diphenyl oxide (MPF)
MPF was synthesized according to the method outlined in Scheme 1. Briefly, there were two steps:
-
The first step was the synthesis of diphenyl ether terminated with methoxyl group (DETM). Methanol, formaldehyde (37 %), sulfuric acid (98 %), and diphenyl ether were added into a 500 mL three-necked flask with a thermometer and condenser at 80 °C for 6 h with stirring. Pouring the crude product into a separator funnel and the sulfuric acid was removed. The remainders were dissolved in toluene. Finally, the solvent was removed by vacuum distillation. Brown yellow liquid was obtained, which was DETM.
-
The second step was the synthesis of phenolic containing diphenyl oxide (MPF). In a three-necked rounded-bottom flask, equipped with a mechanical stirrer, DETM (100 g) and PTSA were added into phenol with stirring. The reaction mixture was heated to 140 °C for some time. Methanol was formed and generated out. Excess phenol and small molecule were distilled under reduced pressure. Then, MPF as a pale yellow product was gained.
Preparation of the MPF/BADCy resins
The mixture of BADCy and MPF with compositions presented in Table 1 was added to a beaker with stirring evenly at 120 °C. The mixture was poured into the preheated (120 °C) mold and degassed at 120 °C for 30 min. Then the mold was moved into a oven for curing according to the following procedure: 160 °C/2 h + 180 °C/2 h + 200 °C/2 h + 220 °C/2 h + 240 °C/2 h. The cured resins were ejected from the molds, then cut and polished according to the the dimension requirements for the property measurements.
Characterization
Fourier transform infrared (FTIR) spectra were recorded on a Nicolet 6700 spectrometer (USA) with KBr pellets. Spectra in the optical range of 400–4000 cm−1 were obtained by averaging 16 scans at a resolution of 4 cm−1.
The absolute molecular weight and molecular weight distribution were determined by gel permeation chromatography (Waters515; Optilab rEX; ViscoStar) (USA) equipped with small angle laser light scattering (Dawn Heleos, USA). The solvent was tetrahydrofuran (THF).
Differential scanning calorimetric (DSC) thermograms were obtained using a TA DSC2910 (USA) instrument under nitrogen atmosphere from ambient to 300 °C, at a heating rate of 10 °C/min.
Dynamic mechanical analysis (DMA) was carried out with a TA Q800 (USA) instrument. The samples were subjected to temperatures scan at a programmed heating rate of 5 °C/min from ambient to 320 °C at a frequency of 1 Hz. The test method was performed by three point bending mode with a tension ratio of 110 %.
Thermogravimetric analysis (TGA) was performed with a Netzsch STA 449F3 (Germany) thermal analyzer at a heating rate of 10 °C/min under nitrogen atmosphere from 30 to 800 °C.
Dielectric measurements were performed with an Agilent E4991A RF (USA) measurement system at room temperature by the two parallel-plate mode at 1 GHz. The applied voltage was 1 V, which was calibrated with polytetrafluoroethylene (PTFE) sample provided by the manufacturer. The dimensions of each sample were (20 ± 0.1) × (20 ± 0.1) × (2 ± 0.1) mm3. Before being tested, samples were dried under vacuum at 100 °C for 8 h. Impact strength of the cured resins was obtained according to GB/T 1843-2008.
A scanning electron microscope (Hitachi S-4800, Japan) was employed to observe the morphology of the fractured surfaces of the samples. The samples were coated with a thin layer of Au before testing.
Results and discussion
Characterization of MPF
FTIR spectra of diphenyl ether, DETM and MPF are shown in Fig. 1. For DETM, the absorption peak due to –OCH3 functional groups is appeared at 1046 cm−1, implying that diphenyl ether has reacted with formaldehyde to form –OCH3 groups. For MPF, the peak at 1046 cm−1 has been absent and the characteristic peak at 3412 cm−1 designated to –OH groups is present. It is due to the reaction of –OCH3 functional groups with phenol to generate out methanol. The absorption peak at 1163 cm−1 is attributed to the C–O–C functional groups. It indicated that phenolic hydroxyl functional group (–OH) has been successfully introduced into the MPF, yielding a novel phenolic resin with diphenyl oxide. The molecular weight of MPF is characterized by GPC equipped with small angle laser light scattering. The M w , M n and M w /M n of MPF were about 5.69 × 104, 2.74 × 104 g/mol and 2.08, respectively.
Curing temperature of MPF/BADCy resins
Figure 2 shows the DSC thermograms of pure BADCy and MPF/BADCy resins. There are two peaks in every thermogram: one was an endothermic peak, for the melting of BADCy, and the other was related with curing reaction. Compared with the curing peak temperature of BADCy (253 °C), the curing peak temperature of MPF/BADCy resins shifted to lower temperatures (i.e., B1: 237 °C; B2: 236 °C; B3: 235 °C; B4: 232 °C).
Thermal stability of MPF/BADCy resins
Thermal stability plays an important role in the applications of thermosetting resins. Here, the thermal stability of the cured BADCy and MPF/BADCy resins were evaluated by TGA. Figure 3 shows TGA profiles of the cured MPF/BADCy resins with different compositions in N2.
The temperature at which 5 wt% weight loss occurs (T d5%) was usually used to evaluate the thermal stability of the thermosetting resins. From Fig. 3, it can be seen that T d5% of the pure BADCy was higher than those of MPF/BADCy resins. The T d5% and the char yield at 800 °C from the TGA curves of the cured MPF/BADCy resins were listed in Table 2. With the increase of the content of MPF, the thermal stability and the char yield at 800 °C of the cured MPF/BADCy resins were reduced owing to the decrease of triazine ring and lower cross-linking density. The T d5% of B3 was only 307 °C, lower than that of B0 by 123 °C. It was maybe due to the presence of many linear long chains without cross-linking in B3. When the ratio of BADCy and MPF was 7:3, the T d5% reached to 371 °C, which is still acceptable in currently printed circuit board (PCB) manufacturing [27, 28].
Dynamic mechanical properties of MPF/BADCy resins
High glass transition temperature (T g) is important for thermosetting resin’s applications. DMA is an effective way for the definition of T g, corresponding to the peak temperature in the plot of tan δ versus temperature. Figures 4 and 5 show the overlay plots of storage modulus and tan δ versus temperature for the cured MPF/BADCy resin samples.
As the content of MPF is increasing, T g of the MPF/BADCy systems is reducing. The decrease of T g can be qualitatively verified by its cross-linking density which is determined according to the rubber elasticity theory as follows [29]:
where G′ is the storage modulus of the cured resin at temperature T (T = T g + 50 °C), R is the gas constant (R = 8.314 J/K/mol), T is the absolute temperature at T g + 50 °C. The T g and cross-linking density (ρ) values of the MPF/BADCy systems are listed in Table 3. It is shown that the cross-linking density of the cured MPF/BADCy resins was depressed with the increase of MPF content. The more MPF in MPF/BADCy systems, the lower was T g. This is consistent with the rule of T d5%, owing to the lower cross-linking density. The T g values obtained in the present study, however, were higher than those reported in the literature [30–32].
Dielectric constant and dielectric loss of MPF/BADCy resins
Excellent dielectric property (lower dielectric constant and dielectric loss) is one of the most desirable properties for next generation electronic devices. Table 4 depicted dielectric constant (D k ) and dielectric loss (D f ) values of the cured MPF/BADCy resins.
From Table 4, D k of the cured MPF/BADCy resins was almost equal to that of the cured BADCy, but D f of the cured MPF/BADCy resins was lower than that of the cured BADCy. The dielectric constant of PPO/BADCy resin in the previous literature [24] was above 3.25. However, in the cured MPF/BADCy system, by incorporating 30 wt% MPF (B2), the dielectric constant and dielectric loss were 3.00 and 0.0062, respectively.
Diphenyl oxide linkage in MPF plays an important role in reducing electronic interactions and diluting dipole concentrations, resulting in low dielectric constant. As the content of MPF was higher than 30 wt%, the dielectric loss of the cured MPF/BADCy resin was elevated again, mainly attributed to the influence of polar functional groups (–OH) outweighing the above account [33].
Impact strength of the cured MPF/BADCy resins
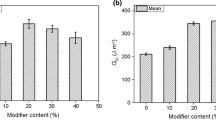
Figure 6 shows the impact strength of the cured MPF/BADCy system with varied MPF content. The impact strength first increased with the addition of MPF, and then decreased with further addition of MPF. The maximum value was 17.77 kJ/m2. Lin et al. [34] has reported the maximum impact strength of 14.10 kJ/m2 in the BADCy/MS systems.
When 10 wt% of MPF was added into the BADCy, the impact strength was three times as large as that of the cured BADCy. The impact strength of B2 was 12.07 kJ/m2, which was twice that of the cured BADCy (BADCy: 6.10 kJ/m2).
SEM results
The increased toughness for the MPF/BADCy resins can be confirmed by the SEM micrographs of the fractured surfaces as shown in Fig. 7a–d. Homogeneous and single-phase structure displays. The surface of the cured BADCy resin (Fig. 7a) had smooth surface, exhibiting a typical brittle feature. By adding MPF into BADCy resin (Fig. 7b–d), a number of tough whirls appeared. The surfaces of these samples showed a typical tough feature, which indicated that MPF plays the role of toughening of MPF/BADCy resin.
From cross-linking density values and SEM micrographs, we could deduce toughening mechanism of MPF/BADCy systems. Figure 8a–d show the schematic diagrams of the microstructures of MPF/BADCy systems. The cured BADCy was composed of triazine rings with high cross-linking density (Fig. 8a) which exhibited low impact strength. When MPF with long chain was introduced into BADCy with small content, there were some interconnected networks between –OCN and –OH units, making the cross-linking density of BADCy a little low (Fig. 8b). At the same time, introduction of diphenyl oxide into the rigid triazine matrix made it flexible. The maximum impact strength value was 17.77 kJ/m2. When the content of MPF was higher, a new semi-IPN between MPF and BADCy molecules was appeared, resulting in lower cross-linking density. The long chain of MPF without cross-linking was not helpful for dissipating the impact energy, which lowers the impact strength again.
Although the impact strength of the cured MPF/BADCy resins decreased with the increase of MPF content, the impact strength of B1, B2 and B3 samples was still higher than that of the cured BADCy. The impact strength of B4 was smaller than that of the cured BADCy. In B4, semi-IPN was the main structure, and cross-linking density was very low, which led to low impact strength.
Comprehensive property of MPF/BADCy/E51 systems
From above discussion when the addition of MPF was below 30 wt%, the comprehensive properties of the modified CE improved. Since MPF is a polymer with high molecular weight, the modified CE with MPF content higher than 30 wt% possessed poor comprehensive properties, due to the low cross-linking density in modified CE. If a third resin with the ability of reacting with CE and MPF was added into the MPF-modified CE, comprehensive properties will be improved again. An epoxy with low cost may be a good modifier for the MPF-modified CE. Therefore, MPF/BADCy/E51 system (based on B3 sample, MPF:BADCy = 5:5) was investigated. The mixtures of BADCy, MPF and E51 with different compositions are presented in Table 5. The curing procedure was the same as that of B3 sample.
Thermal properties of MPF/BADCy/E51 systems are listed in Table 6. It can be seen that T d5% of B3 was only 307 °C, but T d5% of MPF/BADCy/E51 system increased with the increasing of E51 content. The value of T g of MPF/BADCy/E51 systems increased at first and then dropped. From B3-0 to B3-1, MPF/BADCy ratio was constant, and the apparent cross-linking density (ρ) increased with the increasing of E51 content which is corresponded to the effect of E51 on T g and T d5% values. The B3-0.5 as an epoxy-based thermoset resin was different from B3-0, B3-1.5 and B3-1 samples. The apparent cross-linking density (ρ) of B3-0.5 was not comparable with that of B3-0, B3-1.5 and B3-1, but it showed high T d5% value.
Table 7 depicts dielectric constant (D k ) and dielectric loss (D f ) of the cured MPF/BADCy/E51 systems. The B3-1.5 system had a low dielectric constant and its dielectric loss (D f ) was close to that of the pure BADCy. The addition of E51 into B3 was an effective way to decline dielectric constant and dielectric loss, owing to the increase of cross-linking density, again. However, if the content of E51 be high, it will ruin the dielectric properties.
The impact strength of the cured MPF/BADCy/E51 systems was also studied (Fig. 9). With the increase in the content of E51 (from B3-0 to B3-1 samples), the impact strength increased by about 50 % and reached to 12.5 kJ/m2 which was equal to that of B2 sample. For B3-1.5, the impact strength was 11.84 kJ/m2. In MPF/BADCy system, more semi-IPN ruined the impact strength, due to low cross-linking density. When, the appropriate amount of E51 was introduced into MPF/BADCy system, some semi-IPNs transformed to interconnected network to make higher impact strength. As the content of E51 was higher, semi-IPN between MPF and BADCy molecules disappeared and resin became brittle which lowered the impact strength again.
SEM micrographs of the fractured surfaces of MPF/BADCy/E51 are shown in Fig. 10a–d which display a homogeneous and single-phase structure. Compared with B3 sample, there were more tough whirls in MPF/BADCy/E51 resin samples. The surfaces of B3-1.5, B3-1 and B3-0.5 samples showed a typical tough feature [34] which indicated that E51 is helpful for B3 resin sample to be tougher.
MPF/BADCy/E51 system is a new modified CE with good dielectric properties, high impact strength and low cost which will have many applications, especially in printed circuit board (PCB).
Conclusion
In this study, a novel phenolic resin (MPF) with high molecular weight was used as a modifier for CE. When the ratio of MPF and BADCy was 3:7, the cured resin exhibited low dielectric constant (3.00), dielectric loss (0.0062) and high impact strength (12.5 kJ/m2), and good thermal property. Especially, interconnected points between MPF and BADCy with low cross-linking density played an important role in improving toughness. Therefore, high molecular weight phenolic resin with low content can be a good modifier for CE. When the content of MPF was above 30 wt%, MPF/BADCy system showed poor comprehensive properties. To improve MPF/BADCy (MPF: BADCy = 5:5) properties, E51 was added to this system. In the ratio of 50:50:67 for MPF, BADCy and E51, the cured resin exhibited low dielectric constant (2.96), low dielectric loss (0.0078) and high impact strength (11.84 kJ/m2) and its T d5% was 365 °C. Therefore, E51 is a good modifier for MPF/BADCy resin system with high content of MPF which will have many applications, especially in printed circuit board (PCB) manufacturing.
References
Mackenzie PD, Malhotra V (1994) In: Hamerton I (ed) Chemistry and technology of cyanate ester resins, vol 20, 1st edn. Springer, Netherlands, pp 258–281
Biswas M, Capek I, Chern CS, Mathew D, Nair CPR, Ninan KN, Ray SS (2001) New polymerization techniques and synthetic methodologies. Springer, Berlin, pp 1–99
Chen X, Liang G, Gu A, Li Y (2015) Flame retarding cyanate ester resin with low curing temperature, high thermal resistance, outstanding dielectric property and low water absorption for high frequency and high speed printed circuit broads. Ind Eng Chem Res 54:1806–1815
Liu J, Ding N, Xu R, Chen J, Hu B (2011) Cyanate ester resin modified by hydroxyl-terminated polybutadiene: morphology, thermal, and mechanical properties. Polym Eng Sci 51:1404–1408
Suman JN, Kathi J, Tammishetti S (2005) Thermoplastic modification of monomeric and partially polymerized bisphenol A dicyanate ester. Eur Polym J 41:2963–2972
Cho JB, Hwang JW, Cho K, An JH, Park CE (1993) Effects of morphology on toughening of tetrafunctional epoxy resins with poly(ether imide). Polymer 34:4832–4836
Hourston DJ, Lane JM (1992) The toughening of epoxy resins with thermoplastics: 1. Trifunctional epoxy resin-polyetherimide blends. Polymer 33:1379–1383
Bucknall CB, Gilbert AH (1989) Toughening tetrafunctional epoxy resins using polyetherimide. Polymer 30:213–217
Yamanaka K, Inoue T (1989) Structure development in epoxy resin modified with poly(ether sulphone). Polymer 30:662–667
Akay M, Cracknell JG (1994) Epoxy resin–polyethersulphone blends. J Appl Polym Sci 52:663–688
Hu Z, Zhang J, Wang H, Li T, Liu Z, Yu Y (2014) Dual effects of mesoscopic fillers on the polyethersulfone modified cyanate ester: enhanced viscoelastic effect and mechanical properties. RSC Adv 4:34927–34937
Bennett GS, Farris RJ, Thompson SA (1991) Amine-terminated poly(aryl ether ketone)-epoxy/amine resin systems as tough high performance materials. Polymer 32:1633–1641
Li J, Chen P, Yu Q, Ma Z, Ma K, Wang B (2011) Influence of cyanate content on the morphology and properties of epoxy resins with phenolphthalein poly(ether ketone). J Appl Polym Sci 121:598–603
Wang JH, Yan HX, He SB, Yang LI (2008) Influence of the ratio of epoxy/cyanate ester on the curing behavior of epoxy/dicyclopentadiene bisphenol cyanate ester system. High Perform Polym. doi:10.1177/0954008308097424
Gu A (2006) High performance bismaleimide/cyanate ester hybrid polymer networks with excellent dielectric properties. Compos Sci Technol 66:1749–1755
Wu G, Cheng Y, Xie Q, Liu C, Kou K, Zhuo L, Wang Y (2014) Synthesis of a bismaleimide/cyanate ester copolymer containing phenolphthalein functional group with excellent dielectric properties and thermally stable. J Polym Res 21:1022–9760
Ma PC, Liu JF, Fan WF, Meng XS, Yang M (2013) Long chain bismaleimide modified cyanate ester resin and its composites. Chem J Chinese Univ 34:1979–1984
Zhou C, Gu A, Liang G, Yuan L (2011) Novel toughened cyanate ester resin with good dielectric properties and thermal stability by copolymerizing with hyperbranched polysiloxane and epoxy resin. Polym Adv Technol 22:710–717
Jiao J, Zhao LZ, Xia Y, Wang L (2016) Toughening of cyanate resin with low dielectric constant by glycidyl polyhedral oligomeric silsesquioxane. High Perform Polym. doi:10.1177/0954008316649423
Hwang HJ, Hsu SW, Wang CS (2008) Synthesis and physical properties of low-molecular-weight redistributed poly(2,6-dimethyl-1,4-phenylene oxide) for epoxy resin. J Appl Polym Sci 110:1880–1890
Wu SJ (2006) Cure reaction and phase separation behavior of cyanate ester-cured epoxy/polyphenylene oxide blends. J Appl Polym Sci 102:1139–1145
Rusli A, Cook WD, Schiller TL (2014) Blends of epoxy resins and polyphenylene oxide as processing aids and toughening agents. 2: curing kinetics, rheology, structure and properties. Polym Int 63:1414–1426
Huang P, Gu A, Liang G, Yuan L (2011) Curing behavior and dielectric properties of hyperbranched poly(phenylene oxide)/cyanate ester resins. J Appl Polym Sci 121:2113–2122
Gao R, Gu A, Liang G, Dai S, Yuan L (2011) Properties and origins of high-performance poly (phenylene oxide)/cyanate ester resins for high-frequency copper-clad laminates. J Appl Polym Sci 121:1675–1684
Huang P, Gu A, Liang G, Yuan L (2012) Synthesis of epoxy-functionalized hyperbranched poly(phenylene oxide) and its modification of cyanate ester resin. J Appl Polym Sci 123:2351–2359
Lin C, Yuan L, Gu A, Liang G, Wu J (2013) High performance self-healing bismaleimide/diallylbisphenol a/poly(phenylene oxide) microcapsules composites with low temperature processability. Polym Compos 34:335–342
Hou G, Li N, Han H, Huo L, Gao J (2015) Hybrid cationic ring-opening polymerization of epoxy resin/glycidyloxypropyl-polyhedral oligomeric silsesquioxane nanocomposites and dynamic mechanical properties. Iran Polym J 24:299–307
Kimura H, Ohtsuka K, Matsumoto A (2013) New thermosetting resin from benzoxazine and cyanate ester resin. Adv Polym Tech 32:E651–E659
Xie MR, Wang ZG, Zhao YF (2001) Synthesis and properties of a novel, liquid, trifunctional, cycloaliphatic epoxide. J Polym Sci Pol Chem 39:2799–2804
Anuradha G, Sarojadevi M (2008) Synthesis and characterization of poly(arylene ether) containing cyanate ester networks. J Polym Res 15:507–514
Anuradha G, Rakesh S, Sarojadevi M (2009) Synthesis and thermal properties of cyanate esters containing sulfoxide linkage. Polym Eng Sci 49:889–895
Shieh JY, Yang SP, Wu MF, Wang CS (2004) Synthesis and characterization of novel low-dielectric cyanate esters. J Polym Sci Pol Chem 42:2589–2600
Hougham G, Tesoro G, Shaw J (1994) Synthesis and properties of highly fluorinated polyimides. Macromolecules 27:3642–3649
Lin C, Yuan L, Gu A, Chen F, Liang G (2013) High performance cyanate ester resins/reactive porous polymeric microsphere systems with low-temperature processability. Compos Sci Technol 85:148–155
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (contract Grant Number 51003030) for its financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tu, Y., Yu, R., Duan, J. et al. Cyanate ester resin modified by phenolic resin containing diphenyl oxide segments with high molecular weight. Iran Polym J 25, 863–873 (2016). https://doi.org/10.1007/s13726-016-0472-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-016-0472-2