Abstract
Anorectal malignant melanoma (ARMM) is an extremely rare tumor with aggressive biological behavior. It is associated with a very poor prognosis and a 5-year survival rate of less than 10 %. There have therefore been a few reports of patients demonstrating long-term survival. We herein report a valuable case of long-term survival of a patient with ARMM with wide extension into the rectal mucosa, without endoscopic abnormalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant melanoma of the mucous membranes can occur in the anorectal mucosa, oral mucosa, genital mucosa, nasal cavity, and esophagus. Anorectal malignant melanoma (ARMM) is an extremely rare tumor that accounts for 0.4–3.0 % of all malignant melanomas and only 0.1–4.6 % of all anorectal malignant tumors [1–4]. These tumors are often misdiagnosed because of their wide range of macroscopic and microscopic features. ARMM is prone to produce hematogenous and lymphatic metastases, even in early stages. Unfortunately, 70 % of ARMM patients present with invasion of surrounding lymph nodes, and metastasis to inguinal lymph nodes, lungs, liver, brain, and bone at the time of diagnosis, and thus have poor prognoses [5, 6]. The treatment of ARMM remains controversial as a result of its low incidence. We describe a valuable case of a patient with ARMM with wide extension into the lower rectum who experienced long-term survival after surgical treatment and adjuvant chemotherapy.
Case report
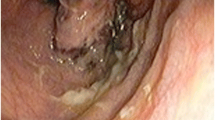
A 72-year-old woman presented at our hospital with fecal occult blood. Colonoscopy revealed a 15-mm submucosal tumor-like lesion in the right wall of the lower rectum and pigmentation of the perianal skin (Fig. 1a–c). We performed a biopsy of this lesion because there was a possibility of rectal cancer. The pathological diagnosis of the tumor was malignant melanoma. Although positron emission tomography–computed tomography scanning showed no infiltration, or nodal or visceral metastases, ARMM close to the dentate line is more likely to metastasize to the inguinal lymph nodes through the lymphatic channels. Therefore, the patient underwent the inguinal lymph node biopsy, which was negative for this tumor. The preoperative tumor stage was IB (cT1bN0M0) [7]. The patient underwent abdominoperineal resection. Microscopic examination resulted in a diagnosis of primary malignant melanoma of the anorectal region, classified as pT1N0M0 and as pathological stage I according to the Japanese classification of colorectal cancer. Hematoxylin–eosin staining of the anorectal specimen showed melanin pigment and a stratified squamous intraepithelial infiltration (Fig. 2a–c). Immunohistochemical analysis revealed that the tumor cells were positive for S-100 protein and HMB-45 and negative for CD45/leukocyte common antigen (Fig. 2d). Malignant melanocytic cells were scattered continuously in the rectal epithelium; there was no satellite lesion (Fig. 3). However, because of the possibility of residual malignant melanocytic cells, we administered adjuvant chemotherapy.
a Colonoscopy showed pigmentation between the anus and the surrounding mass. The tumor surface also showed pigmentation. b Colonoscopy showed tumor lesion of 15 mm on the right side wall of the anus away from the dentate line of the rectum. c Narrow-band imaging colonoscopy showed surface depression. Macroscopic findings were diagnosed as 0–IIa + IIc
The patient’s postoperative course was good. Adjuvant therapy consisted of five courses of dacarbazine (200 mg/day on days 1–5), nimustine (100 mg/day on day 1), vincristine (1 mg/day on day 1), and interferon-β (30,000 units/day on days 1–9) at 3-month intervals. Postoperative adjuvant immunotherapy with interferon-β (3,000,000 units/day on day 1) was administered every month (Fig. 4).
Follow-up endoscopy was performed every year after completion of chemotherapy from May 2010. The patient remained recurrence-free at 5-year follow-up.
Discussion
ARMM normally originates in the transition zone above the dentate line, and is frequently mistaken for benign lesions such as hemorrhoids or rectal polyps. The prognosis is extremely poor, with a 5-year survival rate of less than 10 %, because of both the aggressive biological behavior of the tumor and the usually advanced stage at the time of diagnosis [8, 9]. The typical presentation of ARMM is rectal bleeding and anal pain [10]. Patients may also complain of pruritus, tenesmus, a change in bowel habit, or diarrhea [11]. ARMM is pigmented in the majority of cases, but the lesion appears to be amelanotic in 16–53 % of cases [12]. The diagnosis of ARMM is only established by histopathologic examination. Immunohistochemical staining may be a useful tool in the event of difficulty in establishing a diagnosis, particularly in amelanotic cases with unusual morphologic features [13]. Anti-S-100 protein is the most common immunohistochemical target used in the diagnosis of malignant melanoma, and is highly sensitive for melanocytic differentiation. However, given its lack of specificity, it is used primarily as a screening tool, and HMB-45 and antibodies to Melan-A are the most common melanocyte-specific stains used to diagnose malignant melanoma.
Treatment of ARMM is based essentially on the spread of the disease. Breslow’s tumor thickness, which is the distance from the surface of the epithelium to the point of deepest penetration of the tumor, has also been used as a staging tool [14]. Furthermore, the intraepithelial extension of tumor cells and lymph node metastasis are important prognostic factors in ARMM patients. The territory of the inferior mesenteric artery is an important lymphatic channel for anorectal tumors. The closer a lesion is to the dentate line, the more likely it is to metastasize to the inguinal and obturator lymph nodes through the lymphatic channels to the side [15]. In the present case, we examined the inguinal lymph nodes by preoperative biopsy, and identified the sentinel node by patent blue dye injection and radioisotope examination (99 m-Tc) during surgery. However, examination of the surgically resected specimen unexpectedly revealed malignant and/or atypical melanocytic cells scattered widely in the epithelium of the upper rectum, despite endoscopically non-pigmented rectal mucosa. Surgery remains the cornerstone of treatment for localized ARMM, though controversy still exists over the optimal procedure. Abdominoperineal resection and wide local excision are both options, though the prognosis of ARMM remains very poor, regardless of the surgical approach used. Even in patients with local or locoregional disease, for whom resection is potentially curative, the 5-year survival is only 5–20 %, compared with a 5-year survival of 80 % for cutaneous melanomas [13]. Several chemotherapeutic regimens have been recommended, including interferon-α and cytotoxic chemotherapeutic agents such as cisplatin or dacarbazine, but none has yet shown a survival benefit in patients with melanomas [6, 16]. Further prospective studies are needed to evaluate the efficacy of adjuvant treatment based on chemotherapy or radiotherapy.
At present, there is not enough evidence to make conclusions regarding the usefulness of chemotherapy in patients with ARMM. In this case of ARMM, the patient underwent surgical treatment at an early stage and continued adjuvant chemotherapy successfully. This is an ideal way to achieve long-term survival. To identify the optimal regimen of adjuvant therapy against ARMM, a prospective study should be conducted. Our case of ARMM with wide extension into the lower rectum is extremely valuable because the patient experienced long-term survival after surgical treatment and adjuvant chemotherapy despite the 5-year survival rate of less than 10 % typical of the disease.
References
Thibault C, Sagar P, Nivatvongs S, Ilstrup DM, Wolff BG (1997) Anorectal melanoma: an incurable disease? Dis Colon Rectum 40(6):661–668
Chang AE, Karnell LH, Menck HR (1998) The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 83(8):1664–1678
Heyn J, Placzek M, Ozimek A, Baumgaertner AK, Siebeck M, Volkenandt M (2007) Malignant melanoma of the anal region. Clin Exp Dermatol 32(5):603–607
Klas JV, Rothenberger DA, Wong WD, Madoff RD (1999) Malignant tumors of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer 85(8):1686–1693
Mills SE, Cooper PH (1983) Malignant melanoma of the digestive system. Pathol Annu 18:1–26
Pessaux P, Pocard M, Elias D et al (2004) Surgical management of primary anorectal melanoma. Br J Surg 91:1183–1187
Colon cancer treatment guidelines 2014 of Japanese Society for Cancer of the Colon and Rectum
Row D, Weiser MR (2009) Anorectal melanoma. Clin Colon Ractal Surg 22(2):120–126
Iddings DM, Fleisig AJ, Chen SL, Faries MB, Morton DL (2010) Practice patterns and outcomes for anorectal melanoma in the USA, reviewing three decades of treatment: is more extensive surgical resection beneficial in all patient? Ann Surg Oncol 17:40–44
Morson BC, Volkstadt H (1963) Malignant melanoma of the anal canal. J Clin Pathol 16(2):126–132
Slingluff C, Vollmer R, Seigler H (1990) Anorectal melanoma: clinical characteristics and results of surgical management in twenty-four patients. Surgery 107:1–9
Lagha A, Ayadi M, Krimi S, Chraiet N, Allani B et al (2012) Primary anorectal melanoma: a case report with extended follow-up. Am J Case Rep 13:254–257
Chute DJ, Cousar JB, Mills SE (2006) Anorectal malignant melanoma morphologic and immunohistochemical features. Am J Clin Pathol 126:93–100
Breslow A (1970) Thickness, cross sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg 172:902–908
Kimihiko F (2009) Current status of sentinel lymph node-based nodal ultrastaging in colorectal cancer. Jpn Surg Soc 110(2):73–77
Bullard KM, Tuttle TM, Rothenberger DA, Madoff RD, Baxter NN, Finne CO et al (2003) Surgical therapy for anorectal melanoma. J Am Coll Surg 196(2):206–211
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Umezaki, N., Ishimoto, T., Koba, I. et al. Anorectal malignant melanoma with extensive intraepithelial extension: report of a case. Int Canc Conf J 4, 245–248 (2015). https://doi.org/10.1007/s13691-015-0211-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13691-015-0211-0