Abstract
Four polymeric solutions based on xanthan, high and low molecular weight sulfonated polyacrylamides, and hydrolyzed polyacrylamide were prepared in aqueous solutions and their behaviors in enhanced oil recovery applications were investigated. The effect of thermal aging on polymer solutions was evaluated through rheological measurement. Pendant drop method was also used for measuring the interfacial tension (IFT) between crude oil and brine containing different polymer solutions. Moreover, the zeta potential of the oil reservoir particles treated with oil and polymer was determined by electrophoresis method in a nano-zeta meter instrument. In addition, sand pack and core flooding setup were used for evaluating the effectiveness of the polymer solutions in porous media. Polymer solutions displayed non-Newtonian behavior in almost the whole range of the shear rate applied; a shear thinning behavior was seen. Furthermore, the aging of polymers in formation water decreased the shear viscosity of all the polymers. The oil/water IFT decreased by the addition of polymers to water. The effect of xanthan polymer on zeta potential value was greater than that of the three acrylamide-based polymers. According to sand pack tests, by increasing the polymer concentration, the incremental oil recovery initially increased up to a polymer concentration of 3,500 ppm and then started to fall. Recovery factor increased from 50 to 65 % using the polymer solution in core flooding experiments. By increasing the injection rate from 0.2 to 3 mL/min, the injected fluid had less time to sweep the pores and consequently the amount of recovered oil decreased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Generally, about one-third of original oil in place is recovered by primary and secondary processes and the remaining two-thirds are trapped in reservoirs as residual oil [1]. Today, enhanced oil recovery (EOR) methods are sought to recover residual oil. Water flooding is one of the simplest and less expensive EOR methods; however, it has poor sweep efficiency [2]. Sweep efficiency is mainly affected by mobility ratio and complicated pore structures. Mobility ratio is described by the following equation:
where k rw and k ro are relative permeability of water and oil phases, and \(\mu_{\text{o}}\) and \(\mu_{\text{w}}\) are related to water and oil viscosity, respectively [3]. This equation shows that the mobility ratio could be reduced by an increase in water viscosity or a reduction in water relative permeability, which contributes to higher oil recovery [4, 5].
Mainly, all formations do not have homogeneous structure because of variation in permeability, porosity, and the existence and the orientation of fractures [6]. The existence of natural fractures in oil reservoirs results in an increase in mobility ratio and fingering phenomenon, which eventually leads to poor sweep efficiency [7]. To overcome such problems, chemical materials such as water-soluble polymers as a mobility ratio controller must be utilized. Since polymers influence the area contacted by the displacing agent through increasing the viscosity of the displacing fluid, they improve the mobility ratio [8–11].
Polymer flooding is one of the most effective methods in EOR in the world resulting in producing residual oil that remains after primary production. One of the most crucial criteria to select the appropriate polymer is its ability to generate a viscous solution at the minimum concentration. Ionic polymers fulfill this condition much better in comparison with non-ionic polymers. This can be explained by their conformation occurring in electrolyte solutions. When ionic polymers are dissolved in the presence of an electrolyte in aqueous solution, they are adjusted to decrease the repulsion forces among their own ionic groups. On the other hand, their conformation is rod like, which results in making a solution with a viscosity much higher than the non-ionic systems; however, non-ionic polymers conform to a coil conformation in the solution. Polyacrylamide-based polymers are commonly used to increase the viscosity of the injected water [10–13].
Schurtz and Jewett investigated 61 cases of polymer flooding under different conditions. They understood that polymers could displace oil in such reservoirs at a maximum oil viscosity of 126 cp, a maximum temperature of 109 °C, and a minimum permeability of 20 mD. In addition, they found that the amount of polymer required was a function of water salinity and reservoir rock type. In this way, the higher the water salinity and the clay content in rock, the higher the polymer concentration would reach the desirable viscosity [14].
According to the studies of Wang et al. on the effect of polymer concentration and slug volume, it was concluded that piston-like displacement model for polymer injection in porous media was applicable. They showed that the amount of incremental oil production caused by polymer flooding in heterogeneously layered reservoirs was higher compared to the homogeneous ones. Also, they mentioned that the large polymer slugs with low concentrations produced satisfying results in heterogeneous heavy oil reservoirs. Nevertheless, in homogenous reservoirs, small polymer slugs with low concentrations were useful [15, 16].
Wang and his coworkers [17] suggested the injection of polymers with intermediate molecular weight for EOR in low thickness and permeability formations. In 2009, Rashidi et al. implemented a comprehensive study on salt-tolerant polymers. They concluded that sulfonated polyacrylamides (PAMS) with a high sulfonation degree are less sensitive to shear rate and behave like Newtonian fluids at high NaCl concentrations. Precipitation has also been observed for PAMS copolymers after 7 months of aging at 80 °C. Finally, high temperatures increase retention for both the sulfonated and hydrolyzed polyacrylamide, but only to a small extent [18, 19].
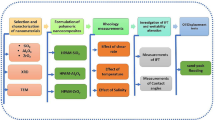
While, as the examples show, the evaluation and application of different polymers for EOR process have been studied by various investigators, there are a few experimental studies that provide an accurate explanation for this phenomenon. The evaluation of the performance of polymers from rheological and interfacial points of view could help with the selection of more effective polymers in porous media as EOR agents. The main aim of this work is to study the performance of different polymers (based on xanthan, high and low molecular weight sulfonated polyacrylamide, and hydrolyzed polyacrylamide) for EOR applications in carbonate-type porous media through laboratory tests. Different analytical methods such as interfacial tension (IFT), rheological measurements, zeta potential, sand pack, and core flooding tests are utilized to study the polymer behavior in EOR processes. To the best of our knowledge, most of the information reported in this paper, such as IFT behavior between a light crude oil phase (APIo = 34) and these polymer solutions, zeta potential value of carbonate particles aged with these polymers and, finally, their performance for EOR purposes in carbonate type sand packs and cores, is new and has not been reported elsewhere.
Experimental
Materials
Sulfonated polyacrylamide with a degree of sulfonation of 25 mol.% and an average molecular weight of 8 × 106 Dalton (AN), sulfonated polyacrylamide with the same degree of sulfonation but higher molecular weights (VM), partially hydrolyzed polyacrylamides with a degree of hydrolysis of 25 mol.%, and an average molecular weight of 16 × 106 Dalton (HM25) were provided by the SNF Company (France).
AN and VM are copolymers of acrylamide and sodium salt of acrylamido-2-methyl-1-propane sulfonic acid. The sulfonated polyacrylamides have higher thermal stability and salt tolerance in oil reservoir conditions than standard hydrolyzed polyacrylamides and are used in oil field applications up to 120 °C.
Xanthan polysaccharide under the trade name of Satiaxane CX 90T was provided by Degussa Texturant System (Germany).
The chemical structure of the above-mentioned polymers is shown in Fig. 1.
Crude oil with an API° of 34 was used as the oil phase. Carbonate particles, produced from one of Iran’s southwest oil reservoir fields during the oil production processes, with a mesh size of 70, were used as the solid phase during zeta potential and sand pack flooding tests.
Rheological measurements
The polymer solutions were prepared by the gradual addition of the polymer powder to formation water (oil reservoir water) while stirring (Table 1) to obtain clear, viscous solutions. The rheological behavior was monitored using a concentric cylinder Physica MCR 501 rheometer (Anton Paar, Austria) equipped with a Peltier device for temperature control. To carry out the rheological tests, the system was first heated to the desired temperature (90 °C), and then the prepared solution was introduced into the heated cylinder and subjected to shear strain. Rheoplus software, associated with the instrument, was utilized for recording and analyzing the flow behavior data. Thermal aging on samples was performed by keeping the polymer solutions in an oven at 90 °C. After 1 week, the rheological behavior of these samples was evaluated and compared with the non-aged samples.
Interfacial properties measurements
Since the presence of polyelectrolytes, which act as surface active agents, affects interfacial properties such as IFT, we need initially to measure IFT by an apparatus with an ability to simulate the temperature and pressure of reservoir conditions on the basis of a standard method such as pendant drop. On the other hand, polymer adsorption and polymer affinity to reservoir rock are substantial factors in the design of a polymer flooding project. Thus, it is necessary to understand the changes in surface charges due to the adsorption of polymers in porous media by zeta potential measurement.
The physical phenomena between two fluids (gas and oil or water and oil) called interfacial tension result from the interactions due to intermolecular forces. In the bulk of a fluid, each molecule is surrounded by equivalent neighboring molecules; hence, the balance of intermolecular forces is zero. A molecule at the interface of two fluids is surrounded by two different neighboring media, which results in an inward force. These molecules are subject to molecular attraction balanced by the fluid resistance to compression. The surface acts like a stretched elastic membrane; therefore, to minimize its energy state, the number of boundary molecules is minimized. In reservoir engineering, oil, water, and gas can coexist in the porous media and the equilibrium of these systems can be ascribed to the interfacial forces. Therefore, the IFT data are required to describe the complete system. The pendant drop method is probably the most convenient, versatile, and popular method to measure the interfacial tension between water and oil phases. The pendant drop method involves the determination of the profile of a drop of one liquid suspended on another liquid at mechanical equilibrium. This profile is determined by the balance between gravity and surface forces.
In this study, IFT was measured by the pendant drop method. This setup, designed by the Schlumberger Company (France), is able to determine IFT at high temperatures and pressures. By using IFT device, a drop of crude oil is raised into the water medium (containing different polymers) (Fig. 2). Then, by the help of IFT Vision software, associated with the instrument, the IFT between crude oil and different polymer solutions is measured. The following equation has been used for calculating the interfacial tension:
where γ, d e, and g represent the IFT, oil drop diameter, and gravity, respectively; Δρ is taken as the density difference between oil and surrounding medium, and H is a shape-dependent parameter. The shape-dependent parameter (H) depends on the value of the “shape factor (S = d s/d e)”. Tables including the set of \(\frac{1}{H}\) versus S values are available in several references [20, 21].
Zeta potential measurements
Zeta potential is defined as an electrical potential which exists at the shear plane of the colloidal particles. These particles are electrically charged because of their ionic characteristics [22, 23]. The zeta potential of the produced particles treated with oil and polymer was measured by a Malvern nano-zeta meter (England). For this purpose, particles were first aged in crude oil at 70 °C for 7 days. The aged particles were then separated from oil phase and dried in a vacuum oven; afterward, the oil-aged particles were placed in different polymer solutions for 3 days at 25 °C. Finally, the polymer-treated particles were dispersed in distilled water at a concentration of 0.1 wt.% using a magnetic stirrer at 25 °C. The final pH of the solutions was adjusted to about 7–8.
Polymer injection in porous media
Sand pack tests
The sand pack assembly for polymer flooding was a stainless steel cylinder with a length of 30.8 cm and an internal diameter of 3 cm packed with sand. The desired particles for packing the sand pack holder (particles with a mesh size of 70) were prepared by grinding and then screening the previously mentioned oil reservoir sand by ball mill. After packing the sand pack holder, both its ends were equipped with stainless steel fluid distributors on which a 200-mesh screen was spot welded to prevent fine sand from flowing out and to provide an even distribution of injected fluid. After assembling the sand-packed apparatus, the sand pack was saturated with synthetic formation water under vacuum. Its pore volume (PV) was about 65 mL. The sand pack was flooded with crude oil to irreducible water saturation (S wir). Then, an initial water flood (2 PV) was conducted until no additional oil was produced by the continuous water injection. Afterward, it was flooded with 0.6 PV of the polymer solution followed by 2 PV of water to recover more and more residual oil. The amount of displaced fluid and differential pressure between the inlet and the outlet during the recovery were recorded. During all the sand pack flooding tests, the injection rate was 0.2 mL/min, the temperature was kept constant at 110 °C using an oven and the back pressure was about 50 bar. The porosity of the sand pack was about 22 %.
Core flooding tests
The general procedure of core flooding was similar to that of the sand pack flooding. At first, the prepared cores were evacuated and saturated with synthetic formation water under vacuum. Its PV was about 30 mL. Then, the cores were placed inside a rubber sleeve in a core holder and flooded with reservoir oil under an overburden pressure of 220 bar outside the rubber sleeve. Afterward, similar to the sand pack tests, 2 PV of water flooding was conducted until no additional oil was produced by the continuous water injection. It was subsequently flooded with 1.2 PV of polymer solution followed by 2 PV of water flooding to recover more residual oil.
During all core flooding tests, the temperature was kept constant at 90 °C using an oven and the back pressure was about 180 bar. The porosity of core was nearly 17 % and the absolute permeability was ~11 mD.
Results and discussion
Rheological behavior
Figure 3 shows the flow curves of different polymer solutions in synthetic formation water before and after thermal aging at 90 °C. This figure indicates that the viscosity of all the polymer solutions continuously decreases with increasing shear rate; in other words, the polymer solutions displays a non-Newtonian behavior in almost the whole range of the shear rate applied and shear thinning effect occurs. This behavior is typical of polymer melts and solutions coming from the disentanglement process and the increase of the average end-to-end distance of the polymeric chains due to shearing.
Additionally, it can be seen from this figure that the shear viscosity decreases after thermal aging for all the polymers. Polyelectrolytes such as xanthan, sulfonated, and hydrolyzed polyacrylamide are built of monomeric units which include an ionizable group, i.e., a group that can dissociate into a chain-fixed cation or anion and a mobile counter-ion bearing the opposite charge in aqueous surroundings. Charges give rise to Coulomb forces. These are much stronger than van der Waals forces and can thus act over longer distances. On dissolving a polyelectrolyte chain, all counter-ions diffuse away and distribute themselves homogeneously in the solvent. As a result, the chain should become fully stretched in a reaction toward the repulsive Coulomb forces between the chain-fixed charges. However, this does not actually happen due to two effects which change the conditions. Firstly, in a polyelectrolyte with ionizable groups at short distances along the chain, for example in every monomeric unit, there arise strong attractive forces on the counter-ions and prevent them from diffusing away. A part of them remain as a condensate in the immediate neighborhood of the polyion, so that the effective charge density is reduced. In addition, the existing chain-fixed charges often do not fully create the associated Coulomb force, but become screened by a shell of counter-ions. This formation of a cloud of opposite charges is found in all electrolytes, polyions, and low molar mass ions and results in a practical disappearance of the Coulomb forces at distances above a certain length. This length, known as the Debye length, also describes the size of the charge compensating cloud. It varies with the ionic strength given by the total concentration of mobile ions [22–24]. The aging of polymers in the electrolyte solution in the presence of cations such as Na+, Mg2+, and Ca2+ makes the screening effect of cations on the anionic groups of these polymer chains more evident and consequently there is less chain expansion, thus leading to lower viscosity.
It can be seen from this figure that the viscosity of xanthan is higher than that of the other polymers after 1 week of thermal aging; this may be due to its higher viscosity before aging. In addition, the viscosity of hydrolyzed polyacrylamide (HM25) is decreased more than that of sulfonated polyacrylamides (VM and AN) during thermal aging; this results from the bigger screening effect of multivalent cations such as Ca2+ on carboxyl groups (–COO−) of hydrolyzed polyacrylamide compared to the sulfonic groups (–SO32−) of sulfonated polyacrylamides.
Interfacial properties
IFT between crude oil and brine is an important variable in water/oil systems used for oil displacements. IFT influences capillary pressure, capillary number, adhesion tension, and the dimensionless time for imbibitions [25]. There is little data available about the IFT between different polymer solutions and crude oil. The effect of different polymers on IFT between crude oil and synthetic formation water is illustrated in Fig. 4. It is obvious that oil/water IFT decreases with the addition of polymers to water.
According to this figure, the effects of polymers on lowering IFT values are different depending on their viscosity and type and number of anionic groups. It is believed that the IFT values between crude oil and aqueous solutions are time dependent. This may be due in part to slow diffusion of some components across the interface and molecular rearrangement at the interface [25]. Easier mass transfer and higher adsorption of anionic groups at oil/water interface are influential in lowering IFT value [26]. In the presence of more viscous polymers, mass transfer rate drops at the interface, which leads to an increase in the time required to reach the minimum IFT. Furthermore, with increasing solution viscosity, the diffusion of polymer functional groups to interface decreases and consequently IFT needs a longer time to reach its lowest value [27].
On this basis, among the polymers of this study, HM25, which has anionic (carboxylic) groups in its molecular chains and low viscosity, acts as the best interfacial tension reducer and decreases the IFT value from 23.7 to 11.1 mN/m. The AN polymer, which has low viscosity similar to HM25 but its carboxylic functional groups are substituted by sulfonated groups, lowers IFT from 23.7 to 12.4 mN/m. Xanthan polymer, which has more anionic groups and higher viscosity than HM25, has lower efficiency than HM25 in lowering IFT and decreases IFT from 23.7 to 13.1 mN/m. Finally, the VM polymer has sulfonated groups and viscosity higher than AN, but with lower efficiency than the latter in lowering IFT from 23.7 to 16.3 mN/m.
The effect of different polymers on the zeta potential value (surface charge) of the carbonate rock particles treated with oil and polymer is shown in Table 2. One of the most important parameters of surface changes in a solid/liquid system is the electrical charge at solid/liquid interfaces. Zeta potential measures the surface charge at the interface of a solid/liquid system, where the mechanism of components interaction at interfaces can be explained. For a solid/electrolyte system, the zeta potential value strongly depends on the composition of the aqueous phase. Surface active groups of the aqueous phase play an important role in adsorption processes at the interface. In addition, it is believed that the adsorption of anionic groups on solid surfaces leads to lower zeta potential value and therefore change of the wettability of the rock surfaces [23, 26]. One can see from Table 2 that for carbonate particles aged in xanthan polymer solution, the zeta potential value is at the lowest level (it is more negative than other polymers and the zeta potential value decreases from −19.6 to −29.6 mV). This behavior is due to the fact that xanthan has two carboxylic groups on its repeating unit and adsorption of these anionic groups onto carbonate rock surface results in significant drop in zeta potential value. On the other hand, we can see from Table 2 that carbonate particles treated with the acrylamide-based polymers (HM25, AN, and VM) have similar zeta potential values. This behavior may be the result of the fact that all of these polymers have only one anionic group on their repeating units. According to zeta potential results, one may conclude that the adsorption of xanthan polymer is higher than those of three acrylamide-based polymers.
Polymer injection in porous media
Sand pack flooding
Based on previous evaluations, the VM polymer has relatively similar adsorption behavior onto carbonate rock, but higher viscosity than the other acrylamide-based polymers after thermal aging. The xanthan polymer, in spite of its higher viscosity after 1 week of thermal aging at 90 °C, has higher adsorption on carbonate rock compared to the VM polymer and its stability at higher temperatures (higher than 90 °C) is lower than that of sulfonated polyacrylamides [28]. Therefore, it seems that the VM polymer is a good candidate for flooding experiments in porous media.
The effects of water flooding and flooding using different concentrations of the VM polymer on oil recovery in sand pack are shown in Fig. 5. It can be seen that about 40 % of oil is recovered after 2 PV of water flooding. The injection of 0.6 PV of polymer solution followed by 2 PV of water flooding has a significant effect on oil recovery. The injection of solutions containing 900, 2,000, 3,500, 5,000, 7,000, and 9,000 ppm of the VM polymer into sand pack increases oil recovery by about 6, 11, 16, 55, 9, and 10 %, respectively. In general, adding polymer to water can decrease water mobility ratio; therefore, it can delay the breakthrough process during water flooding and thereby improve sweep efficiency and ultimate oil recovery.
According to Fig. 5, by increasing polymer concentration up to 3,500 ppm, incremental oil recovery initially increases and then drops. By increasing polymer concentration, the viscosity of solutions increases and therefore their effectiveness in sweeping residual oil rises. However, at higher polymer concentrations, a huge amount of high molecular weight VM polymer chains could not enter into small pores containing residual oil, and therefore these pores could not be swept by injected polymer solutions; thus, the percentage of incremental oil recovery decreases. From an economic point of view, oil companies cannot afford to apply concentrated solutions of polymer flooding to a real field project. Therefore, according to the sand pack experimental results, the VM polymer solution with a concentration of 3,500 ppm is herein selected for core flooding tests.
Core flooding
As mentioned in the previous section, a polymer solution containing 3,500 ppm of VM polymer is selected for core flood tests. At this stage, two core experiments are designed under more realistic conditions. The results of oil recovery by sequential water flooding, polymer flooding, followed by water flooding again are depicted in Fig. 6.
In the first experiment, the injection rate was 0.2 mL/min. After 2 PV water flooding, 50 % of oil was recovered. The injection of 1.2 PV of polymer solution containing 3,500 ppm of the VM polymer followed by 2 PV water flooding increased the recovery factor from 50 to 65 %. This result may be attributed to the role of polymers in lowering the mobility ratio of water and improving its sweep efficiency. In fact, a highly viscous displacing agent can cause delay in the breakthrough process. Therefore, the polymer solution has more time to sweep the residual oil remaining in the core. Increasing the injection rate from 0.2 to 3 mL/min decreased the recovery factor (Fig. 6). By raising the injection rate, breakthrough occurs sooner and the injected fluids have less time to sweep the pores; as a result, the amount of recovered oil decreases. These results revealed the importance of polymer flooding in oil recovery processes.
Conclusions
In the current study, the behavior of four different polymers for EOR application was investigated through evaluations such as interfacial tension, rheological, zeta potential, sand pack, and core flooding tests. All the polymer solutions displayed non-Newtonian behavior in almost the whole range of shear rates applied, which was followed by shear thinning. The aging of polymers in synthetic formation water made the screening effect of cations on the anionic groups of the polymer chains more evident, and consequently less chain expansion occurred which led to lower viscosity. The oil/water IFT decreased by the addition of polymers to water; the effects of polymers on lowering IFT were different depending on polymer viscosity and the type and number of anionic groups. The effect of xanthan polymer on zeta potential value was more significant than the effect of three acrylamide-based polymers. According to the polymer flooding results in the sand pack experiment, by increasing polymer concentration up to 3,500 ppm, the incremental oil recovery initially increased and then dropped. Using a polymer solution containing 3,500 ppm of the VM polymer in the core flooding experiments increased recovery factor from 50 to 65 %. By raising the injection rate from 0.2 to 3 mL/min, the injected fluid had less time to sweep the pores and consequently the amount of recovered oil was decreased. This work emphasizes the importance of polymer evaluations before using polymer flooding in oil recovery processes.
References
Wu Y, Shuler PJ, Blanco M, Tang Y, Goddard WA (2006) A study of wetting behavior and surfactant EOR in carbonates with model compounds. In: SPE 99612, SPE/DOE symposium on improved oil recovery, Tulsa
Donaldson EC, Chilingarian VG, Yen TF, Sharma MK (1989) Developments in petroleum sciences: enhanced oil recovery. Elsevier B.V, Amsterdam
Anupom S, Arun B, Inamul H (2003) Water soluble acrylamidomethyl propane sulfonate (AMPS) copolymer as an enhanced oil recovery chemical. Energy Fuels 17:683–688
Sorbie KS (1991) Polymer improved oil recovery. Blackie and Son, Glasgow
Needham RB, Doe PH (1987) Polymer flooding review. J Can Pet Technol 39:1503–1507
Van den Hoek PJ (2004) Impact of induced fractures on sweep and reservoir management in pattern floods. In: SPE 90968, SPE annual technical conference and exhibition, Houston
Shedid ASh (2006) Influences of fracture orientation on oil recovery by water and polymer flooding processes: an experimental approach. J Pet Sci Eng 50:285–292
Levitt DB, Pope GA (2008) Selection and screening of polymers for enhanced-oil recovery. In: SPE113845, SPE/DOE symposium on improved oil recovery, Tulsa
Moradi AA, Cleveland DH (1987) Development and evaluation of EOR polymers suitable for hostile environments: II-copolymers of acrylamide and sodium AMPS. In: SPE16273, SPE annual technical conference and exhibition, Houston
Taylor KC, Nasr-El-Din HA (1995) Water-soluble hydrophobically associating polymers for improved oil recovery: a literature review. In: SPE29008, SPE international symposium on oilfield chemistry, San Antonio
Lynch EJ, McWilliams DC (1969) Mobility control with partially hydrolyzed polyacrylamide a reply to Emil Burcik. J Pet Technol 21:1247–1248
Mezzomo RF, Luvizotto JM, Palagi CL (2001) Improved oil recovery in Carmopolis field: R&D and field implementations. SPE Reserv Eval Eng 4:4–10
Sandiford BB (1964) Laboratory and field studies of water floods using polymer solutions to increase oil recoveries. J Pet Technol 16:917–922
Jewett RL, Schurz GF (1970) Polymer flooding-a current appraisal. J Pet Technol 22:675–684
Wang GC, Caudle B (1970) Effects of polymer concentrations, slug size and permeability stratification in viscous water floods. In: SPE 2927, fall meeting of the society of petroleum engineers of AIME, Houston
Fulin Y, Demin W, Chunling K, Xinguang S, Weijie L, Gang W (2006) Study on high-concentration polymer flooding to further enhance oil recovery. In: SPE101202, SPE annual technical conference and exhibition, San Antonio
Wang D, Li Sh, Fan Ch, Li J, Wu H, Dusseault MB (2007) Flooding thin low-permeability layers with a new salt-resistant, medium-molecular-weight polymer. In: SPE109627, SPE annual technical conference and exhibition, Anaheim
Rashidi M, Blokhus AM, Skauge A (2010) Viscosity study of salt tolerant polymers. J Appl Polym Sci 117:1551–1557
Rashidi M, Blokhus AM, Skauge A (2010) Viscosity and retention of sulfonated polyacrylamide polymers at high temperature. J Appl Polym Sci 119:3623–3629
Danesh A (1998) PVT and phase behavior of petroleum reservoir fluids. Elsevier Science B.V, Amsterdam
Firoozabadi A (1999) Thermodynamics of hydrocarbon reservoirs. McGraw-Hill, New York
Elmofty SE, Shokir EM (2003) Applying electrophoresis technique to study adsorption of surface active agents on reservoir rocks. In: SPE85649, 27th annual SPE international conference and exhibition, Abuja
Jarrahian Kh, Seiedi O, Sheykhan M, Vafaie Sefti M, Ayatollahi Sh (2012) Wettability alteration of carbonate rocks by surfactants: a mechanistic study. Colloids Surf A 410:1–10
Strobl G (2007) The physics of polymers. Springer, Berlin
Buckley JS, Fan T (2005) Crude oil/brine interfacial tensions. In: Proceedings of international symposium of the society of core analysts, Toronto
Liu Q, Dong M, Yue X, Hou J (2008) Synergy of alkali and surfactant in emulsification of heavy oil in brine. Colloids Surf A 273:219–228
Daoshan L, Shouliang L, Liu Y, Demin W (2004) The effect of biosurfactant on interfacial tension and adsorption loss of surfactant in ASP flooding. Colloids Surf A 244:53–60
Stahl GA, Schulz DN (1988) Water-soluble polymers for petroleum recovery. Plenum Publishing Co, New York
Acknowledgments
The authors would like to express their appreciation for the financial support by the Research and Technology Directorate/National Iranian Oil Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aalaie, J., Jarrahian, K., Ghorashi, S. et al. The performance of polymer solutions in enhanced oil recovery: studies on their rheological, interfacial, and porous media behaviors. Iran Polym J 23, 827–834 (2014). https://doi.org/10.1007/s13726-014-0269-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-014-0269-0