Abstract
Purpose of Review
This narrative review appraises research data on the potentially harmful effect of obesity and insulin resistance (IR) co-existence with type 1 diabetes mellitus (T1DM)-related cardiovascular (CVD) complications and evaluates possible therapeutic options.
Recent Findings
Obesity and IR have increasingly been emerging in patients with T1DM. Genetic, epigenetic factors, and subcutaneous insulin administration are implicated in the pathogenesis of this coexistence. Accumulating evidence implies that the concomitant presence of obesity and IR is an independent predictor of worse CVD outcomes.
Summary
The prevalence of obesity and IR has increased in patients with T1DM. This increase can be partly attributed to general population trends but, additionally, to iatrogenic weight gain caused by insulin treatment. This association might be the missing link explaining the excess CVD burden observed in patients with T1DM despite optimal glycemic control. Data on newer agents for type 2 diabetes mellitus (T2DM) treatment are unraveling novel ways to challenge this aggravating coexistence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction–T1DM
It has been almost 100 years since Leonard Thompson got treated with the first insulin injection and consequently avoided death for nearly a year. Since that era, the treatment of type 1 diabetes (T1DM) has been revolutionized, dramatically increasing the life expectancy of patients, which, back in 1923, used to be close to 1 year after presenting symptoms emerged [1]. Despite the improvement, however, the mortality of people with T1DM remains higher than in the average population, mainly due to a twofold increase in cardiovascular mortality [2]. Furthermore, while the incidence of microvascular complications such as nephropathy has been reduced after the implementation of intensive insulin treatment [3], less impressive progress has been made regarding cardiovascular complications, implying that apart from hyperglycemia, other mechanisms might participate as well [4].
Obesity is a well-recognized risk factor for cardiovascular disease. The incidence of obesity worldwide has reached outstanding proportions in past years, giving rise to the term “obesity pandemic,” with the western way of sedentary living and hypercaloric dieting being the main culprits [5].
Stereotypically, patients with T1DM are thought to be lean young persons, yet this traditional image is being challenged nowadays by the increasing coexistence of both overweight and obesity in more than 20% of individuals with the disease [6, 7]. Several valid questions arise from this observation: what are the causes of such a phenotype change? Is it predominantly driven by environmental factors, or is it mostly an iatrogenic side effect resulting from the intensive treatment with insulin? Is overweight/obesity a culprit in the excess cardiovascular incidence of people with T1DM? And finally, what therapeutic options can we use to tackle this situation?
Therefore, the aim of the review was to appraise research data on the potentially harmful effect of obesity and insulin resistance (IR) co-existence T1DM related cardiovascular disease (CVD) complications and evaluate possible therapeutic options.
T1DM and Cardiometabolic Complications
Several cohort studies have identified that people with T1DM have a shorter life expectancy than their age-matched general population. Specifically, an extensive Scottish cohort study [8] of 24,691 patients showed a reduction in life expectancy by 13 and 11 years for women and men, respectively. Another sizeable Australian cohort study [9] found similar results (12.2 years of life expectancy loss). CVD is the main reason for this excess mortality in T1DM [10], especially in individuals past middle age [8, 9], with ischemic heart disease being the most common complication. Women with T1DM appear to have a similar incidence of CVD with men, even in the premenopausal period, indicating that the disease eliminates the hormonal cardiovascular protection of the female gender. Other factors, such as the age of diabetes onset [11], duration of diabetes, and patients’ age, were also independent predictors of incident CVD.
A well-established treatable causative factor of CVD in T1DM is hyperglycemia. Results from the DCCT (Diabetes Control and Complications Trial) randomized control trial (RCT) indicated that after 6.5 years in the trial, patients in the intensive insulin treatment group had an HbA1c approximately 2% lower than in the conventional arm. This effect on glycemia resulted in a lower incidence of microvascular complications [12]. At the end of the study, there was also a numerical trend in reducing CVD outcomes. Still, due to the small number of events, it failed to reach statistical significance [13]. The population of DCCT continued participating in a follow-up observational study, the Epidemiology of Diabetes Interventions and Complications study (EDIC). After 17 years of observation, participants in the DCCT intensive treatment arm had a 42% reduction in CVD incidence [14]. Surprisingly, this improvement occurred despite the equalization of HbA1c values between the two trial arms during EDIC. Protection from CVD was attributed to a carry-over effect of prior improved glycemic control during DCCT, giving birth to the term “metabolic memory.”
The correlation between glycemic control and CVD was reproduced in some observational studies, such as the Finnish Diabetic Nephropathy (FinnDiane) Study [15] and the Swedish registry study [2]. Yet, no significant interaction was reported in other studies [16, 17••], the longest of which is the Pittsburgh Epidemiology of Diabetes Complications Study (EDC). EDC is a large prospective observational study that enrolled patients with T1DM, diagnosed between 1950 and 1980. An EDC earlier analysis of CVD predictors found an independent association of CVD with albumin excretion rate (AER) but was not able to demonstrate a protective effect of better glycemic control. This disparity with the DCCT study may be explained when the differences between studies are closely examined.
As a real-life study, EDC included participants with more CVD-related risk factors (i.e., hypertension, dyslipidemia) and longer diabetes duration at baseline. Such patients were excluded from the DCCT. On the other hand, EDC was an observational study with the disadvantage of having a higher probability of bias than an RCT, such as DCCT. Finally, HbA1, a less accurate and standardized glycemia marker and not HbA1c, was used in EDC. Despite previous differences, a more recent EDC analysis using HbA1c, derived from HbA1 using a regression equation, revealed a significant correlation between hyperglycemia and CVD incidence but highlighted the importance of albumin excretion rate (AER) as the most influential independent predictor [18]. While the role of hyperglycemia in CVD is quite well recognized, its influence cannot account for the total incidence of CVD in people with T1DM [19]. Other traditional risk factors, such as dyslipidemia, hypertension, smoking, and–perhaps most importantly–nephropathy, are also implicated. Nephropathy closely follows hyperglycemia as the second stronger predictor of CVD in T1DM [19]. It may be further subclassified as microalbuminuria, macroalbuminuria and overt glomerular filtration rate (GFR) reduction. These specific facets of diabetic nephropathy can occur either as consecutive events or independently of each other. Thankfully, the results from epidemiological studies show that post DCCT, there is a marked decline in the incidence of nephropathy worldwide, mainly attributed to the application of intensive glycemic treatment in most patients with T1DM [20].
Dyslipidemia is also a classical risk factor for CVD, with low-density lipoprotein cholesterol (LDL-C) levels being the most important biomarker for its diagnosis and treatment. Unfortunately, data on dyslipidemia diagnosis and management mainly come from trials in T2DM and the general population. Therefore, their results may not be generalized in patients with T1DM. Indeed, it would appear that LDL-C influence in CVD incidence is less prominent in T1DM than in T2DM [21, 22]. Deficient and very high HDL-C levels, especially in women with T1DM, also seem to increase coronary artery disease (CAD) [23]. Hypertension is also tightly linked with T1DM, having a higher prevalence than in the age-matched general population [24]. Alarmingly, 5.9% of young children and adolescents with the disease have hypertension, according to the SEARCH trial results [25]. Hypertension can be a cause and, more frequently, a consequence of nephropathy, and as shown in the results of EURODIAB, it is positively correlated with disease duration [26]. Smoking has repeatedly been shown to correlate positively with CVD outcomes in the T1DM population and remains the first preventable cause of death [27].
The shared pattern that emerges from most study reports is that the influence of conventional risk factors on CVD incidence is becoming more critical with increasing age and longer disease duration. Therefore, diagnostic and therapeutic vigilance on hypertension and dyslipidemia management should be amplified as the patient with type 1 diabetes ages. Unfortunately, data from a recent study of the Swedish National Diabetes Register show that after adjustment of for the previously mentioned factors, individuals with T1DM still have twice the incidence of myocardial infarction and heart failure hospitalization than age and sex-matched individuals from the general population [28••]. This finding highlights the fact that despite impressive improvements in the management of CV risk factors, there are still unidentified pathways leading to an unacceptably high incidence of CVD in patients with T1DM.
Diabetes as a Continuous Spectrum
The Many Faces of Diabetes
Classification of diseases has been helpful for physicians who are required to make decisions regarding the diagnosis and treatment of a specific disease with certainty. In diabetes, the definition of opposite poles, with one being the insulin-deficient type 1 patient and the other the insulin-resistant type 2, paves quite a straightforward and physician-reassuring path regarding critical therapeutic choices, such as the initiation of insulin treatment. Alas, despite best efforts to give clear-cut definitions for diabetes, some cases may not be adequately categorized, casting doubt and much uncomfortable uncertainty regarding clinical decision-making.
Intermediate Cases of Diabetes
Nowadays, T1DM is usually diagnosed in lean individuals of young age, based on the presence of specific islet auto-antibodies and absence of residual insulin secretion, as measured by C-peptide levels. Several notable exceptions exist in the above definition, as well as in its individual parameters. A typical example refers to individuals, usually of African or Asian origin, who present with insulin deficiency and ketosis but are antibody-negative, and are usually termed as idiopathic or T1B diabetes [29]. Further adding to the confusion, soon after T1DM diagnosis, the requirement for exogenous insulin waxes and wanes for a particular–usually short–period of time. This phenomenon is termed as the “honeymoon period” and is actually a partial remission of the disease. Its pathophysiology is not entirely understood, possibly indicating an interplay between reduced insulin requirements and β cell preservation and implying that other factors apart from autoimmunity may be implicated in the pathogenesis of the disease. Another indicator of the interconnection between insulin requirements and residual insulin secretion in patients with T1DM, irrespectively of autoimmune damage, is that children with T1DM, overweight and obesity and insulin resistance (IR) exhibit higher C-peptide levels than those of age-matched normal and underweight individuals [30]. In the SEARCH for Diabetes in Youth study, obesity correlated with earlier age of T1DM diagnosis in children with reduced C-peptide levels [31]. Similarly, an association of weight and earlier age of diagnosis of T1DM was shown in a study of 9248 children from Germany and Austria [32•].
Intermediate cases can also be considered in patients diagnosed with T2DM (T2D) since, in some of them, near-complete pancreatic exhaustion eventually develops, while others are prone to the development of ketosis at or soon after diagnosis [33]. Another gray-zone category is that of latent autoimmune diabetes in adults (LADA). In patients with LADA, the disease presents in antibody-positive adults without ketosis, with sparing of insulin secretion for a longer time than in patients with T1DM, having, therefore, a milder but highly variable clinical course [34].
Based on the above examples mentioned, some authors support that the increased insulin demand in the setting of IR associated with the rise of childhood obesity causes β-cell exhaustion, which, coupled with autoimmunity, results in the earlier development of T1DM [29,30,31, 35]. This notion is known as the “accelerator hypothesis” and may explain why children with T1DM have become progressively heavier at diagnosis over the past 20 years and also why the heavier children develop T1DM at an earlier age [35]. This hypothesis may also explain the observed global rise in T1DM incidence during the last decades [36].
Double Diabetes
Metabolic syndrome is the combined clinical term encompassing dysglycemia, dyslipidemia, central obesity, hypertension, and, recently, non-alcoholic fatty liver disease [37]. The critical component in the metabolic syndrome pathogenesis is IR, a T2DM hallmark. However, it is less well recognized that, according to several recent studies, many patients with T1DM nowadays also meet the metabolic syndrome’s criteria [38]. This combination of T2DM features in individuals with T1DM gave rise to the concept of “double diabetes” (DD).
As observed almost 30 years ago, individuals with DD usually have a family history of T2D and are more likely to be overweight [39]. The DD phenotype may even be found in children as young as five years of age [40]. The actual percentage of people with T1DM who may be classified as DD varies among studies, depending–among others–on the metabolic syndrome definition. In one of the initial reports describing DD, approximately 16% in a cohort of 448 T1DM patients had a history of T2D in their families [39]. These individuals had higher HbA1c values, increased body weight, and received greater insulin doses than those without a family history of T2D. Similar associations were found in the more recent Finnish Diabetic Nephropathy Study [41]. The highest level of evidence comes from the DCCT study [42]. Participants with a family history of T2D in the study’s intensive treatment arm exhibited a higher degree of central obesity, increased triglyceride values, and total daily insulin dose.
Several studies have examined the influence of DD on microvascular and macrovascular complications. Data from a retrospective analysis of the EDC found that positive family history of T2DM was a predictor of almost two times higher incidence of CVD, specifically coronary artery disease (CAD) (HR 1.89, 95% CI 1.27, 2.84). Yet, the effect was diminished after adjustment for cofactors [43]. Regarding microvascular complications, the prospective EURODIAB study found a correlation between a positive family history of T2DM and a higher percentage of albuminuria only in women.
The exact definition of DD remains challenging because while a family history of T2DM is easy to record, the precise selection of defining metabolic biomarker(s) is hard to establish. Despite being a widely used term, metabolic syndrome has at least four different definitions [44]. The WHO definition has been proven as the most useful predictor of CVD [45] in 3783 people with T1DM enrolled in the prospective Finnish Diabetic Nephropathy (FinnDiane) Study [46]. Its description includes any distortion in glucose metabolism (diabetes or prediabetes defined as fasting glucose ≥ 110 mg/dl) plus any 2 of the following: hypertension, dyslipidemia (increased triglycerides or low HDL-C values), central obesity, and microalbuminuria (i.e., albumin/creatine ratio ≥ 30 μg/mg). This definition carries a problematic clinical relevance since a patient having only one of these parameters would be considered as “non-metabolic,” and thus of low risk. Such a classification is another example of dichotomous thinking, not considering individual differences such as the stage of hypertension or the level of dyslipidemia. Due to the confusion created by the several definitions and the low incorporation in everyday practice, the term metabolic syndrome may not be optimal for defining T2D features in patients with type 1 diabetes.
The Role of Insulin Resistance
The pathophysiologic basis tying the individual components of the metabolic syndrome is IR, which is also a critical metabolic disorder in T2DM. IR is a measurable quality, and the gold standard measurement method is the euglycemic-hyperinsulinemic clamp protocol [47]. While the protocol is highly accurate in defining IR, it is also time-consuming and laborious. It requires trained personnel for execution, making it not practical for clinical work and is primarily reserved for research. An accurate and easy-to-use marker of IR for patients with T1DM was created from an analysis of the EDC data, based on clamp results from 24 patients enrolled in the study, the so-called estimated glucose disposal rate (eGDR) [48]. The computation of eGDR is done through the following equation of easily collected clinical markers: 24.31 − (12.22 × waist to hip ratio) − (3.29 × hypertension) − (0.57 × HbA1C); units are mg/kg/min, hypertension taking the value of 0 or 1 on absence and presence, respectively. Lower eGDR values indicate higher IR. Indeed, IR measured through the eGDR was a central predictor of CVD outcomes, specifically CAD and peripheral vascular disease, in a retrospective analysis of EDC data [17••]. Another independent retrospective analysis of the DCCT cohort showed that patients with higher eGDR (calculated using BMI instead of the waist to hip ratio) at baseline had a lower risk chance of developing retinopathy (HR: 0.75, 95% CI 0.69–0.81), kidney disease (HR: 0.88, 95% CI 0.80–0.96) and CVD (HR: 0.70, 95% CI 0.56–0.88) [49••]. Interestingly, in the same analysis, the metabolic syndrome (IDF definition) [50] did not show any prognostic significance regarding CVD. Finally, individual components of IR, such as the waist to hip ratio (WHR) and triglycerides, were predictive of microvascular events in the prospective analysis of the EURODIAB study [51].
Collectively, it appears that eGDR serves as a better surrogate of IR than metabolic syndrome and can be used to define the group of patients with DD.
Type 1 Diabetes, Obesity, and IR
Physiology of Energy Balance in T1DM
Energy balance is defined as the combined result of energy influx and efflux created through physiologic consumption and metabolism of nutrients. This balance is tightly regulated and kept constant under homeostatic conditions to maintain bodyweight. The resting energy expenditure primarily drives energy balance; the term refers to the energy used by the organism to maintain vital functions during sleep and bed rest. Resting energy expenditure comprises as much as 70% of the total daily energy expenditure. The remaining 30% includes energy spent on activities (exercise, work, etc.) plus the thermal energy produced by food consumption [52].
Due to the lack of endogenous insulin production, coupled with exogenous insulin treatment, energy balance is quite distinct in people with T1DM. In the absence of the anabolic stimulus of insulin, people with T1DM before initiating insulin are in a catabolic state resulting in weight loss and wasting. One of the critical factors leading to weight loss before insulin treatment initiation is glycosuria. It has been calculated that loss of glucose in the urine can amount to 300 to 400 kcal per day [53]. On the other hand, insulin deficiency removes insulin’s postprandial inhibitory effect on glucagon production. Hyperglucagonemia, in turn, leads to augmented hepatic glucose production and, as a consequence, to increased protein turnover to support gluconeogenesis [54, 55]. In a supervised experiment of 10 patients with T1DM, uncontrolled hyperglycemia resulted in increased energy demands. Following intravenous insulin commencement, these effects normalized [56].
Under physiological conditions, insulin is secreted by the beta cells, in direct proportion to food intake (mainly carbohydrates) and the amount of body fat [57, 58]. Secreted insulin results, primarily through actions in the central nervous system, in higher levels of satiety, with the goal of body weight maintenance. Exogenous insulin bypasses this homeostatic mechanism, as it is usually injected in doses often miscalculated, based only on carbohydrate counting and irrespective of the body’s fatty tissue. Simultaneously, insulin-induced hypoglycemia, a frequent adverse effect of exogenous insulin therapy, negates the increased satiety signal from high insulin doses and may lead to increased food consumption [58]. Glucagon also reduces appetite in healthy individuals [59]. This response appears to be maintained in patients with T1DM, although it is impaired in people with obesity [60]. The exact overall effect of glucagon on hunger levels in people with obesity and T1DM remains unknown.
Epidemiology of Obesity in Type 1 Diabetes
Data regarding the prevalence of obesity at baseline in both the DCCT and EDC studies truly justify the historical description of the exclusively lean patient with T1DM. Specifically, participants with obesity accounted for 1% and 3.4% of the respective cohorts at recruitment. However, obesity prevalence after 12 years of EDIC follow-up increased to 31% in the previously intensively treated group of the DCCT. Similarly, a sevenfold increase (22.7%) was observed after 18 years in the EDC [61, 62•]. In both studies, obesity rate changes were of greater magnitude than the corresponding increase in the general population [61, 62•]. The SEARCH for Diabetes in Youth (SEARCH) study noted a significant increase in overweight, but not obesity prevalence in young individuals with T1DM compared to healthy participants of the National Health and Nutrition Examination Survey (NHANES). Registry data from European (Germany, Austria) and American children with at least 1 year of T1DM duration showed that 24% and 12%, respectively, met the WHO criteria for either overweight or obesity. Importantly, these prevalence figures were higher than the corresponding national and international reference values [63]. Taken together, epidemiological findings suggest that the global trend towards higher obesity rates increases similarly or even at a faster rate in patients with T1DM than in the general population [64].
Pathophysiology of Obesity in Type 1 Diabetes
The Role of Genetics
There are several indications that the genetic background of a patient with T1DM plays a role in the subsequent development of obesity (Fig. 1). Specifically, during the DCCT, intensively treated patients with a family history of T2DM had more pronounced central weight gain, as well as several other obesity-related risk factors at the end of the trial [42]. Intriguingly, there was no association between family history of T2DM and obesity in the control group of the DCCT [42]. Another cross-sectional study that examined the anthropometric parameters of both young patients with T1DM (n = 234) and their parents found a robust correlation between youth and parental BMI, raising the possibility of genetically influenced obesity [65].
For many years, the influence of genes in the development of obesity has been an area of extensive research. More than 100 obesity-related genes have been discovered, yet their overall effect on BMI appears not to be very strong [66]. Some of the best recognized genes implicated in the pathophysiology of obesity are the fat mass and obesity-associated (FTO) gene and the melanocortin 4 receptor (MCR) genes [67]. These genes mainly influence weight by modifying hunger/satiety levels in the central nervous system. Genetic analysis of 1119 children of Polish origin with T1DM revealed an association of their BMI with the A allele of the FTO rs9939609 gene only, while no other of the studied genes showed any such association [68]. The same FTO polymorphism was associated with higher BMI (but not with nephropathy) in a study evaluating 1,110 patients for the genetic linkage of obesity and kidney disease in type1 diabetes [69]. A more recent study by the same Polish group further examined the combined influence of FTO and clinical factors (disease duration, metabolic control, sex) in obesity prevalence in children with and without T1DM. An independent association between obesity and clinical characteristics was shown, but not with FTO polymorphism [70].
A single nucleotide polymorphism (SNP) in the genetic locus of the transcription factor 7 like 2 (TCF7L2) has been found to be strongly correlated with T2DM [71]. A recent study by Redondo et al. evaluated the effect of this SNP on adolescents (n = 810) with newly diagnosed Τ1DM. This analysis showed that this SNP was correlated, in children older than 12 years of age, with the presence of a single rather than multiple autoantibodies, a higher level of C-peptide, and lower mean glucose [72]. Hence, describing a milder clinical phenotype of T1DM, the presence of this SNP may potentially result in a T2DM-associated genetic influence.
A common problem associated with genetic studies, which are essentially observational studies, most often of cross-sectional design, is that causality between a SNP and a disease or a characteristic cannot be reliably established. Given the myriad of factors influencing the final phenotype, a method that could diminish the confounding effect is needed. The Mendelian randomization method was developed to address this need [73]. Briefly, the Mendelian randomization method builds on the fact that genotype is inherited randomly from the parents. The random distribution of genes with a well-known function (phenotype) is examined in populations of an observational study to determine causality with a potentially changeable exposure on the outcome of interest. In turn, this randomness reduces the confounder effect, creating an RCT analogous to a genetic study [74]. A Mendelian randomization study examined the presence of 23 SNPs known to be correlated with childhood obesity in 5913 cases of children with T1DM and demonstrated a positive relationship between obesity and the risk for T1DM [75]. Specifically, an increase of 1 standard deviation of BMI was associated with a 32% increase in the risk of developing T1DM. However, a substantial limitation was the absence of a known mechanism related to gene function, and their corresponding influence on the incidence of obesity.
The Role of Epigenetics
Environmental factors, such as diet, stress levels, and exercise habits, interact with genes and influence gene expression without actually changing the sequence of the DNA. This elaborate modification formed by the gene-environment contact is called epigenetics [76]. Several observations point to the importance of epigenetic modification in the pathogenesis of T1DM. Monozygotic twin studies show that the probability of both siblings being affected by T1DM is approximately 50% [77]. This discordance (only one twin being affected) implies that apart from purely genetic factors, the environment also influences disease development. The three primary mechanisms through which the environment exerts epigenetic changes are DNA methylation, histone level changes, and specific microRNAs (miRNA) [78].
DNA methylation has traditionally been linked with inhibition of gene expression. Excessive DNA methylation of lipid and inflammatory loci has been described as a consequence of obesity, and such patterns were able to predict the future development of T2DM [79]. Further evidence of the cause and effect relationship between obesity with DNA methylation was provided by the restoration of the methylation pattern following gastric bypass surgery [80] and lighter methylation patterns found in successful weight-loss maintainers compared to individuals with obesity [81].
Changes at the histone level reveal several causal patterns of association between epigenetic modification and T1DM. An increase in histone H3 lysine 9 was found in the lymphocytes of patients with type1 diabetes compared with controls [82]. In vitro and in vivo experiments showed evidence that upregulation of nuclear factor-κB (NFκB)-p65 gene, a gene known to be upregulated by hyperglycemia, was mediated at the histone level through increased methylation of H3K4 combined with reduced methylation of H3K9 after exposure to increased glucose concentrations [83]. Finally, in an epigenetic analysis of the DCCT/EDIC study, patients in the intensive treatment arm free of complications were compared to patients in the conventional treatment group who developed nephropathy with or without retinopathy after 10 years of follow-up. The analysis revealed considerable histone enrichment of H3K9Ac in the monocytes of the conventional group [84]. This epigenetic effect was associated with higher HbA1c values and genetic loci connected to the NFκB inflammatory pathway [84]. These epigenetic patterns persisted in a consequent analysis after 17 years of follow-up in EDIC, providing evidence for the epigenetic formation of metabolic memory [85].
MiRNAs are small non-coding RNA molecules that exert influence through posttranscriptional modification, primarily by inhibition of gene expression [86]. The relationship of miRNAs with obesity and T2DM has been described in several studies [87]. One of the largest, the Framingham Heart Study (FHS) Offspring, with data from 2317 people, showed an association of 16 miRNAs with traditional metabolic risk factors such as BMI, visceral obesity, and IR [88]. Specific examples of increased levels of miRNA have also been shown in patients with T1DM. Hyperexpression of miR-326 was found in lymphocytes of people with T1DM and correlated with an increased concomitant presence of glutamic acid decarboxylase antibodies (GADA) and insulinoma-associated (IA-2) autoantibody (IA-2A) [89]. Evidence regarding the relationship between ongoing autoimmunity and miRNA was also shown in another study by Yang et al., where under-expression of miR-146a in patients with T1DM was connected to higher autoantibody titers [90]. A systematic review and bioinformatic analysis revealed 11 miRNAs consistently elevated in patients with T1DM compared to controls [91], which were associated with transcriptional pathways of apoptosis, immune response, and insulin production, implying their potential use as disease biomarkers.
The Role of Subcutaneous Insulin Administration and Intensive Glycemic Control
Insulin has a well-documented anabolic action [92]. Physiologically increased insulin concentration lead to increased lipid synthesis, reduced protein breakdown, and a reduction in metabolic rate, leading to a net effect of weight gain. On the other end of the spectrum, an insulin deficiency state, such as uncontrolled T1DM, leads to muscle and fat tissue wasting to compensate with alternative metabolic fuels for the decreased glucose utilization in cells [93]. Furthermore, the loss of the natural insulin anabolic signal and the increased energy expenditure/fluid loss caused by glycosuria also contribute to the overall weight loss. Therefore, it is reasonable to expect that restoring insulin signals in patients with T1DM will result in weight gain (re-gain) due to the catabolic state's reversion. Yet, peripheral subcutaneous insulin administration does not mimic the natural route of insulin secretion, bypassing its first pass through the liver, which, on average, removes ≈50% of the secreted insulin [94]. First pass loss leads to the exposure of peripheral tissues to supraphysiologic insulin concentrations, potentially increasing anabolic signals and promoting weight gain [95].
Indeed, it would appear that one of the foremost determinants of the increase in overweight/obesity percentages found in patients with T1DM in both the EDC and the DCCT/EDIC was the consolidation of intensive insulin treatment as the optimal therapeutic approach in the management of hyperglycemia. The higher mean weight gain of 4.75 kg in the intensively treated group in DCCT provides proof of concept-evidence that higher insulin doses eventually lead to more significant weight gain [96]. Intensive insulin treatment in the DCCT was associated with a 73% increase in the risk of becoming overweight, and 25% of the intensively treated patients had BMI > 30 kg/m2 at the end of the study [97]. The effect of intensive insulin treatment on weight gain was pronounced despite proper dietician consulting [98]. The EDC study provided further such evidence, at baseline only 7% of the cohort was using multiple insulin injections (≥ 3 insulin injections per day) or an insulin pump; this percentage increased to 82% by 2004–2007 with a concomitant sevenfold augmentation in obesity prevalence [62•]. The most significant increase in weight in both the EDC and the DCCT was observed in participants with higher HbA1c baseline values [62•, 99]. This finding might seem to support the hypothesis that in participants with uncontrolled diabetes, weight gain may be (at least partly) a weight-regain. However, if that were the case, one would expect the BMI of participants with the highest baseline HbA1c to be lower than that of the rest of the cohort. Nonetheless, baseline BMIs were actually similar between the different quadrants of future weight gainers, implicating that weight gain occurring in the high baseline HbA1c population was not simply a re-gain phenomenon. Another intriguing observation was that the quadrant of participants with the highest weight gain had worse glycemic control at baseline than the rest of the study patients, despite the similar total daily insulin dose, implying a higher baseline IR in this group [99].
The Role of Hypoglycemia
A well-recognized frequent side effect of insulin treatment in patients with T1DM is hypoglycemia [100]. Results from the DCCT showed a threefold increase in severe hypoglycemia in the intensively versus the conventional treatment group [97]. It has been estimated that patients treated with intensive insulin therapy in the “real world” spend about 10% of their time at plasma glucose levels less than 60 mg/dl [101]. One of the first symptoms of hypoglycemia is increased hunger, and patients often consume more than the proposed 15 g of carbohydrate to satiate [102]. Given the higher frequency of hypoglycemia in intensively treated patients and the resulting increased carbohydrate consumption, gaining weight seems like a reasonable consequence. A correlation between the frequency of hypoglycemia and weight gain was again evident in the DCCT during the 1st year of follow-up for both mild (r = 0.21, P < 0.05) and severe episodes (r = 0.18, P < 0.05) [103]. A more recent analysis found no evidence of severe hypoglycemia-induced weight gain after the completion of DCCT. Even the combined severe hypoglycemic episodes of the two study arms accounted for only 2% of participants' weight gain during the whole study [42]. Yet, milder hypoglycemia, which might contribute substantially to increased food intake, was not examined. The exact threshold of mild hypoglycemia has been debated and revised from scientific communities over the years [104,105,106]. The biggest issue with a clear-cut definition is that non-severe symptomatic hypoglycemia occurs at different thresholds between patients, depending on several factors such as current glycemic control, the presence of autonomic neuropathy, concomitant medication use, etc. Without being able to quantify the effect of mild hypoglycemia on extra food consumption, no definitive conclusion on the relationship between hypoglycemia and weight gain can be drawn. Hopefully, the recent consensus [107] on hypoglycemia from both the European and American diabetes associations will help to better define and study hypoglycemia-related endpoints in future studies.
Exercise is an integral part of any intervention aiming at weight control and an essential part of both type 1 and T2DM management. In particular, aerobic exercise reduces blood glucose by increasing GLUT 4 transporters on muscle cells and facilitates the subsequent influx of glucose into the cells [108]. While data on exercise and T1DM are not as abundant as in type 2, it is considered an effective means of controlling body weight and improving insulin sensitivity [109]. The high frequency of hypoglycemia in people with T1DM causes associated fear, leading to the adoption of “protective” behaviors such as insulin dose omission, excess consumption of calories, and exercise avoidance [110]. Indeed, a study by Brazeau et al. in 100 patients with T1DM showed that fear of hypoglycemia was the most significant barrier to increase physical activity [111]. Reduced exercise in patients with T1DM to circumvent hypoglycemia may contribute to weight gain, increased IR, and a reduced sense of well-being. The exact effect of exercise avoidance on the bodyweight of people with T1DM has not been adequately examined in prospective studies and remains an area for further research.
The Role of Exogenous Insulin in Generating Insulin Resistance Independently of Obesity
Glucotoxicity in uncontrolled diabetes is a well-studied phenomenon [112]. The term encompasses direct damage caused by increased blood glucose concentrations on β-cells coupled with organ-specific increases in IR, resulting in reduced glucose utilization and defective glycogen synthesis [113]. The initiation of exogenous insulin treatment in inulin naïve patients with T1DM improves insulin sensitivity in the short term, often leading to a transient decrease in insulin needs and even complete remission of diabetes [114]. It has been reported that insulin sensitivity is 40% higher in patients achieving remission than those who do not [104]. When, however, insulin sensitivity of individuals with longer T1DM duration (2–32 years) was examined, it was surprisingly found to be 40% lower than in matched healthy controls. Therefore, it would appear that in the long run, the attempt to counteract the toxic effect of increased blood glucose with exogenous insulin administration lowers tissue sensitivity to insulin.
Exogenous insulin treatment does not follow the natural physiological pathway into the portal vein. It, thus, avoids the first pass insulin removal by the liver, creating a hypo-insulinemic environment in the portal circulation and a hyper-insulinemic environment in the periphery. This phenomenon has been historically recognized in the late 50 s by several reports that found a 50% reduction in circulating insulin after its first pass through the liver [115, 116]. Later research in the 70 s showed an even higher insulin removal rate, with the portal concentration of insulin being even three times as high as in the peripheral circulation [117, 118]. The introduction of the euglycemic hyperinsulinemic clamp protocol gave researchers a valuable tool to assess the effect of supraphysiologic peripheral insulin concentrations (such as observed in T1DM) on IR. Specifically, the induction of peripheral hyperinsulinemia during the implementation of such protocols was shown to decrease healthy volunteers' insulin sensitivity by 14–20% compared to saline infusion [119, 120].
Furthermore, several experiments in patients with T1DM have consistently validated that higher insulin concentrations in the periphery cause an average increase of ~40% in IR [121, 122], depending on the population studied. In the more recent coronary artery Calcification in T1DM (CACTI) study, which examined the effect of IR on coronary artery calcification, people with T1DM had higher levels of IR than controls. Most importantly, this difference remained significant after adjusting for traditional elements of the metabolic syndrome and associations with hyperglycemia [123]. A recent experiment aimed to clarify whether hyperglycemia or iatrogenic hyperinsulinemia is the primary driver of IR in T1DM. The investigators compared patients with T1DM (who are hyperinsulinemic and hyperglycemic) to individuals with monogenic diabetes due to partial deficiency of the glucokinase enzyme (Maturity-Onset Diabetes of the Young – GSK-MODY) who are normoinsulinemic and hyperglycemic and to healthy controls (normoglycemic and normoinsulinemic). The two diabetic groups were matched in terms of BMI and HbA1c. IR was assessed through hyperinsulinemic-euglycemic clamps. The experiment showed a 22% higher IR level in patients with T1DM than in participants with GSK-MODY and a 29% higher level than in healthy controls. Further multivariate analysis showed that high plasma insulin concentration and not hyperglycemia was the chief contributor to IR induction in the T1DM group [124].
Insulin Pump and Obesity
Continuous subcutaneous insulin infusion through an insulin pump is increasingly becoming the preferred way of insulin administration in intensively treated patients with T1DM [125]. Several trials have examined the association between insulin pump usage and weight gain. Some initial retrospective studies reported an increase in weight gain with insulin pumps compared to multiple daily injections (MDI) [126, 127]. However, more recent studies and meta-analyses did not demonstrate any effect of insulin pumps on gaining weight over MDI [128, 129]. In the “real world,” a registry study with 1258 patients from two diabetes centers evaluated the effect of insulin pumps on body weight in adults transitioning to pumps from MDI after 1 year of follow-up. No change in weight was observed for the combined cohort, while a modest weight gain of 1.1 ± 0.3 kg (Steno Diabetes Center) and 1.7 ± 1.1 (Joslin Diabetes Center) was observed only in participants with baseline HbA1c ≥ 9% [130]. An intriguing finding of a recent study in children and adolescents with T1DM, transitioning from MDI to insulin pump therapy is the association of weight gain with higher basal insulin dose [131], despite similar levels of total daily insulin. This difference was observed after 1 year of follow-up. Under physiological conditions, human metabolism relies almost equally on bolus and basal insulin secretion from the pancreas [132]. Patients with T1DM using a high percentage of basal insulin do not adequately mimic the physiologic route to glycemic control. Hence, the reconstitution of normal insulin secretion could alleviate excessive weight gain in patients with T1DM using insulin pumps.
The Association Between Insulin Resistance, Obesity, and Complications in Type 1 Diabetes
The “Obesity Paradox” in Type 1 Diabetes
BMI has been traditionally considered as a “classic” risk factor for cardiovascular events, cardiovascular death, and overall mortality. Yet, surprisingly, back in 2002, Gruber et al. demonstrated in a prospective study a correlation between better angioplasty outcomes and increased patient weight (n = 9633) [133]. Since then, several studies have reported improved cardiovascular outcomes in patients with higher BMI values (usually in the overweight or grade I obesity range) than in individuals of average or low BMI, an observation collectively known as the obesity paradox [134, 135]. Similarly, improved survival benefits of obesity have been in other critical illnesses like sepsis [136, 137]. Several mechanisms have been proposed as the underlying cause(s) of this odd finding. Briefly, an increase in body weight could result from an increase in muscle tissue rather than fat, possibly leading to a more favorable metabolic profile. In the great majority of epidemiological studies, however, the single biomarker used to assess adiposity is BMI, which does not reflect differences between fat and muscle tissue, neither accounts for gender, age, and tribe. Hence, BMI suffers from the same disadvantage as many other biomarkers do, performing well in large population studies but failing to account for individual differences [138]. Reverse causality has also been implicated as an explanatory factor in some obesity paradox studies, especially those showing a “protective” effect of higher BMI on mortality from cancer and heart failure, as those two conditions are often causally associated with loss of body weight [139]. On the other hand, some potential protective mechanisms associated with higher BMI have been proposed, including lower levels of prothrombotic factors (i.e., thromboxane B2) [140], higher ghrelin production, which may possibly be associated with improved cardiac function [141], and a presumably suppressed inflammatory environment [142] as indicated by decreased tumor necrosis factor-a levels in a few studies [127]. However, these associations include a high degree of selection bias or are subjects of several confounding effects [143, 144]. In addition, controlling for smoking is essential, given that smokers have lower mean body weight than nonsmokers due to the anorexigenic effect of smoking. Additionally, smoking cessation is most often accompanied by an increase in body weight [145]. Therefore, not controlling for smoking may falsely attribute a cardiovascular protective impact on BMI, while cardioprotection is, in fact, mediated either through the absence or the cessation of tobacco usage.
Evidence for the obesity paradox has been found in studies of patients with T1DM. The first study describing BMI’s protective effect in people with T1DM was the New Jersey study of 725 African Americans, which showed a higher mortality risk in participants with lower BMI, after 2 years of follow-up [146]. Results indicating a U-shaped relationship of BMI to overall mortality were observed after 18–20 years of follow-up in the EDC (n = 655) [147]. Initial analysis of the DCCT/EDIC data did not show an increased incidence of cardiovascular events in the group that gained the most weight than the rest of the participants. After 20 years of follow-up, the plot lines depicting cardiovascular incidence events began to diverge, indicating a higher incidence in patients in the intensive arm of the DCCT that had gained enough weight during the study to be classified as belonging to the extensive weight gain group [148]. This difference was mainly due to increased coronary angiography procedures operated in individuals with higher BMI. In contrast, no difference was observed regarding the 3-point major adverse cardiac events (MACE) (nonfatal MI or nonfatal stroke or CVD-related death). Such a discrepancy could be attributed to the difference between silent versus evident coronary artery disease. Apart from being performed during an acute coronary syndrome as part of the emergency management, coronary angiography is often performed for diagnostic purposes or screening for silent CAD. In this case, referral of patients for the procedure is decided taking into account symptoms and clinical risk factors, including obesity. Therefore, individuals with obesity may be more frequently evaluated for silent coronary disease due to their phenotype, a so-called referral bias [149, 150]. An earlier analysis of patients with T1DM from the Swedish National diabetes registry (n = 17 499) did not find an independent association between increased BMI and myocardial infarction (MI) or coronary disease [151]. A low BMI value (≤ 18.5 kg/m2) was associated with increased mortality from CAD and death from any cause, possibly indicating the presence of comorbidities. A more recent analysis of 26,215 patients with T1DM from the same registry demonstrated a minor increase in the risk with increasing BMI values for major CVD, heart failure, and death from any cause only in male patients [152]. Still, no difference in outcomes was found in overweight and female patients. After a median follow-up of 13.7 years in the FinnDiane study (n = 5836), the obesity paradox was demonstrated in overall mortality in T1DM patients with nephropathy (lowest mortality was associated with a BMI between 25.9 to 26.1 kg/m2), while no such an association was evident in patients without nephropathy (lowest mortality BMI = 24.3 to 24.8 kg/m2) [153].
Considering all the evidence mentioned above, it is reasonable to assume that despite the widespread acceptance of BMI for categorizing obesity, its use sometimes creates substantial confusion when investigating the association between adiposity and cardiovascular morbidity/mortality in patients with T1DM. This issue could be resolved by introducing other adiposity measurements both in everyday practice and clinical research, optimally those that can also serve as an IR indicator. Waist circumference, waist-hip ratio, waist-to-height ratio, and finally, waist circumference adjusted BMI have been shown to improve the prediction of cardiovascular outcomes in the general population when compared to BMI [154,155,156]. Incorporating markers that better describe the combined effect of obesity and IR could help in the paradox’s resolution.
Microvascular Complications
Despite the individual pros and cons of obesity and IR markers, their presence has been repeatedly associated with T1DM complications (Table 1). In particular, patients with overweight and T1DM compared to those with normal weight had a higher prevalence of neuropathy and retinopathy in a cross-sectional study with 592 participants [157]. A large (n = 1172) prospective multinational study by Tesfaye et al. found that after 7.3 ± 0.6 years of follow-up, BMI was an independent predictor of neuropathy development in T1DM [158•]. The metabolic syndrome, defined by the NCEP criteria [159], as well as its individual components was associated with 3.75 higher odds of having nephropathy, after adjusting for age, gender, tobacco use, and HbA1c, in the multicenter cross-sectional Finish study for nephropathy (FinnDiane) [160]. Similarly, the metabolic syndrome used to characterize DD was correlated with a higher prevalence of microvascular complications (retinopathy 32.4% versus 21.7%, nephropathy 28.3% versus 17.8%, P < 0.001) in an Austrian-German multicenter cross-sectional study of 7926 patients with T1DM [161]. Insulin sensitivity measured in several observational studies of small sample size either by the eGDR or by the euglycemic-hyperinsulinemic clump has been repeatedly associated with overt microvascular complications and microalbuminuria [162,163,164]. Results from the EDC study also confirm that reduced eGDR significantly predicted overt nephropathy after 6 to 10 years of follow-up [165]. Finally, the highest level of evidence regarding microvascular deterioration under the influence of obesity/IR emanates from the analysis of DCCT/EDIC. After 9 years of follow-up, participants’ higher baseline IR (eGDR) was a robust independent predictor of future microvascular (retinopathy, nephropathy) complications. On the other hand, though, both the metabolic syndrome, defined by the IDF consensus criteria [166] and daily insulin dose were not [49••].
Macrovascular Complications
It has been proposed that the presence of obesity/IR in T1DM (Table 2) may be the missing link that explains those patients’ twofold higher risk for macrovascular events, despite attaining reasonable glycemic control [167]. Indeed, IR measured through the eGDR was a central predictor of hard CVD outcomes (myocardial infarction, CAD death, or coronary stenosis on angiography), specifically CAD, in an analysis of the EDC, using 10-year follow-up data [17••]. Additionally, a separate analysis from the same cohort attempted to evaluate the relationship of eGDR with the development of peripheral vascular disease after ten years of follow-up [168]. It demonstrated that lower baseline eGDR (indicating higher IR levels) was positively associated with the development of lower extremity arterial disease, defined as claudication, presence of foot ulcer, or lower extremity amputation. Finally, a more recent EDC analysis, 25 years after the study’s initiation, showed that eGDR was independently correlated with overall CVD incidence (fatal CVD, non-fatal MI, non-fatal cerebrovascular event, revascularization, angina, or ischemic changes in ECG), but not with the “classical” 3-point major adverse cardiovascular events (MACE) (fatal CVD, non-fatal MI, or non-fatal cerebrovascular event) [18]. This finding raises the possibility that eGDR’s influence on CVD incidence at longer follow-ups is mediated primarily by an increase in softer endpoints. Yet, we must not disregard the possibility that since no formal power calculation has been reported for the EDC, these results might also stem from a lack of adequate sample size to examine the association of eGDR and CVD. An independent post hoc analysis of the DCCT cohort also showed that patients with higher baseline eGDR (calculated using BMI instead of waist to hip ratio) had a lower risk (HR: 0.7, 95% CI 0.56–0.88) of developing CVD (angina, MI, coronary revascularization, ischemic changes in ECG) [49••]. Several other studies with a cross-sectional design have also attempted to evaluate the influence of IR/obesity on CVD development. Specifically, a multicenter diabetes clinic-based survey called Metascren assessed the prevalence of two different metabolic syndrome definitions (AHA/NHLBI [169], IDF [38]), in 638 Italian patients with T1DM [38] and examined their association with diabetes complications. The IDF definition was significantly associated with increased CVD outcomes by an OR of 2.05 (P = 0.03), whereas the association of the AHA/NHLBI definition with CVD did not reach statistical significance. The number of participants with CVD complications in the Metascren was somewhat low (n = 59), and this precludes any definitive explanation on the different metabolic syndrome definition discrepancy. A more extensive German cross-sectional study of 1241 participants with T1DM, using a somewhat broad definition of the metabolic syndrome, showed an independent association with peripheral artery disease (OR:2.28, 95% CI 1.38–3.76) [170]. Data from a registry study conducted in Austria and Germany demonstrated a higher presence of CVD-related endpoints (CAD 8.0% versus 3.0%, stroke 3.6% versus 1.6%, diabetic foot syndrome 5.5% versus 2.1%) in people with T1DM and metabolic syndrome (NCEP definition) [161] than those without it. Prospectively collected data in an Australian cohort of 501 people with T1DM found a progressive increase in CVD prevalence (angina, MI, revascularization, cerebrovascular event) by an OR of 1.14 (95% CI 1.04–1.24) per unit increase in BMI [171].
Taken together, data from the existing prospective and cross-sectional studies indicate that there is accumulating but still not convincing evidence regarding a causal association between insulin resistance and hard CVD outcomes in patients with type 1 diabetes. The relatively low number of events in prospective cohorts (mainly from the DCCT and EDC) and confounders in cross-sectional analyses are the main obstacles preventing more definitive conclusions from being drawn.
Atherosclerotic Disease
In an attempt to predict and thus prevent future CVD outcomes, the detection of subclinical atherosclerosis has received considerable attention over the past 30 years. Coronary artery calcification (CAC) identified by computed tomography (CT) and quantified by the Agatston score is a widely used marker for subclinical atherosclerosis [172]. CAC presence has been associated with a history of MI and the prevalence of angiographic stenosis ≥ 50%, but not with angina pectoris or ischemic ECG changes, in male participants of the EDC study [173]. The Coronary Artery Calcification in T1DM (CACTI) study examined the prevalence of CAC in 652 asymptomatic participants with T1DM and 764 healthy control subjects [174]. The study found a higher prevalence of CAC in people with diabetes across all the age-group comparisons to healthy subjects. Moreover, lower eGDR were positively (OR: 1.6, 95% CI 1.2–2.0) associated with increased CAC. The relationship between IR and high CAC was further verified by a euglycemic-hyperinsulinemic clamp study in 87 participants (40 with and 47 without T1DM) of the CATCI [123]. Similarly, results from a Brazilian cross-sectional survey of 100 consecutive patients showed that lower eGDR was associated with increased CAC presence irrespective of age, gender, and diabetic nephropathy [175]. The possible influence of obesity/IR on CAC progression was also examined in a follow-up analysis focusing on participants with T1DM [175]. Patients were further characterized based on the presence of metabolic risk factors (hypertension, high triglycerides, low HDL-C, increased CRP) as metabolically normal or abnormal. In this asymptomatic cohort, CAC presence was evaluated at baseline and after 6 years of follow-up. Overweight and obesity were shown to be independently associated with CAC at baseline. In comparison, obesity and IR (eGDR) but not overweight were found to predict CAC progression in the follow-up evaluation. Interestingly, the predictive ability of obesity for CAC was significant only in those deemed metabolically abnormal. The DCCT/EDIC also examined CAC progression showing that the WHR, but not BMI, was an independent predictor of CAC both at recruitment and at the time of CT scan evaluation [176]. A surprising finding came from a cross-sectional assessment in a subset of patients from the EDC study (n = 315). While the presence of CAC was correlated with BMI, visceral and peripheral adiposity and WHR, after adjustment for potential confounders, the severity of CAC was not associated with any of the obesity markers and even had an inverse relationship with them. While this finding might seem paradoxical, it should be noted that the CT scan compared to modern modalities, has a relatively low discriminatory ability to differentiate between unstable and inactive coronary plague, possibly accounting for controversies between studies [177]. The use of 18F‐NaF Positron Emission Tomography in future studies, a modality that can identify inflammation and microcalcifications (the hallmarks of plague rapture), may serve to clarify discrepancies in the relationship between obesity/IR and subclinical atherosclerosis.
Another frequently used modality for assessing subclinical atherosclerosis is the ultrasonographic evaluation of carotid intima-media thickness (IMT). The IMT measurement has been shown to be an independent predictor for future CVD events in general population studies [178, 179]. Higher IMT values have been reported in patients with T1DM, even at younger ages, in several cross-sectional studies, compared to healthy controls [178, 180,181,182,183]. BMI was significantly associated with IMT in univariate analysis in all those studies, but was not retained in the final multivariable models, after correction for obesity-related comorbidities. Participants from the DCCT/EDIC were also evaluated for subclinical atherosclerosis using IMT during the 1st year of the EDIC phase and after six years of follow-up [184]. Their respective IMT values were subsequently compared to 222 age and sex-matched healthy volunteers. Although no difference was found between the two populations during the first year of the study, after 6 years, a significantly greater progression of IMT in the T1DM group was observed, particularly in patients randomized in the conventional treatment arm. Evaluation for IMT progression predictors, revealed that several traditional risk factors like smoking, dyslipidemia, and hypertension but not BMI were associated with faster subclinical atherosclerosis progression. A more recent prospective study conducted in children and adolescents (n = 150) with T1DM (16.45 ± 2.59 years-old at baseline) also revealed a significant increase in IMT values after four years of follow-up. Diabetes duration, hypertension, and BMI at baseline were all independent predictors of plaque augmentation [185]. In accordance with these findings, BMI was the only modifiable risk factor associated with subclinical atherosclerosis in 298 patients with T1DM of young age (mean age 13.3 ± 2.9 years) in the SEARCH CVD Study [186].
Collectively, it appears that BMI and IR exert substantial influence on subclinical atherosclerosis in younger patients, underlying the importance of early interventions against obesity/IR development.
HF
Type 1 diabetes is linked with an average twofold increased risk of developing heart failure (HF) [187]. Nonetheless, the existence of a so-called diabetic cardiomyopathy as a distinct clinical entity is still a matter of debate and is usually defined as “ventricular dysfunction in the absence of coronary artery disease and hypertension” [188]. The most common echocardiographic findings in asymptomatic patients with T1DM are related to left ventricular diastolic dysfunction [189]. However, more recent studies have demonstrated the presence of early impaired systolic function with the use of speckle tracking echocardiography in T1DM patients, even in the absence of diastolic dysfunction [190].
Obesity and IR have been recognized as substantial risk factors for HF development, particularly HF with preserved ejection fraction in the general population and in T2DM [191, 192]. Furthermore, the risk of HF has been described to increase in a graded manner across BMI categories [193]. Lind et al. prospectively followed 20,985 patients of relatively young age (mean 38.6 years) with T1DM, recorded in the Swedish national diabetes registry, until hospital admission for HF, death, or end of the study [194]. A total of 3% of the initial cohort was admitted for HF over a median follow-up of nine years. Smoking, hypertension, and BMI were shown to be significant modifiable predictors of risk for HF. A subsequent analysis of the same group of patients revealed that the risk of hospitalization for HF was increased in the previously described graded manner across obesity categories (HRs 1.55 for BMI 30–35 kg/m2, and 2.90 for BMI ≥ 35 kg/m2) [195]. However, there was no increased risk (HR 0.94, 95% CI 0.78–1.12) in overweight patients. In another study investigating HF incidence in the entire Scottish population, higher BMI (as a continuous variable) was independently associated with incident hospitalization for HF in individuals with T1DM (HR: 1.22, 95% CI 1.04–1.45).
The effect of IR on exercise capacity of T1DM youth was evaluated by Nadeau et al. In this well-designed study, 12 adolescents with T1DM were compared to 12 healthy volunteers matched for age, daily activity level, and BMI values [196•]. Both groups underwent IR measurement through a euglycemic-hyperinsulinemic clamp protocol, treadmill assessment of cardiopulmonary fitness, cardiac echocardiography, and determination of body composition by dual-energy X-ray absorptiometry. As expected, higher IR levels were reported in youths with diabetes, despite having similar BMIs and body composition to the control group. There was also a significant decrease in peak oxygen consumption, indicating compromised cardiopulmonary fitness. Finally, echocardiography revealed that, despite their young age, participants with T1DM had findings of increased left ventricular volume and diastolic dysfunction, evident by the higher filling pressure at rest.
Is There a Role for Antidiabetic Medications in Tackling IR in Type 1 Diabetes?
Metformin
Given the proven benefits on hepatic glucose production and, less prominently, on peripheral IR, its low cost and safe profile, it is not surprising that metformin has been tested in several trials since the mid-80 s as add-on therapy in patients with T1DM [197]. Variable study designs and conflicting results have led to the need for conducting meta-analysis studies. The first was based on data from five controlled studies in T1DM patients with BMI ≥ 25 kg/m2 or those characterized as insulin resistant (total daily insulin dose ≥ 0.9 u/kg), and reported a small improvement with metformin adjuvant use in glycemic control (−0.27% on HbA1c) as well as a reduction in total daily insulin dose and improvement in blood lipids, but no effect on BMI [198]. A Cochrane review evaluated 60 studies in which metformin was added to insulin therapy in T1DM [199]. Due to the low-quality design issues in most of the trials, the authors included only two studies in the statistical analysis. The only benefit observed was a 10% reduction in daily insulin dose, while no effect was found regarding BMI or cholesterol values. A more recent meta-analysis by Liu et al., with data from 8 RCTs, found a significant reduction in weight with metformin use (mean difference −2.41 kg, 95% CI −4.17 to −0.65). Improvement was also noted in lipid control in conjunction with a small but significant decrease (mean difference −1.36 U, P = 0.004) in total daily insulin needs, while no sustained effect was found regarding HbA1c [200].
The largest RCT so far, performed in 428 adults with T1DM with at least three additional CVD risk factors indicated that metformin, as an add-on treatment to insulin, results in significant (but clinically negligible) reductions in weight (−1.17 kg, −1.66 to −0.69; P < 0.0001) and LDL-C (−0.13 mmol/L, −0.24 to −0.03; P = 0.0117) over 3 years [201]. At the same time, a transient initial decrease of HbA1c was not sustained by the 6th month of the study. No effect on endothelial function, progression of mean IMT or retinopathy was noticed. In all abovementioned studies, the use of metformin caused little, if any, increase in hypoglycemic events. Of note, there are currently no randomized studies examining the effect of metformin on CVD outcomes in the T1DM population.
Taken together, the observations from studies with metformin in T1DM do not support its widespread use in this population. However, its use on an individualized, case-by-case basis, for example, in insulin-resistant individuals with obesity or women with polycystic ovary syndrome, could be reasonable.
Pramlintide
The hormone amylin is a small peptide co-secreted with insulin by β pancreatic cells in response to meal consumption. As a result of the β cell autoimmune destruction, amylin is also deficient in patients with T1DM [202]. The main physiological effects of amylin include postprandial inhibition of glucagon secretion, slowing of gastric emptying, and hypothalamic reduction of appetite [203]. A synthetic analog of amylin, pramlintide, is the only FDA approved glucose lowering agent apart from insulin in T1DM, aiming mainly to reduce postprandial glucose excursions.
Early animal studies suggested that amylin may reduce glucose uptake and increase hepatic glucose production [204, 205]; later studies, however, as well as a few studies in humans, have been inconclusive [206]. Studies with pramlintide in patients with T1DM have shown that the drug has no effect on insulin sensitivity [206].
Overall, pramlintide appears to affect BMI reduction positively; in three RCTs performed in individuals with T1DM, body weight was reduced by 1.0–1.3 kg after 13–26 weeks [207], while the placebo-subtracted weight difference reached 2.5 kg in one study [182]. Nevertheless, its use requires multiple daily injections with meals adding more burden to the already perplexing treatment schedule of patients with T1DM. Furthermore, a relatively higher incidence of nausea and a higher incidence in severe hypoglycemia associated with its use, as described by a meta-analysis of 3 pramlintide RCT, further diminishes its appeal as an adjuvant treatment for obesity [208].
SGLT-2 I Inhibitors
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are a class of antidiabetic drugs currently approved mainly for T2DM. Their glucose-lowering effect is produced from an increase in urinary glucose excretion caused by the inhibition of SGLT2 transporter in the proximal renal tubule [209]. Apart from a moderate reduction in blood glucose values, SGLT 2i exert nephroprotective and cardioprotective actions, significantly reducing hospitalization for HF and renal function deterioration [210]. Furthermore, their use is associated with a decrease in body weight and blood pressure [209]. The impact of SGLT2 inhibitors as an add-on treatment on insulin therapy in people with T1DM has been evaluated in a few clinical trials and one meta-analysis [211]. Bodyweight decreased with SGLT-2i (mean difference vs. placebo: −2.76 kg, −1.11, −4.40), as well as total daily insulin dose, and HbA1c (mean difference vs. placebo: −0.39%, −0.25 to −0.51). All of these benefits came without an increase in severe hypoglycemia. Despite these impressive results, substantial concern exists regarding the possibility of ketoacidosis (mostly euglycemic), which was observed in 6.5% of the participants receiving an SGLT-2i but in none in the placebo group [211].
There are no specific data regarding the effect of SGLT-2i on insulin sensitivity in patients with T1DM. Although they are not considered insulin-sensitizing drugs, SGLT-2i may indirectly improve insulin action through several mechanisms, such as weight loss, reduction of glucotoxicity and lipotoxicity, and reduction of oxidative stress and inflammation [212]. Notably, there are currently no published studies of SGLT-2i in T1DM with hard endpoints. Based on the existing short-term clinical trials, dapagliflozin received an EMA (European Medical Agency) approval as the first add-on treatment to insulin for the treatment of certain patients with type 1 diabetes in Europe.
Collectively, while SGLT 2 inhibitors have proven beneficial effects on BMI and daily insulin dose, their incorporation to the treatment plan of T1DM should proceed with extreme caution due to the increased risk of euglycemic ketoacidosis.
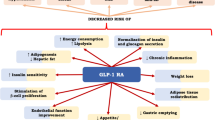
GLP-1RA
Glucagon-like peptide 1 (GLP-1) agonists are injectable peptides that correct high blood sugar levels without causing hypoglycemia through: (1) enhancement of insulin secretion in a glucose-dependent manner, (2) inhibition of glucagon release, (3) promotion of satiety, and (4) slowing of gastric emptying [213]. Apart from the substantial improvement of glycemia observed in patients with T2DM, treatment with GLP-1RA causes a significant reduction in BMI [214]. Some long-acting members of the class also confer substantial protection from CVD events [215,216,217]. This advantageous therapeutic profile is coupled with little safety concerns, with the main side effect being related to the gastrointestinal system, mainly nausea [214].
Although GLP-1Rs are not typical insulin-sensitizers, they may exert a beneficial indirect effect on insulin action through their remarkable weight loss effect but also via pleiotropic effects on insulin-resistance inducing pathways [218]. Exenatide, a short-acting GLP1RA, was evaluated as an adjuvant of insulin treatment in a small crossover trial (6 months on exenatide followed by 6 months off) in 14 adult patients with T1DM. These patients were assessed for IR through a euglycemic-hyperinsulinemic clamp protocol at the end of each period. After being on the drug, participants exhibited significant improvements in IR, accompanied by an average reduction of 4.2 kg in weight, as well as a reduction in daily insulin dose. At the same time, no significant effect was shown in HbA1c [219]. A more recent and larger RCT (n = 108) evaluated the impact of exenatide as an add-on treatment in people with T1DM for 26 weeks [220]. While the primary endpoint (i.e., reduction in HbA1c) was not reached, treatment with exenatide resulted in a significant decrease in weight (−4.4 kg) without any treatment-related serious adverse events. Liraglutide, a long-acting agent with proven CVD benefit, has also been tested on top of insulin in an RCT of 100 patients with T1DM and BMI ≥ 25 g/m2, who had failed to reach their glycemic target (HbA1c > 8%) [221]. There was no significant improvement in glycemic control with liraglutide, but a substantial improvement in body weight and reduced insulin requirements was noted, without any significant safety issues. Collectively, GLP1RAs exert a robust reduction in weight and daily inulin dose in patients with T1DM, with a neutral effect in glycemia. No agent of this class has been approved for the treatment of T1DM. Nevertheless, due to their excellent safety profile and effect on obesity and IR, these drugs could be used in the future as an adjuvant treatment in individual cases, particularly in those characterized by obesity and DD.
Concluding Remarks
A specific subcategory of patients with T1DM will develop obesity/IR, probably through the complex interaction of genetic predisposition and exogenous insulin administration. These patients often referred to as having DD, are at greater risk for development of complications. Improved identification of patients with DD can be made by incorporating easily calculated measurements of insulin resistance, such as the eGDR and the waist to hip ratio, into common clinical practice. Finally, pharmacologic strategies used to tackle obesity/IR in type 2 diabetes may be useful as adjunctive to insulin treatment in T1DM. Further research in this subpopulation is needed in order to tailor diabetes management and improve expectancy and quality of life.
Abbreviations
- AER:
-
Albumin excretion rate
- CVD:
-
Cardiovascular disease
- CAD:
-
Coronary artery disease
- CACTI:
-
Calcification in Type 1 Diabetes Mellitus Study
- DCCT:
-
Diabetes Control and Complications Trial
- DD:
-
Double diabetes
- EDC:
-
Pittsburgh Epidemiology of Diabetes Complications Study
- EDIC:
-
Epidemiology of Diabetes Interventions and Complications study
- eGDR:
-
Estimated glucose disposal rate
- FinnDiane:
-
Finnish Diabetic Nephropathy Study
- FTO:
-
Fat mass and Obesity-associated
- GADA:
-
Glutamic acid decarboxylase antibodies
- GFR:
-
Glomerular filtration rate
- GLP-1:
-
Glucagon-like peptide 1 agonists
- GSK-MODY:
-
Glucokinase Enzyme Related-Maturity-Onset Diabetes of the Young
- HF:
-
Heart failure
- HOMA:
-
Homeostatic model assessment
- IDF:
-
International Diabetes Federation
- LADA:
-
Latent autoimmune diabetes in adults
- LDL-C:
-
Low-density lipoprotein cholesterol
- MACE:
-
Major adverse cardiovascular events
- MCR:
-
Melanocortin 4 receptor gene
- MDI:
-
Multiple daily injections
- miRNA:
-
MicroRNAs
- MS:
-
Metabolic syndrome
- NCEP:
-
National Cholesterol Education Program
- NFκB:
-
Nuclear factor-κB
- IR:
-
Insulin resistance
- IMT:
-
Carotid intima-media thickness insulin resistance
- RCT:
-
Randomized controlled trial
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- SGLT2i:
-
Sodium-glucose cotransporter 2 inhibitors
- SNP:
-
Single nucleotide polymorphism
- WHR:
-
Waist to hip ratio
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Poynton FJ. Five cases of diabetes mellitus in young children. Br Med J. 1923;1(3242):277–9. https://doi.org/10.1136/bmj.1.3242.277.
Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Glycemic control and cardiovascular disease in 7,454 patients with type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes Care. 2010. https://doi.org/10.2337/dc10-0398 Published online.
Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003. https://doi.org/10.2337/diacare.26.4.1258 Published online.
Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD009122.pub2 Published online.
Kopp W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes Targets Ther. 2019. https://doi.org/10.2147/DMSO.S216791 Published online.
Minges KE, Whittemore R, Weinzimer SA, Irwin ML, Redeker NS, Grey M. Correlates of overweight and obesity in 5529 adolescents with type 1 diabetes: The T1D Exchange Clinic Registry. Diabetes Res Clin Pract. 2017. https://doi.org/10.1016/j.diabres.2017.01.012 Published online.
Baskaran C, Volkening LK, Diaz M, Laffel LM. A decade of temporal trends in overweight/obesity in youth with type 1 diabetes after the Diabetes Control and Complications Trial. Pediatr Diabetes. 2015;16(4):263–70. https://doi.org/10.1111/pedi.12166.
Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a scottish cohort with type 1 diabetes, 2008–2010. J Am Med Assoc. 2015. https://doi.org/10.1001/jama.2014.16425 Published online.
Huo L, Harding JL, Peeters A, Shaw JE, Magliano DJ. Life expectancy of type 1 diabetic patients during 1997–2010: a national Australian registry-based cohort study. Diabetologia. 2016. https://doi.org/10.1007/s00125-015-3857-4 Published online.
Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001. https://doi.org/10.1007/PL00002934 Published online.
Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018. https://doi.org/10.1016/S0140-6736(18)31506-X Published online.
Shamoon H, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Published online.
The Diabetes Control, Complications Trial (DCCT) Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the diabetes control and complications trial. Am J Cardiol. 1995. https://doi.org/10.1016/S0002-9149(99)80683-3 Published online.
Nathan DM, Cleary PA, Backlund JYC, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005. https://doi.org/10.1056/NEJMoa052187 Published online.
Wadén J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009. https://doi.org/10.2337/db09-0693 Published online.
Klein BEK, Klein R, McBride PE, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med. 2004. https://doi.org/10.1001/archinte.164.17.1917 Published online.
•• Orchard TJ, Olson JC, Erbey JR, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh epidemiology of diabetes complications study. Diabetes Care. 2003. https://doi.org/10.2337/diacare.26.5.1374 Published online. This prospective cohort study showed that IR, as measured by the eGDR, was a strong predictor of hard CVD endpoints, such as MI or CAD death in people with T1D during the 10-year follow-up period.
Miller RG, Costacou T, Orchard TJ. Risk factor modeling for cardiovascular disease in type 1 diabetes in the pittsburgh epidemiology of diabetes complications (EDC) study: a comparison with the diabetes control and complications trial/epidemiology of diabetes interventions and complication. Diabetes. 2019. https://doi.org/10.2337/db18-0515 Published online.
Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014. https://doi.org/10.1056/NEJMoa1408214 Published online.
Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. J Am Med Assoc. 2005. https://doi.org/10.1001/jama.294.14.1782 Published online.
Hero C, Svensson AM, Gidlund P, Gudbjörnsdottir S, Eliasson B, Eeg-Olofsson K. LDL cholesterol is not a good marker of cardiovascular risk in Type 1 diabetes. Diabet Med. 2016. https://doi.org/10.1111/dme.13007 Published online.
Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH. Relationship between risk factors and mortality in type 1 diabetic patients in europe: The eurodiab prospective complications study (PCS). Diabetes Care. 2008. https://doi.org/10.2337/dc08-0107 Published online.
Costacou T, Evans RW, Orchard TJ. High-density lipoprotein cholesterol in diabetes: Is higher always better? J Clin Lipidol. 2011. https://doi.org/10.1016/j.jacl.2011.06.011 Published online.
De Ferranti SD, De Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014. https://doi.org/10.2337/dc14-1720 Published online.
Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for diabetes in youth study: Rationale, findings, and future directions. Diabetes Care. 2014. https://doi.org/10.2337/dc14-0574 Published online.
Karamanos B, Porta M, Songini M, et al. Different risk factors of microangiopathy in patients with Type I diabetes mellitus of short versus long duration. The EURODIAB IDDM Complications Study. Diabetologia. 2000. https://doi.org/10.1007/s001250050053 Published online.
United States Department of Health and Human Services. The Health Consequences of smoking–50 years of progress A Report of the Surgeon General. United States Department of Health and Human Services; 2014. Published online.
•• Rawshani A, Rawshani A, Franzén S, et al. Range of risk factor levels: control, mortality, and cardiovascular outcomes in type 1 diabetes mellitus. Circulation. 2017. https://doi.org/10.1161/CIRCULATIONAHA.116.025961 Published online. Recent large Swedish National Diabetes Register study, with a follow-up time of 10 years, showing that compared to the control population, people with T1D have twice the risk for MI, even after attaining the corresponding targets for all traditional CVD-related risk factors.
American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015. https://doi.org/10.2337/dc15-S005 Published online.
Redondo MJ, Rodriguez LM, Escalante M, O’Brian Smith E, Balasubramanyam A, Haymond MW. Beta cell function and BMI in ethnically diverse children with newly diagnosed autoimmune type 1 diabetes. Pediatr Diabetes. 2012. https://doi.org/10.1111/j.1399-5448.2012.00875.x Published online.
Dabelea D, D’Agostino RB, Mayer-Davis EJ, et al. Testing the accelerator hypothesis: Body size, β-cell function, and age at onset of type 1 (autoimmune) diabetes. Diabetes Care. 2006. https://doi.org/10.2337/diacare.29.02.06.dc05-1339 Published online.
• Knerr I, Wolf J, Reinehr T, et al. The “accelerator hypothesis”: relationship between weight, height, body mass index and age at diagnosis in a large cohort of 9,248 German and Austrian children with type 1 diabetes mellitus. Diabetologia. 2005. https://doi.org/10.1007/s00125-005-0033-2 Published online. The "accelaartor hypothesis" postulates that the increased insulin demands caused by obesity lead to faster deterioration of beta cell function in people predisposed to develop T1D. With data from 116 diabetes clinics, this prospective observational study showed that higher BMI was associated with earlier T1D onset in youth and adolescents aged 0–20 years.
Umpierrez GE. Ketosis-prone type 2 diabetes: time to revise the classification of diabetes. Diabetes Care. 2006. https://doi.org/10.2337/dc06-1870 Published online.
Østergaard JA, Laugesen E, Leslie RD. Should there be concern about autoimmune diabetes in adults? Current Evidence and Controversies. Curr Diab Rep. 2016. https://doi.org/10.1007/s11892-016-0780-0 Published online.
Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetologia. 2001. https://doi.org/10.1007/s001250100548 Published online.
Patterson CC, Dahlquist GG, Gyürüs E, et al. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009. https://doi.org/10.1016/S0140-6736(09)60568-7 Published online.
Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020. https://doi.org/10.1016/j.jhep.2020.03.039 Published online.
Bonadonna RC, Cucinotta D, Fedele D, Riccardi G, Tiengo A. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: results from Metascreen, a multicenter diabetes clinic-based survey. Diabetes Care. 2006. https://doi.org/10.2337/dc06-0942 Published online.
Teupe B, Bergis K. Epidemiological evidence for “double diabetes.” Lancet. 1991. https://doi.org/10.1016/0140-6736(91)90988-2 Published online.
Libman IM, Becker DJ. Coexistence of type 1 and type 2 diabetes mellitus: “Double” diabetes? Pediatr Diabetes. 2003. https://doi.org/10.1034/j.1399-5448.2003.00012.x Published online.
Thorn LM, Forsblom C, Waden J, et al. Effect of parental type 2 diabetes on offspring with type 1 diabetes. Diabetes Care. 2009. https://doi.org/10.2337/dc08-0472 Published online.
Purnell JQ, Dev RK, Steffes MW, et al. Relationship of family history of type 2 diabetes, hypoglycemia, and autoantibodies to weight gain and lipids with intensive and conventional therapy in the diabetes control and complications trial. Diabetes. 2003. https://doi.org/10.2337/diabetes.52.10.2623 Published online.
Erbey JR, Kuller LH, Becker DJ, Orchard TJ. The association between a family history of type 2 diabetes and coronary artery disease in a type 1 diabetes population. Diabetes Care. 1998. https://doi.org/10.2337/diacare.21.4.610 Published online.
Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011. https://doi.org/10.1186/1741-7015-9-48 Published online.
World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. World Health Organization. 1999. https://apps.who.int/iris/handle/10665/66040.
Thorn LM, Forsblom C, Wadén J, et al. Metabolic syndrome as a risk factor for cardiovascular disease, mortality, and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2009. https://doi.org/10.2337/dc08-2022 Published online.
Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987. https://doi.org/10.2337/diab.36.8.914 Published online.
Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000. https://doi.org/10.2337/diabetes.49.4.626 Published online.
•• Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the diabetes control and complications trial. Diabetes Care. 2007. https://doi.org/10.2337/dc06-1982 Published online. Data analysis from this RCT has shown that higher IR at baseline predicted both the development of micro-and macrovascular disease in people with T1D. Further on, it also found an increase in MS prevalence among study participants, particularly those receiving intensive insulin treatment, during the nine years of follow-up.
The IDF consensus worldwide definition of the metabolic syndrome. Obe Metab. 2005. https://doi.org/10.14341/2071-8713-4854 Published online.
Porta M, Sjoelie AK, Chaturvedi N, et al. Risk factors for progression to proliferative diabetic retinopathy in the EURODIAB Prospective Complications Study. Diabetologia. 2001. https://doi.org/10.1007/s001250100030 Published online.
Lam YY, Ravussin E. Analysis of energy metabolism in humans: a review of methodologies. Mol Metab. 2016. https://doi.org/10.1016/j.molmet.2016.09.005 Published online.
Jacob AN, Salinas K, Adams-Huet B, Raskin P. Potential causes of weight gain in type 1 diabetes mellitus. Diabetes Obes Metab. 2006. https://doi.org/10.1111/j.1463-1326.2005.00515.x Published online.
Charlton MR, Nair KS. Role of hyperglucagonemia in catabolism associated with type 1 diabetes: Effects on leucine metabolism and the resting metabolic rate. Diabetes. 1998. https://doi.org/10.2337/diabetes.47.11.1748 Published online.
Nair KS. Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. J Clin Endocrinol Metab. 1987. https://doi.org/10.1210/jcem-64-5-896 Published online.
Nair KS, Halliday D, Garrow JS. Increased energy expenditure in poorly controlled Type 1 (insulin-dependent) diabetic patients. Diabetologia. 1984. https://doi.org/10.1007/BF00253494 Published online.
Woods SC, Porte D, Bobbioni E, et al. Insulin: Its relationship to the central nervous system and to the control of food intake and body weight. Am J Clin Nutr. 1985. https://doi.org/10.1093/ajcn/42.5.1063 Published online.
Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc B Biol Sci. 2006. https://doi.org/10.1098/rstb.2006.1858 Published online.
Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Am J Physiol Regul Integr Comp Physiol. 1992. https://doi.org/10.1152/ajpregu.1992.262.6.r975 Published online.
Arafat AM, Weickert MO, Adamidou A, et al. The impact of insulin-independent, glucagon-induced suppression of total ghrelin on satiety in obesity and type 1 diabetes mellitus. J Clin Endocrinol Metab. 2013. https://doi.org/10.1210/jc.2013-1635 Published online.
Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care. 2014. https://doi.org/10.2337/dc13-2112 Published online.
• Conway B, Miller RG, Costacou T, et al. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabet Med. 2010. https://doi.org/10.1111/j.1464-5491.2010.02956.x Published online. This observational study showed that during the 18 years of follow-up, the prevalence of overweight and obesity significantly increased in participants with T1D. Initiation of intensive insulin treatment regiments during the study was an independent predictor of increased weight gain.
Dubose SN, Hermann JM, Tamborlane WV, et al. Obesity in youth with type 1 diabetes in Germany, Austria, and the United States. J Pediatr. 2015. https://doi.org/10.1016/j.jpeds.2015.05.046 Published online.
Buzzetti R, Zampetti S, Pozzilli P. Impact of obesity on the increasing incidence of type 1 diabetes. Diabetes Obes Metab. 2020. https://doi.org/10.1111/dom.14022 Published online.
Sands ALP, Higgins LA, Mehta SN, Nansel TR, Lipsky LM, Laffel LMB. Associations of youth and parent weight status with reported versus predicted daily energy intake and hemoglobin A1c in youth with type 1 diabetes mellitus. J Diabetes Sci Technol. 2013. https://doi.org/10.1177/193229681300700131 Published online.
Chesi A, Grant SFA. The genetics of pediatric obesity. Trends Endocrinol Metab. 2015. https://doi.org/10.1016/j.tem.2015.08.008 Published online.
Loos RJF, Lindgren CM, Li S, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008. https://doi.org/10.1038/ng.140 Published online.
Łuczyński W, Fendler W, Ramatowska A, et al. Polymorphism of the FTO gene influences body weight in children with type 1 diabetes without severe obesity. Int J Endocrinol. 2014. https://doi.org/10.1155/2014/630712 Published online.
Gu HF, Alvarsson A, Brismar K. The common FTO genetic polymorphism rs9939609 is associated with increased BMI in type 1 diabetes but not with diabetic nephropathy. Biomark Insights. 2010. https://doi.org/10.4137/bmi.s4599 Published online.
Luczyński W, Szypowska A, Glowińska-Olszewska B, Szadkowska A, Bossowski A. Disease associated clinical factors and FTO polymorphism: effect on body mass in children with type 1 diabetes mellitus. Pediatr Diabetes. 2014. https://doi.org/10.1111/pedi.12091 Published online.
Grant SFA, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006. https://doi.org/10.1038/ng1732 Published online.
Redondo MJ, Geyer S, Steck AK, et al. TCF7L2 genetic variants contribute to phenotypic heterogeneity of type 1 diabetes. Diabetes Care. 2018. https://doi.org/10.2337/dc17-0961.
Gray R, Wheatley K. How to avoid bias when comparing bone marrow transplantation with chemotherapy. Bone Marrow Transplant. 1991.
Smith GD. Mendelian randomization for strengthening causal inference in observational studies: application to gene × environment interactions. Perspect Psychol Sci. 2010. https://doi.org/10.1177/1745691610383505 Published online.
Censin JC, Nowak C, Cooper N, Bergsten P, Todd JA, Fall T. Childhood adiposity and risk of type 1 diabetes: a Mendelian randomization study. PLoS Med. 2017. https://doi.org/10.1371/journal.pmed.1002362 Published online.
Dupont C, Armant DR, Brenner CA. Epigenetics: definition, mechanisms and clinical perspective. Semin Reprod Med. 2009. https://doi.org/10.1055/s-0029-1237423 Published online.
Kaprio J, Tuomilehto J, Koskenvuo M, et al. Concordance for Type 1 (insulin-dependent) and Type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia. 1992. https://doi.org/10.1007/BF02221682 Published online.
Al Aboud NM, Jialal I. Genetics, Epigenetic Mechanism. StatPearls Publishing; 2018.
Dayeh T, Tuomi T, Almgren P, et al. DNA methylation of loci within ABCG1 and PHOSPHO1 in blood DNA is associated with future type 2 diabetes risk. Epigenetics. 2016. https://doi.org/10.1080/15592294.2016.1178418 Published online.
Barres R, Kirchner H, Rasmussen M, et al. Weight loss after gastric bypass surgery in human obesity remodels promoter methylation. Cell Rep. 2013. https://doi.org/10.1016/j.celrep.2013.03.018 Published online.
Huang YT, MacCani JZJ, Hawley NL, Wing RR, Kelsey KT, McCaffery JM. Epigenetic patterns in successful weight loss maintainers: A pilot study. Int J Obes. 2015. https://doi.org/10.1038/ijo.2014.213 Published online.
Miao F, Smith DD, Zhang L, Min A, Feng W, Natarajan R. Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation an epigenetic study in diabetes. Diabetes. 2008. https://doi.org/10.2337/db08-0645 Published online.
Brasacchio D, Okabe J, Tikellis C, et al. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes. 2009. https://doi.org/10.2337/db08-1666 Published online.
Miao F, Chen Z, Genuth S, et al. Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes. 2014. https://doi.org/10.2337/db13-1251 Published online.
Zhuo C, Feng M, Andrew DP, et al. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc Natl Acad Sci USA. 2016. https://doi.org/10.1073/pnas.1603712113 Published online.
Ross JS, Carlson JA, Brock G. miRNA: The new gene silencer. Am J Clin Pathol. 2007. https://doi.org/10.1309/2JK279BU2G743MWJ Published online.
Ling C, Rönn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019. https://doi.org/10.1016/j.cmet.2019.03.009 Published online.
Shah R, Murthy V, Pacold M, et al. Extracellular RNAs are associated with insulin resistance and metabolic phenotypes. Diabetes Care. 2017. https://doi.org/10.2337/dc16-1354 Published online.
Sebastiani G, Grieco FA, Spagnuolo I, Galleri L, Cataldo D, Dotta F. Increased expression of microRNA miR-326 in type 1 diabetic patients with ongoing islet autoimmunity. Diabetes Metab Res Rev. 2011. https://doi.org/10.1002/dmrr.1262 Published online.
Yang M, Ye L, Wang B, et al. Decreased miR-146 expression in peripheral blood mononuclear cells is correlated with ongoing islet autoimmunity in type 1 diabetes patients. J Diabetes. 2015. https://doi.org/10.1111/1753-0407.12163 Published online.
Assmann TS, Recamonde-Mendoza M, De Souza BM, Crispim D. MicroRNA expression profiles and type 1 diabetes mellitus: systematic review and bioinformatic analysis. Endocr Connect. 2017. https://doi.org/10.1530/EC-17-0248 Published online.
Dimitriadis G, Mitron P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011. https://doi.org/10.1016/S0168-8227(11)70014-6 Published online.
Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J. Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J Clin Invest. 1995. https://doi.org/10.1172/JCI118000 Published online.
Chap Z, Ishida T, Chou J, et al. First-pass hepatic extraction and metabolic effects of insulin and insulin analogues. Am J Physiol Endocrinol Metab. 1987. https://doi.org/10.1152/ajpendo.1987.252.2.e209 Published online.
Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes -causes, effects and coping strategies. Diabetes Obes Metab. 2007. https://doi.org/10.1111/j.1463-1326.2006.00686.x Published online.
Nathan DM. Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the diabetes control and complications trial. Diabetes Care. 2001. https://doi.org/10.2337/diacare.24.10.1711 Published online.
Diabetes Control and Complications Trial Research Group. Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care. 1995. https://doi.org/10.2337/diacare.18.11.1415 Published online.
Anderson EJ, Richardson M, Castle G, et al. Nutrition interventions for intensive therapy in the Diabetes Control and Complications Trial. The DCCT Research Group. J Am Diet Assoc. 1993 Published online.
Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: Results from the DCCT. J Am Med Assoc. 1998. https://doi.org/10.1001/jama.280.2.140 Published online.
Ratzki-Leewing A, Harris SB, Mequanint S, et al. Real-world crude incidence of hypoglycemia in adults with diabetes: results of the InHypo-DM Study. Canada BMJ Open Diabetes Res Care. 2018. https://doi.org/10.1136/bmjdrc-2017-000503 Published online.
Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003. https://doi.org/10.2337/diacare.26.6.1902 Published online.
Merwin RM, Moskovich AA, Dmitrieva NO, et al. Disinhibited eating and weight-related insulin mismanagement among individuals with type 1 diabetes. Appetite. 2014. https://doi.org/10.1016/j.appet.2014.05.028 Published online.
Wing RR, Cleary PA. Weight gain associated with intensive therapy in the diabetes control and complications trial. Diabetes Care. 1988. https://doi.org/10.2337/diacare.11.7.567 Published online.
Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV. Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes. 1988. https://doi.org/10.2337/diab.37.7.901 Published online.
Boyle PJ, Schwartz NS, Shah SD, Clutter WE, Cryer PE. Plasma glucose concentrations at the onset of hypoglycemic symptoms in patients with poorly controlled diabetes and in nondiabetics. N Engl J Med. 1988. https://doi.org/10.1056/nejm198806093182302 Published online.
Cryer PE. Preventing hypoglycaemia: what is the appropriate glucose alert value? Diabetologia. 2009. https://doi.org/10.1007/s00125-008-1205-7 Published online.
Ratner RE. Hypoglycemia: new definitions and regulatory implications. Diabetes Technol Ther. 2018. https://doi.org/10.1089/dia.2018.0113 Published online.
Hayashi T, Wojtaszewski JFP, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol Endocrinol Metab. 1997. https://doi.org/10.1152/ajpendo.1997.273.6.e1039 Published online.
Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia Published online. 2012. https://doi.org/10.1007/s00125-011-2403-2.
Martyn-Nemeth P, Schwarz Farabi S, Mihailescu D, Nemeth J, Quinn L. Fear of hypoglycemia in adults with type 1 diabetes: impact of therapeutic advances and strategies for prevention - a review. J Diabetes Complicat. 2016. https://doi.org/10.1016/j.jdiacomp.2015.09.003 Published online.
Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008. https://doi.org/10.2337/dc08-0720 Published online.
Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress. World J Gastroenterol. 2009. https://doi.org/10.3748/wjg.15.4137 Published online.
Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990. https://doi.org/10.2337/diacare.13.6.610 Published online.
Yki-Järvinen H, Koivisto VA. Natural course of insulin resistance in type I diabetes. N Engl J Med. 1986. https://doi.org/10.1056/nejm198607243150404 Published online.
Madison LL, Unger RH, Kaplan N. The relationship between the mechanism of action of the sulfonylureas and the secretion of insulin into the portal circulation. Ann NY Acad Sci. 1959. https://doi.org/10.1111/j.1749-6632.1959.tb39579.x Published online.
Samols E, Ryder JA. Studies on tissue uptake of insulin in man using a differential immunoassay for endogenous and exogenous insulin. J Clin Invest. 1961. https://doi.org/10.1172/JCI104435 Published online.
Blackard WG, Nelson NC. Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion. Diabetes. 1970. https://doi.org/10.2337/diab.19.5.302 Published online.
Horwitz DL, Starr JI, Mako ME, Blackard WG, Rubenstein AH. Proinsulin, insulin and C-peptide concentrations in human portal and peripheral blood. J Clin Invest. 1975. https://doi.org/10.1172/JCI108047 Published online.
Rizza RA, Mandarino LJ, Genest J, Baker BA, Gerich JE. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia. 1985. https://doi.org/10.1007/BF00279918 Published online.
Marangou AG, Weber KM, Boston RC, et al. Metabolic consequences of prolonged hyperinsulinemia in humans. Evidence for induction of insulin insensitivity. Diabetes. 1986. https://doi.org/10.2337/diab.35.12.1383 Published online.
Donga E, Dekkers OM, Corssmit EPM, Romijn JA. Insulin resistance in patients with type 1 diabetes assessed by glucose clamp studies: systematic review and meta-analysis. Eur J Endocrinol. 2015. https://doi.org/10.1530/EJE-14-0911 Published online.
Kaul K, Apostolopoulou M, Roden M. Insulin resistance in type 1 diabetes mellitus. Metabolism. 2015. https://doi.org/10.1016/j.metabol.2015.09.002 Published online.
Schauer IE, Snell-Bergeon JK, Bergman BC, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes the CACTI study. Diabetes. 2011. https://doi.org/10.2337/db10-0328 Published online.
Gregory JM, Smith TJ, Slaughter JC, et al. Iatrogenic hyperinsulinemia, not hyperglycemia, drives insulin resistance in type 1 diabetes as revealed by comparison with GCK-MODY (MODY2). Diabetes. 2019. https://doi.org/10.2337/db19-0324 Published online.
Van Den Boom L, Karges B, Auzanneau M, et al. Temporal trends and contemporary use of insulin pump therapy and glucose monitoring among children, adolescents, and adults with type 1 diabetes between 1995 and 2017. Diabetes Care. 2019. https://doi.org/10.2337/dc19-0345.
Bruttomesso D, Pianta A, Crazzolara D, et al. Continuous subcutaneous insulin infusion (CSII) in the veneto region: efficacy, acceptability and quality of life. Diabet Med. 2002. https://doi.org/10.1046/j.1464-5491.2002.00750.x Published online.
Joubert M, Morera J, Vicente A, Rod A, Parienti JJ, Reznik Y. Cross-sectional survey and retrospective analysis of a large cohort of adults with type 1 diabetes with long-Term continuous subcutaneous insulin infusion treatment. J Diabetes Sci Technol. 2014. https://doi.org/10.1177/1932296814537040 Published online.
Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012. https://doi.org/10.7326/0003-4819-157-5-201209040-00508 Published online.
REPOSE Study Group. Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised trial (REPOSE). BMJ. 2017. https://doi.org/10.1136/bmj.j1285 Published online.
Mehta SN, Andersen HU, Abrahamson MJ, et al. Changes in HbA1c and weight following transition to continuous subcutaneous insulin infusion therapy in adults with type 1 diabetes. J Diabetes Sci Technol. 2017. https://doi.org/10.1177/1932296816658900 Published online.
Boucher-Berry C, Parton EA, Alemzadeh R. Excess weight gain during insulin pump therapy is associated with higher basal insulin doses. J Diabetes Metab Disord. 2016. https://doi.org/10.1186/s40200-016-0271-5 Published online.
Kruszynska YT, Home PD, Hanning I, Alberti KGMM. Basal and 24-h C-peptide and insulin secretion rate in normal man. Diabetologia. 1987. https://doi.org/10.1007/BF01788901 Published online.
Gruberg L, Weissman NJ, Waksman R, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: The obesity paradox? J Am Coll Cardiol. 2002. https://doi.org/10.1016/S0735-1097(01)01802-2 Published online.
Wang L, Liu W, He X, et al. Association of overweight and obesity with patient mortality after acute myocardial infarction: a meta-analysis of prospective studies. Int J Obes. 2016. https://doi.org/10.1038/ijo.2015.176 Published online.
Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004. https://doi.org/10.1016/j.jacc.2003.11.039 Published online.
Karampela I, Chrysanthopoulou E, Christodoulatos GS, Dalamaga M. Is there an obesity paradox in critical illness? Epidemiologic and metabolic considerations. Curr Obes Rep. 2020. https://doi.org/10.1007/s13679-020-00394-x Published online.
Karampela I, Christodoulatos GS, Dalamaga M. The role of adipose tissue and adipokines in sepsis: inflammatory and metabolic considerations, and the obesity paradox. Curr Obes Rep. 2019. https://doi.org/10.1007/s13679-019-00360-2 Published online.
Casas-Vara A, Santolaria F, Fernández-Bereciartúa A, González-Reimers E, García-Ochoa A, Martínez-Riera A. The obesity paradox in elderly patients with heart failure: analysis of nutritional status. Nutrition. 2012. https://doi.org/10.1016/j.nut.2011.10.006 Published online.
Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005. https://doi.org/10.1001/archinte.165.1.55 Published online.
Graziani F, Biasucci LM, Cialdella P, et al. Thromboxane production in morbidly obese subjects. Am J Cardiol. 2011. https://doi.org/10.1016/j.amjcard.2011.01.053 Published online.
Khatib M, Simkhada P, Gode D. Cardioprotective effects of ghrelin in heart failure: from gut to heart. Hear Views. 2014. https://doi.org/10.4103/1995-705x.144792 Published online.
Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013. https://doi.org/10.1530/JOE-12-0232 Published online.
Robinson WR, Furberg H, Banack HR. Selection bias: A missing factor in the obesity paradox debate. Obesity. 2014. https://doi.org/10.1002/oby.20666 Published online.
Preston SH, Stokes A. Obesity paradox: Conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiology. 2014. https://doi.org/10.1097/EDE.0000000000000075 Published online.
Nuttall FQ. Body mass index: Obesity, BMI, and health: a critical review. Nutr Today. 2015. https://doi.org/10.1097/NT.0000000000000092 Published online.
Roy M, Rendas-Baum R, Skurnick J. Mortality in African-Americans with Type 1 diabetes: The New Jersey 725. Diabet Med. 2006. https://doi.org/10.1111/j.1464-5491.2006.01901.x Published online.
Conway B, Miller RG, Costacou T, et al. Adiposity and mortality in type 1 diabetes. Int J Obes. 2009. https://doi.org/10.1038/ijo.2009.75 Published online.
Purnell JQ, Braffett BH, Zinman B, et al. Impact of excessive weight gain on cardiovascular outcomes in type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes Care. 2017. https://doi.org/10.2337/dc16-2523 Published online.
Niraj A, Pradahan J, Fakhry H, Veeranna V, Afonso L. Severity of coronary artery disease in obese patients undergoing coronary angiography: “Obesity Paradox” revisited. Clin Cardiol. 2007. https://doi.org/10.1002/clc.20113 Published online.
Rubinshtein R, Halon DA, Jaffe R, Shahla J, Lewis BS. Relation between obesity and severity of coronary artery disease in patients undergoing coronary angiography. Am J Cardiol. 2006. https://doi.org/10.1016/j.amjcard.2005.11.061 Published online.
Vestberg D, Rosengren A, Eeg-Olofsson K, et al. Body mass index as a risk factor for coronary events and mortality in patients with type 1 diabetes. Open Hear. 2018. https://doi.org/10.1136/openhrt-2017-000727 Published online.
Edqvist J, Rawshani A, Adiels M, et al. BMI, mortality, and cardiovascular outcomes in type 1 diabetes: findings against an obesity paradox. Diabetes Care. 2019. https://doi.org/10.2337/dc18-1446 Published online.
Dahlström EH, Sandholm N, Forsblom CM, et al. Body mass index and mortality in individuals with type 1 diabetes. J Clin Endocrinol Metab. 2019. https://doi.org/10.1210/jc.2019-00042 Published online.
Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020. https://doi.org/10.1038/s41574-019-0310-7 Published online.
Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 05 could be a suitable global boundary value. Nutr Res Rev. 2010. https://doi.org/10.1017/S0954422410000144 Published online.
Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012. https://doi.org/10.1111/j.1467-789X.2011.00952.x Published online.
De Block CEM, De Leeuw IH, Van Gaal LF. Impact of overweight on chronic microvascular complications in type 1 diabetic patients. Diabetes Care. 2005. https://doi.org/10.2337/diacare.28.7.1649 Published online.
• Tesfaye S, Chaturvedi N, Eaton SEM, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005. https://doi.org/10.1056/nejmoa032782 Published online. This large prospective study of 1172 patients with T1D showed that baseline BMI, a potentially modifiable factor, was an independent predictor for the development of neuropathy.
Bartlett JG. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults. Infect Dis Clin Pract. 2001. https://doi.org/10.1097/00019048-200106000-00021 Published online.
Thorn LM, Forsblom C, Fagerudd J, et al. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care. 2005. https://doi.org/10.2337/diacare.28.8.2019 Published online.
Merger SR, Kerner W, Stadler M, et al. Prevalence and comorbidities of double diabetes. Diabetes Res Clin Pract. 2016. https://doi.org/10.1016/j.diabres.2016.06.003 Published online.
Chillarón JJ, Goday A, Flores-Le-Roux JA, et al. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab. 2009. https://doi.org/10.1210/jc.2009-0960 Published online.
Girgis CM, Scalley BD, Park KEJ. Utility of the estimated glucose disposal rate as a marker of microvascular complications in young adults with type 1 diabetes. Diabetes Res Clin Pract. 2012. https://doi.org/10.1016/j.diabres.2012.02.004 Published online.
Yip J, Mattock MB, Morocutti A, Sethi M, Trevisan R, Viberti G. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993. https://doi.org/10.1016/0140-6736(93)91943-G Published online.
Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int. 2002. https://doi.org/10.1046/j.1523-1755.2002.00507.x Published online.
Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome - a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006. https://doi.org/10.1111/j.1464-5491.2006.01858.x Published online.
Gregory JM, Cherrington AD, Moore DJ. The peripheral peril: Injected insulin induces insulin insensitivity in type 1 diabetes. Diabetes. 2020. https://doi.org/10.2337/dbi19-0026 Published online.
Olson JC, Erbey JR, Forrest KYZ, Williams K, Becker DJ, Orchard TJ. Glycemia (or, in women, estimated glucose disposal rate) predict lower extremity arterial disease events in type 1 diabetes. Metabolism. 2002. https://doi.org/10.1053/meta.2002.30021 Published online.
Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. Circulation. 2005. https://doi.org/10.1161/circulationaha.105.169404 Published online.
Reindel J, Zander E, Heinke P, Kohnert K-D, Allwardt C, Kerner W. Metabolisches Syndrom bei Patienten mit Diabetes mellitus Typ 1. The metabolic syndrome in patients with type 1 diabetes mellitus. Associations with cardiovascular risk factors and cardiovascular morbidity. Herz. 2004. https://doi.org/10.1007/s00059-004-2606-0 Published online.
Price SA, Gorelik A, Fourlanos S, Colman PG, Wentworth JM. Obesity is associated with retinopathy and macrovascular disease in type 1 diabetes. Obes Res Clin Pract. 2014. https://doi.org/10.1016/j.orcp.2013.03.007 Published online.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990. https://doi.org/10.1016/0735-1097(90)90282-T Published online.
Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ. Coronary calcium in adults with type 1 diabetes: a stronger correlate of clinical coronary artery disease in men than in women. Diabetes. 2000. https://doi.org/10.2337/diabetes.49.9.1571 Published online.
Dabelea D, Kinney G, Snell-Bergeon JK, et al. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance?: The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003. https://doi.org/10.2337/diabetes.52.11.2833 Published online.
Rodrigues TC, Biavatti K, Almeida FK, Gross JL. Coronary artery calcification is associated with insulin resistance index in patients with type 1 diabetes. Brazilian J Med Biol Res. 2010. https://doi.org/10.1590/S0100-879X2010007500109 Published online.
Cleary PA, Orchard TJ, Genuth S, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes. 2006. https://doi.org/10.2337/db06-0653 Published online.
Wang Y, Osborne MT, Tung B, Li M, Li Y. Imaging cardiovascular calcification. J Am Heart Assoc. 2018. https://doi.org/10.1161/JAHA.118.008564 Published online.
O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999. https://doi.org/10.1056/nejm199901073400103 Published online.
Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation. 1997. https://doi.org/10.1161/01.CIR.96.5.1432 Published online.
Yamasaki Y, Kawamori R, Matsushima H, et al. Atherosclerosis in carotid artery of young IDDM patients monitored by ultrasound high-resolution B-mode imaging. Diabetes. 1994. https://doi.org/10.2337/diab.43.5.634 Published online.
Margeirsdottir HD, Stensaeth KH, Larsen JR, Brunborg C, Dahl-Jørgensen K. Early signs of atherosclerosis in diabetic children on intensive insulin treatment: a population-based study. Diabetes Care. 2010. https://doi.org/10.2337/dc10-0505 Published online.
Larsen JR, Brekke M, Bergengen L, et al. Mean HbA1c over 18 years predicts carotid intima media thickness in women with type 1 diabetes. Diabetologia. 2005. https://doi.org/10.1007/s00125-005-1700-z Published online.
Ogawa Y, Uchigata Y, Iwamoto Y. Progression factors of carotid intima-media thickness and plaque in patients with long-term, early-onset type 1 diabetes mellitus in Japan: simultaneous comparison with diabetic retinopathy. J Atheroscler Thromb. 2009. https://doi.org/10.5551/jat.1701 Published online.
Purnell JQ, John EH, Cleary PA, et al. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interven. Circulation. 2013. https://doi.org/10.1161/CIRCULATIONAHA.111.077487 Published online.
Dalla Pozza R, Beyerlein A, Thilmany C, et al. The effect of cardiovascular risk factors on the longitudinal evolution of the carotid intima medial thickness in children with type 1 diabetes mellitus. Cardiovasc Diabetol. 2011. https://doi.org/10.1186/1475-2840-10-53 Published online.
Shah AS, Dabelea D, Fino NF, et al. Predictors of increased carotid intima-media thickness in youth with type 1 diabetes: The SEARCH CVD study. Diabetes Care. 2016. https://doi.org/10.2337/dc15-1963 Published online.
McAllister DA, Read SH, Kerssens J, et al. Incidence of hospitalization for heart failure and case-fatality among 3.25 million people with and without diabetes mellitus. Circulation. 2018. https://doi.org/10.1161/CIRCULATIONAHA.118.034986 Published online.
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972. https://doi.org/10.1016/0002-9149(72)90595-4 Published online.
Acar G, Akcay A, Sokmen A, et al. Assessment of atrial electromechanical delay, diastolic functions, and left atrial mechanical functions in patients with type 1 diabetes mellitus. J Am Soc Echocardiogr. 2009. https://doi.org/10.1016/j.echo.2009.03.028 Published online.
Jȩdrzejewska I, Król W, Światowiec A, et al. Left and right ventricular systolic function impairment in type 1 diabetic young adults assessed by 2D speckle tracking echocardiography. Eur Heart J Cardiovasc Imaging. 2016. https://doi.org/10.1093/ehjci/jev164 Published online.
Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001. https://doi.org/10.1097/00000441-200104000-00003.
Savji N, Meijers WC, Bartz TM, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Hear Fail. 2018. https://doi.org/10.1016/j.jchf.2018.05.018 Published online.
Clark K. Obesity and the risk of heart failure. J Insur Med. 2003. https://doi.org/10.1056/nejmoa020245 Published online.
Lind M, Bounias I, Olsson M, Gudbjörnsdottir S, Svensson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20 985 patients with type 1 diabetes: An observational study. Lancet. 2011. https://doi.org/10.1016/S0140-6736(11)60471-6 Published online.
Vestberg D, Rosengren A, Olsson M, Gudbjörnsdottir S, Svensson AM, Lind M. Relationship between overweight and obesity with hospitalization for heart failure in 20,985 patients with type 1 diabetes: a population-based study from the Swedish National Diabetes Registry. Diabetes Care. 2013. https://doi.org/10.2337/dc12-2007 Published online.
• Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010. https://doi.org/10.1210/jc.2009-1756 Published online. This cross-sectional study has shown that youth with T1D demonstrated higher IR and worse exercise capacity compared to age-matched people without diabetes.
Vella S, Buetow L, Royle P, Livingstone S, Colhoun HM, Petrie JR. The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia. 2010. https://doi.org/10.1007/s00125-009-1636-9 Published online.
Pang TTL, Narendran P. Addressing insulin resistance in type 1 diabetes. Diabet Med. 2008. https://doi.org/10.1111/j.1464-5491.2008.02493.x Published online.
Abdelghaffar S, Attia AM. Metformin added to insulin therapy for type 1 diabetes mellitus in adolescents. Cochrane Database Syst Rev. 2009. https://doi.org/10.1002/14651858.CD006691.pub2 Published online.
Liu C, Wu D, Zheng X, Li P, Li L. Efficacy and safety of metformin for patients with type 1 diabetes mellitus: a meta-analysis. Diabetes Technol Ther. 2015. https://doi.org/10.1089/dia.2014.0190 Published online.
Petrie JR, Chaturvedi N, Ford I, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017. https://doi.org/10.1016/S2213-8587(17)30194-8 Published online.
Scherbaum WA. The role of amylin in the physiology of glycemic control. Exp Clin Endocrinol Diabetes. 1998. https://doi.org/10.1055/s-0029-1211958 Published online.
Hoogwerf BJ, Doshi KB, Diab D. Pramlintide, the synthetic analogue of amylin: Physiology, pathophysiology, and effects on glycemic control, body weight, and selected biomarkers of vascular risk. Vasc Health Risk Manag. 2008. https://doi.org/10.2147/vhrm.s1978 Published online.
Cooper GJS, Leighton B, Dimitriadis GD, et al. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc Natl Acad Sci USA. 1988. https://doi.org/10.1073/pnas.85.20.7763 Published online.
Young AA, Cooper GJS, Carlo P, Rink TJ, Wang MW. Response to intravenous injections of amylin and glucagon in fasted, fed, and hypoglycemic rats. Am J Physiol Endocrinol Metab. 1993. https://doi.org/10.1152/ajpendo.1993.264.6.e943 Published online.
Schmitz O, Nyholm B, Orskov L, Gravholt C, Møller N. Effects of amylin and the amylin agonist pramlintide on glucose metabolism. Diabet Med. 1997 Jun; 14 Suppl 2:S19–23. Retrieved from https://onlinelibrary.wiley.com/toc/10969136/14/S2.
Younk LM, Mikeladze M, Davis SN. Pramlintide and the treatment of diabetes: a review of the data since its introduction. Expert Opin Pharmacother. 2011. https://doi.org/10.1517/14656566.2011.581663 Published online.
Lee NJ, Norris SL, Thakurta S. Efficacy and harms of the hypoglycemic agent pramlintide in diabetes mellitus. Ann Fam Med. 2010. https://doi.org/10.1370/afm.1174 Published online.
Brown E, Rajeev SP, Cuthbertson DJ, Wilding JPH. A review of the mechanism of action, metabolic profile and haemodynamic effects of sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2019. https://doi.org/10.1111/dom.13650 Published online.
Rangaswami J, Bhalla V, De Boer IH, et al. Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: a scientific statement from the American Heart Association. Circulation. 2020. https://doi.org/10.1161/CIR.0000000000000920 Published online.
El Masri D, Ghosh S, Jaber LA. Safety and efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors in type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018. https://doi.org/10.1016/j.diabres.2018.01.004 Published online.
Yaribeygi H, Sathyapalan T, Maleki M, Jamialahmadi T, Sahebkar A. Molecular mechanisms by which SGLT2 inhibitors can induce insulin sensitivity in diabetic milieu: a mechanistic review. Life Sci. 2020. https://doi.org/10.1016/j.lfs.2019.117090 Published online.
Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012. https://doi.org/10.1038/nrendo.2012.140 Published online.
Sfairopoulos D, Liatis S, Tigas S, Liberopoulos E. Clinical pharmacology of glucagon-like peptide-1 receptor agonists. Hormones. 2018. https://doi.org/10.1007/s42000-018-0038-0 Published online.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. Drug Ther Bull. 2016. https://doi.org/10.1056/nejmoa1603827 Published online.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019. https://doi.org/10.1016/S0140-6736(19)31149-3 Published online.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016. https://doi.org/10.1056/nejmoa1607141 Published online.
Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: Systematic review and meta-analyses of randomised controlled trials. BMJ. 2012. https://doi.org/10.1136/bmj.d7771 Published online.
Sarkar G, Alattar M, Brown RJ, Quon MJ, Harlan DM, Rother KI. Exenatide treatment for 6 months improves insulin sensitivity in adults with type 1 diabetes. Diabetes Care. 2014. https://doi.org/10.2337/dc13-1473 Published online.
Johansen NJ, Dejgaard TF, Lund A, et al. Efficacy and safety of meal-time administration of short-acting exenatide for glycaemic control in type 1 diabetes (MAG1C): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020. https://doi.org/10.1016/S2213-8587(20)30030-9 Published online.
Dejgaard TF, Frandsen CS, Hansen TS, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2016. https://doi.org/10.1016/S2213-8587(15)00436-2 Published online.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Georgios Karamanakos, Maria Dalamaga declare that they have no conflict of interest. Alexander Kokkinos: Served on advisory boards, received travel grants, or research support from Eli Lilly, Sanofi, Novo Nordisk, MSD, Astra Zeneca, ELPEN Pharma, Boehringer-Ingelheim, Bausch Health, Ethicon, Galenica, and Epsilon Health. Stavros Liatis: I have received research support and/or consulting or speaker honoraria from Astra-Zeneca, Boehringer-Ingelheim, MSD, Novartis, Novo Nordisk, Sanofi, Eli Lilly, and ELPEN, during the last three years.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on The Obesity Epidemic: Causes and Consequences
Rights and permissions
About this article
Cite this article
Karamanakos, G., Kokkinos, A., Dalamaga, M. et al. Highlighting the Role of Obesity and Insulin Resistance in Type 1 Diabetes and Its Associated Cardiometabolic Complications. Curr Obes Rep 11, 180–202 (2022). https://doi.org/10.1007/s13679-022-00477-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-022-00477-x