Abstract
Purpose of Review
To describe what is known about the association between obesity and attention-deficit hyperactivity disorder (ADHD) in children along with the co-occurring conditions of sleep dysfunction, loss of control/binge eating disorder (LOC-ED/BED), and anxiety.
Recent Findings
Obesity and ADHD share common brain pathways (hypothalamic, executive, and reward centers) with pathophysiology in these areas manifesting in partial or complete expression of these diseases. Sleep dysfunction, LOC-ED/BED, and anxiety share similar pathways and are associated with this disease dyad.
Summary
The association of obesity and ADHD with sleep dysfunction, LOC-ED/BED, and anxiety is discussed. An algorithm outlining decision pathways for patients with obesity and with and without ADHD is presented. Future research exploring the complex pathophysiology of both obesity and ADHD as well as co-occurring conditions is needed to develop clinical guidelines and ultimately assist in providing the best evidence-based care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Evidence of an association between obesity and attention-deficit/hyperactivity disorder (ADHD) has grown in the past 20 years. Studies exploring this association through the lens of genetics and physiology have increased our understanding of the connection between these two disease states. Particularly in children, the specific manifestations of this association continue to evolve.
The disease of obesity is a manifestation of dysfunction of normal (homeostatic) energy regulation resulting in metabolic derangements and excess energy storage. The disease is heterogeneous, with a biological basis, genetic phenotypes, and system redundancy. ADHD is a disease characterized by persistent, excessive, and functionally damaging levels of overactivity, inattention, and impulsivity [1]. Multiple conditions are associated with obesity and ADHD with potential causation, directionality, and interaction still to be determined [2,3,4,5,6,7].
In this review, the association between obesity and ADHD is examined along with co-occurring diagnoses (sleep disorders, loss of control eating disorder/binge eating disorder (LOC-ED/BED), and anxiety) which may impact obesity and/or ADHD through shared pathophysiologic pathways. The roles of inflammation and genetics are discussed. Finally, we offer obesity management strategies for children with and without ADHD.
Definition
Obesity
The World Health Organization defines overweight and obesity as abnormal or excessive adipose accumulation that presents a risk to health [8]. Weight varies by age and sex in children; standardized growth charts track children’s weight by growth percentiles [9]. Classifications of overweight and obesity by BMI percentile include overweight (85th–94th percentiles), obesity (class 1; > 95th percentile to 120% of 95th percentile), and severe obesity (class 2: > 120–140% of the 95th percentile; class 3: > 140% of the 95th percentile) [10, 11].
Attention-Deficit/Hyperactivity Disorder
Attention-deficit/hyperactivity disorder is defined as the presence of inattention and/or hyperactivity/impulsivity symptoms that impair functioning [12]. The median age of ADHD diagnosis is 7 years, with approximately one-third of children diagnosed before 6 years of age [13]. In terms of developmental trajectory, data suggest that hyperactive/impulsive symptoms decline over time, whereas inattention symptoms persist [14]. Symptoms and impairment have been identified through adolescence and into adulthood [15].
The reported prevalence of ADHD varies in children from 2 to 18% depending on measurement and population. A meta-analysis of 175 studies identified the estimated worldwide pooled prevalence of ADHD as 7.2% [16]. Prevalence numbers from community-based samples were found to be higher (7.2–15.5%) [17, 18]. ADHD may present with variety of risk factors, symptom expression, co-occurring disorders, executive functioning impairments, and/or developmental trajectories.
Association Between Obesity and ADHD
Cortese (2019) provides an understanding of the complex history of the association between ADHD and obesity along with a historical timeline [19••]. In a 2016 review of pooled data from 41 studies (48,161 subjects with ADHD and comparison group of 679,975 subjects without ADHD), Cortese and colleagues found an association between obesity and ADHD for both children and adults. In those patients with ADHD, there was an increased pooled prevalence of obesity in 70% of adults and 40% in children [20].
In contrast, a separate review by Nigg (2016) found a less robust relationship between ADHD and obesity with only 1/3 of the studies illustrating an association that reached significance (OR 1.22) and with no evidence of a significant relationship among preadolescents [21]. This review differed from Cortese et al. (2016) as it included pooled studies where criteria for obesity were BMI values as dimensional scores rather than obesity as a category, a metabolic disorder impacting weight (example: diabetes), or a diagnosis of disordered eating. The researchers did find that the association between ADHD and obesity was twice as large in adults compared to children and noted that few studies focus primarily on preadolescents. The authors suggest that obesity/ADHD association research focus on subpopulations within each disease based on age, sex, and comorbid conditions which they hypothesized might demonstrate a stronger association.
Overall, there is more evidence for ADHD preceding obesity, rather than obesity preceding ADHD [22,23,24]. A study by Khalife and colleagues, specifically focused on children, suggested that teacher-reported ADHD symptoms in childhood predicted adolescent obesity, but obesity did not predict ADHD [22]. ADHD may lead to obesity through shared pathophysiology, response inhibition, and loss of control eating, among other mechanisms. Seymour et al. found a significant overlap of neural circuitry between the two conditions with functional abnormalities found in reward, response inhibition, emotional processing, and regulation [25]. Studies also suggest that obesity may lead to ADHD [26, 27] or that the link between ADHD and metabolic syndrome/obesity is bidirectional [28].
Basic Physiology of Obesity/ADHD
Knowledge of normal and pathophysiology of obesity and ADHD is necessary to understand the reported association between the two diseases. Shared pathways may not only explain the association but perhaps offer opportunities for early screening and shared treatment strategies. There are multiple pathways known and probably unknown that impact ADHD and obesity. This section highlights pathways involved in neurohormonal signaling to the hypothalamic, cognitive, and reward centers of the brain.
Obesity Physiology Pathways
An increased understanding of the pathophysiology and complex pathways involved in excess adiposity resulting from dysregulation of the energy regulation system (ERS) has expanded the current assessment and treatment approach for obesity.
The hypothalamus receives input from the macro- and microenvironment including biological, behavioral, developmental, and psychological mediators that all impact the ERS. Key areas in the arcuate nucleus of the hypothalamus receive the input signals that determine satiety and hunger. Messages are relayed to 2nd order neurons within the hypothalamus and disseminated to pertinent areas of the brain and the vagus nerve to adjust food intake and energy expenditure. [29••, 30, 31].
The prefrontal cortex (PFC) region of the brain manages executive function: attention, planning, problem solving, and impulse control. Several studies suggest that an association between PFC deficits and increased adiposity may impair cortical functions that normally inhibit short-term rewards. [32,33,34,35]. Heinitz et al. found that left dorsolateral prefrontal cortex (DLPFC) activity is reduced in patients with obesity in response to food stimuli and postprandial state perhaps due to less impulse control via deregulated inhibitory mechanisms impacting eating patterns and food choice [36]. Transcranial stimulation of the left DLPFC resulted in decreased hunger and urge to eat, highlighting the important role of the DLPFC in eating patterns. The exact mechanism of the DLPFC is not completely understood, particularly the directionality of the relationship between adiposity and DLPFC activity [37].
Poor executive function within the PFC can result in compromised self-control, potentially leading to consistently unhealthy eating behaviors [38]. A study by Bruce et al. (2010), which included children ages 10–17 years, found greater premeal stimulation of the PFC as well as greater postmeal stimulation of the orbitofrontal cortex in subjects with obesity versus normal weight. The study was limited by a small sample size [39].
The reward center plays a role in obesity pathophysiology through dopamine receptors which influence reward seeking and motivation-related feeding behaviors. Stice et al. (2013) noted in adults that a reduction in D2 dopamine receptors was associated with a higher BMI supporting the theory that lower D2 receptor activity may promote feeding and increase the risk of obesity [40]. The authors hypothesized that individuals with hypo-functioning of the dorsal striatum in the reward center may overeat to compensate for this deficiency, known as reward deficiency syndrome (RDS). The RDS consists of genetic and epigenetic influences leading to dysfunction of the reward center presenting as hypo-dopaminergic function [41]. Blum et al. (2014) propose that hypo-dopaminergic functioning is due to reduced downregulation of dopamine receptors and suboptimal release of dopamine into the reward center leading to increased cravings [41].
Münzberg and colleagues proposed an integrative model to explain the interaction between the hypothalamic/homeostatic and reward/hedonic mechanisms [37]. This model emphasizes the interconnectedness of both mechanisms acting in a coordinated fashion to receive energy availability signals to regulate energy balance. Münzberg’s model includes bidirectional communication between the hypothalamus and reward center operating at the unconscious level. These pathways are complex and therefore represent an area of ongoing robust research which will add significantly to our understanding of the pathophysiology of obesity [29••, 37, 42, 43].
ADHD Physiology Pathways
Similar to obesity pathophysiology, reviews of the neurobiology of ADHD have identified various alterations in brain anatomy, brain functioning, and neurochemical factors in cognitive and reward centers [44,45,46]. With regard to brain anatomy, studies have identified morphological alterations in the cerebellum, temporoparietal lobes, basal ganglia, and corpus callosum in individuals with ADHD relative to controls [47, 48]. Studies have also demonstrated global thinning of the cortex (including the prefrontal cortex) in children and adolescents with ADHD, reductions in the density of the DLPFC, reduction in surface area, and decreased cortical folding [49, 50]. Rate of cortical thinning is correlated with hyperactivity and impulsivity symptom severity [51]. Anomalies in structures such as the hippocampus [52] and thalamus [53] have been identified in children and adolescents with ADHD, relative to controls.
A meta-analysis of children with ADHD that pooled functional magnetic resonance imaging (fMRI) studies identified significant hypoactivity in frontal regions including anterior cingulate, dorsolateral prefrontal, inferior prefrontal, and orbitofrontal cortices, as well as related regions such as portions of the basal ganglia, thalamus, and parietal cortices [54]. These findings are similar to those identified in structural imaging studies. Data also suggests the presence of abnormal connectivity between the amygdala and prefrontal cortex among youth with ADHD [52].
Shaw and colleagues (2007) found that the ordered sequence of development throughout the cortex was similar in children with ADHD and those without; however, the median age at which 50% of the cortical points achieved peak thickness in children with ADHD was 10.5 years, while in typically developing controls, it was 7.5 years [55]. The delay was greatest in the prefrontal regions important for the control of cognitive processes, including attention, executive functioning, and motor planning [55]. Neuroimaging studies have demonstrated a relationship between the maturation of the prefrontal cortex (and related circuitry) and reduced ADHD symptoms with development [56,57,58,59], as well as changes in executive functioning [60, 61].
With regard to neurochemistry, evidence from psychopharmacologic studies provide support for involvement of dopaminergic and adrenergic systems (reward centers). Stimulants, the first-line treatment of ADHD, block the reuptake of dopamine and norepinephrine into the presynaptic neuron, and amphetamines promote the release of dopamine and norepinephrine into the extra-neuronal space [62, 63]. Connected to our understanding of the relationship between ADHD and obesity, patients with reported ADHD symptoms and greater dopaminergic activation in reward centers of the brain are found to have increased hedonic eating patterns and more likely to have higher BMIs [19••, 41, 64]. These studies outline multiple mechanisms that may contribute to dysregulation of the reward center which may explain greater dopaminergic activation needed to overcome the suboptimal release of dopamine noted in some variants. Perhaps for certain phenotypes of patients presenting with both obesity and ADHD, this additional factor may contribute to the pathophysiology.
In sum, studies of youth with ADHD have identified a variety of brain differences that are thought to underlie symptom trajectory and severity. Our review suggests that prefrontal/cortical functioning and reward centers in the brain are involved in attentional dysregulation. There is less understanding of any hypothalamic pathophysiology among youth with ADHD. What data we do find suggest that links between hypothalamic pathophysiology and ADHD depend upon other co-occurring processes and social determinant factors that may lead to the presentation of ADHD symptoms [45••]. For example, in a study of post institutionalized children, hypocortisolism (as measured by diurnal slope and reactivity) mediated the relationship between early adversity and later externalizing/ADHD symptoms in kindergarten [46].
Obesity/ADHD Shared Pathways
The above discussion suggests that the shared neurobiological pathways of obesity and ADHD include abnormal reward center responses, dopamine and norepinephrine presynaptic availability, and related alterations in impulse control/executive functioning. Knowledge of these pathways allows for a more detailed biological understanding of why attentional dysregulation and energy dysregulation occur together and may help providers and families to appreciate the potential impact of jointly treating symptoms. For example, in children with obesity and ADHD, inattention and impulsivity may manifest as inconsistency with implementing intensive lifestyle goals and difficulty inhibiting urges to eat. Disorganization and planning deficits may also impact success in following specific dietary recommendations (e.g., forgetting to pack a lunch or forgetting to bring healthy snacks when away from home).
Clinically, there are several related conditions, which may further elucidate the connections between obesity and ADHD. Evidence exists that sleep dysfunction, LOC-ED/BED, and anxiety are present at higher rates among children with obesity and ADHD [65,66,67,68]. The presence of these conditions occurring simultaneously and/or longitudinally (one condition preceding another) suggests that interventions tailored to address these conditions concurrently may be more effective and produce more positive outcomes for patients. With this in mind, we outline the current understanding of the connections between sleep dysfunction, LOC-ED/BED, and anxiety with obesity and ADHD.
Shared Pathways of Obesity and ADHD with Associated Conditions
Sleep Dysfunction
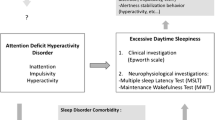
Sleep dysfunction, ranging from mild to severe, can impact the presentation of both obesity and ADHD [65]. Clinically, children with obesity have higher risk for sleep disorders which can negatively impact obesity treatment responses if left undiagnosed and untreated. Sleep disorders such as obstructed sleep apnea and sleep disordered breathing share neural pathophysiology with obesity and ADHD which may exacerbate ADHD symptoms of inattention, impulsivity, and poor executive function creating treatment challenges.
Sleep dysfunction may be the primary cause of these symptoms rather than ADHD. Addressing sleep may resolve attentional symptoms as well as improve obesity management goals.
Several papers report that sleep curtailment results in increased appetite [66, 69, 70]. Tankersley et al. (1998) and Aygun et al. (2005) describe biological links involving leptin and sleep dysfunction in patients with obesity and ADHD [71, 72]. In subjects with obesity, excess fat mass contributes to leptin resistance which can impact respiratory response. Halbower et al. (2006) hypothesized that respiratory issues during sleep may result in neuronal injury in key brain areas [73]. This is particularly important during childhood development and may be expressed as behavioral symptoms seen in patients with ADHD [65].
Circadian rhythm dysregulation can present as excessive daytime sleepiness (EDS). Research suggests that patients with ADHD with the circadian preference “eveningness type” (later bed and awakening time) were associated with a higher BMI [74]. Turkoglu and Cetin (2019) conducted a study of chronotype (circadian) preferences as a mechanism linking ADHD symptoms to obesity in children and adolescents [75]. In the “eveningness type” subset, 61.90% had obesity compared to those in the “morningness type” where 86.84% had a normal BMI. The authors found a correlation between BMI percentile scores and ADHD index scores.
LOC-ED/BED
LOC-ED/BED patterns also co-occur in patients with both obesity and ADHD [76, 77] highlighted in reward center and dopamine receptor function and with prefrontal cortex dysregulation of executive function [67•]. An interplay between hormones acting metabolically in the dorsolateral striatum below the level of conscious awareness may lead to symptoms seen in LOC-ED/BED [29••, 78]. Elevated cortisol from chronic stress contributes to insulin resistance and abnormal satiety cues [79•]. Stress accelerates habitual control suggesting that neuroadaptations resulting from exposure to chronic stress may enhance the expression of habit such as loss of control and binge eating patterns [80].
Anxiety
Obesity is a significant risk factor for anxiety in children and adolescents. Data from Lindberg and colleagues (2020) studying children and adolescents found that 43% of girls with obesity had a higher risk of anxiety as compared to girls of normal weight with similar findings in boys. The authors suggest that environmental, genetic, and/or physiological factors may be a link between obesity and anxiety [68]. There is also evidence of ADHD symptoms predicting emotional symptoms, including anxiety, from childhood up to young adulthood [81••], with the co-occurrence of ADHD and anxiety symptoms being identified in children as young as preschool age [82]. The combination of ADHD and anxiety symptoms appears to result in unique patterns of impairment with children performing worse on working memory tasks and reporting more physical anxiety symptoms than children with ADHD but not anxiety [83]. Because of the clear associations between obesity, ADHD, and anxiety in the pediatric population, clinicians are advised to consider each disorder independently and to coordinate treatment of these coexisting diseases.
Role of Inflammation
There is growing data regarding chronic inflammation that may link ADHD and obesity together. Inflammation may have a negative impact on the brain centers of attention and executive function through the impact of cortisol and insulin resistance. Studies suggest shared metabolic perturbations, including insulin resistance and abnormal leptin and ghrelin levels, through stress and sleep dysfunction in both disease states, as well as changes in leptin and adiponectin impacted by changes in inflammatory cytokines [19••, 84]. Elevated inflammatory markers (IL-6, TNF-alpha) are related to worsening hyperactivity and impulsivity symptoms seen in ADHD as well as insulin resistance seen in obesity [27, 79•]. Additionally, researchers note an association between inflammatory cytokines and ADHD symptoms in children and adolescents with obesity [27].
Genetic Underpinnings
Several research studies have uncovered a genetic association between obesity and ADHD. A study by Barker and colleagues (2019) found a relationship between polygenic risk scores (PRS) for ADHD impulsivity and elevated BMI [85•]. Patte and colleagues (2016) found that patients with ADHD and genetic profiles associated with increased dopamine receptor activation in the reward center were associated with elevated BMI and reward-based eating patterns [64]. Hanc et al. (2018) noted that overweight in boys with ADHD was linked to polymorphism in three candidate genes (DRD4, SNAP25, 5HTR2A) [86]. Finally, Albayrak and colleagues found an association with risk alleles for obesity and the ADHD traits of inattention, hyperactivity, and impulsivity justifying further research into a shared genetic background of ADHD and obesity [87]. Ongoing genetic research, particularly for pediatric severe obesity and ADHD, will improve clinical management as well as reduce stigma allowing providers and patients to approach these disorders with improved understanding and informed decision-making.
Discussion
There is increasing evidence for the relationship between obesity and ADHD in the pediatric population. Our brief review of the pathophysiology of both diseases reveals potential shared pathways. A more detailed understanding of these pathophysiologic intersections of obesity and ADHD can allow for improvement in clinical application and management for both diagnoses. Understanding of pathophysiology may reduce bias and stigma for both disorders, which is vital to engage patients and families, as well as clinical providers, involved in the care of these patients. Enhanced awareness can facilitate recommended evaluation for other co-occurring disorders including sleep dysregulation, LOC-ED/BED, and anxiety. Providers working across multiple specialties should collaboratively implement focused screening, assessment, and shared treatment strategies for these co-occurring conditions.
Movement toward precision medicine will assist in identifying unique phenotypes of symptoms that require tailored treatment modalities. For example, early identification of patients presenting with obesity with or without ADHD may represent such phenotypes. The research presented here highlights the increased risk of developing obesity in patients with ADHD, those with sleep dysfunction, as well as an awareness that obesity and ADHD present increased risk for sleep dysfunction, LOC-ED/BED, and anxiety. This knowledge assists clinicians to address these co-occurring conditions with appropriate screening and discussion with the family using a collaborative model with professional colleagues for symptom management.
Treatment Recommendations
Based on the evidence reviewed, we have identified clinical information that is essential to gather, along with focused screening tools, to best assess for obesity, ADHD, and frequently co-occurring disorders (Table 1). We also present an algorithm outlining decision pathways for patients with obesity and with and without ADHD (Fig. 1). Essential for an understanding of how best to address patients with obesity and ADHD is whether these patients are impacted by sleep dysfunction/disorders. There is evidence that specific sleep patterns among those with ADHD are associated with increased risk for obesity (e.g., “eveningness type) [74, 75]. Furthermore, investigators hypothesize that treating sleep dysfunction could eliminate ADHD symptoms as illustrated in an early study by Chervin and colleagues where 81% of youth with ADHD and evidence of snoring achieved resolution of attention symptoms once sleep issues were addressed [88]. Considering shared pathophysiology in addressing multiple diagnoses may lead to a single intervention with dual benefits. For example, if a patient with obesity in a pediatric weight management clinic screens positive for both attention/learning concerns as well as sleep dysfunction, the provider may recommend a sleep study. If positive and successfully treated, data suggests for some patients, this could improve attention as well as obesity by addressing the sleep disordered breathing shared across both diagnoses. A further understanding of sleep dysfunction in relation to increased risk of ADHD and obesity suggests that there may be unique pathways of symptoms and unique phenotypes which would guide management strategies.
The literature also suggests that LOC-ED/BED is common among children and adolescents with ADHD as well as with obesity [37, 67•]. From a pathophysiology standpoint, executive functioning deficits, immediate reward signaling, and/or metabolic derangement may lead to reward center dysregulation, driving the relationship among LOC-ED/BED, ADHD, and obesity. With this knowledge, providers have an opportunity to screen for signs of LOC-ED/BED when children present with attentional dysregulation and/or obesity, which can improve and prioritize care. Awareness of the need to ask about eating patterns, satiety and hunger signaling, binge eating, and frequent food cravings in these cohorts can initiate further discussion about symptoms that may otherwise not be a focus of the consult or intervention. A notable example of treatment targeting multiple symptoms would be the use of lisdexamfetamine, FDA approved for children with ADHD and for adults with ADHD and/or BED, as an evidence informed medication for both ADHD and LOC-ED/BED [89,90,91,92]. Evidence suggests that a significant number of children with obesity who seek treatment meet criteria for anxiety and that eating pathology is significantly associated with severity of anxiety symptoms [93]. Thus, pediatric providers may also consider screening for co-occurring mental health symptoms, particularly when obesity and LOC-ED/BED are both present.
It should be noted that the behavioral and lifestyle strategies critical for obesity management may be especially challenging for children with ADHD and their families to implement in daily life. With this in mind and in conjunction with the evidence base for ADHD intervention strategies [94] and clinical practice guidelines [95], there would be a strong recommendation for providers to be cognizant of the need for structure and predictability within any obesity intervention program. Examples of increased structure could include a specific plan or hierarchy of lifestyle education that is provided to families, as well as a consistent flow to appointments (e.g., initially starting with basic education and transitioning to implementation of the strategies for each family). Consideration may be given to patient goals with providers encouraging changes that may be easier for the family initially and then moving to goals that are more challenging. Similarly, goals will be more readily achievable, if they are short term (e.g., assessing change over a one-week period) and can be easily adjusted over time (e.g., discussing challenges that may have arisen and making changes if the initial goal is not feasible). Patients and providers will likely need to identify strategies for managing executive dysfunction including opportunities for daily reminders (e.g., phone alert to take medication at a specific time), utilizing organization supports (e.g., planners, snack lists), and introducing schedules that maximize the likelihood of follow through/completion (e.g., times of day to be physically active). Strategies for managing attentional dysregulation may also be necessary for addressing behavior in clinic with providers encouraging movement breaks and providing incentives for participating in lifestyle education/curriculum. While these ideas are not an exhaustive list of recommendations, the above discussion should alert providers to the types of considerations necessary for supporting optimal outcomes for children with obesity and ADHD.
Future Research
The opportunity to increase education around the relationship between obesity and ADHD and the key co-occurring disorders outlined in this review supports earlier screening and enhanced interventions that may change the trajectory for both disorders. Our relatively limited understanding of the complex pathophysiology connecting obesity and ADHD demands additional research and clinical trials to better elucidate pathophysiology. This understanding will lead to the development of clinical guidelines and algorithms to assist clinicians in providing the best evidence-based care. These algorithms will inform practical clinical interventions, direct order of intervention implementation, and indicate when to initiate a multifaceted approach team approach.
Conclusion
The co-occurrence of pediatric obesity and ADHD is increasingly supported in scientific literature and suggests shared pathophysiology. Increased knowledge and awareness about the relationship between ADHD and obesity can direct intervention efforts and improve health outcomes, particularly if providers are aware of the increased likelihood of sleep dysregulation, LOC-ED/BED, and anxiety. This review provides a screening/assessment, referral, and treatment algorithm to guide interdisciplinary teams and primary medical homes. Both developmental and obesity interdisciplinary teams can serve as key resources to the primary care provider when patients are presenting with these complex diagnoses to build on therapies and offer additional insights and expertise. It is our hope that this targeted multimodal approach will allow a more coordinated and cohesive plan across healthcare systems to address disease dysregulation from multiple co-occurring diseases in children and adolescents.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. 2018;5:175–86. https://doi.org/10.1016/S2215-0366(17)30167-0.
Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176–85.
Kim J, Lee GH, Sung SM, Jung DS, Pak K. Prevalence of attention deficit hyperactivity disorder symptoms in narcolepsy: a systematic review. Sleep Med. 2020;65:84–8. https://doi.org/10.1016/j.sleep.2019.07.022.
Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92(2):251–65. https://doi.org/10.1016/j.mayocp.2016.09.017.
Ranum BM, Wichstrøm L, Pallesen S, Falch-Madsen J, Halse M, Steinsbekk S. Association between objectively measured sleep duration and symptoms of psychiatric disorders in middle childhood. JAMA Netw Open. 2019;2(12):e1918281. Published 2019 Dec 2. https://doi.org/10.1001/jamanetworkopen.2019.18281.
Sciberras E, Efron D, Patel P, Mulraney M, Lee KJ, Mihalopoulos C, et al. Does the treatment of anxiety in children with attention-deficit/hyperactivity disorder (ADHD) using cognitive behavioral therapy improve child and family outcomes? Protocol for a randomized controlled trial. BMC Psychiatry. 2019;19(1):359. https://doi.org/10.1186/s12888-019-2276-3.
Sharma V, Coleman S, Nixon J, Sharples L, Hamilton-Shield J, Rutter H, et al. A systematic review and meta-analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years. Obes Rev. 2019;20(10):1341–9. https://doi.org/10.1111/obr.12904.
Obesity. World Health Organization. https://www.who.int/health-topics/obesity#tab=tab_1 Updated 2020. Accessed Jun 30, 2020.
Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27.
Gulati AK, Kaplan DW, Daniels SR. Clinical tracking of severely obese children: a new growth chart. Pediatrics. 2012;130(6):1136–40.
Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–712. https://doi.org/10.1161/CIR.0b013e3182a5cfb3.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington: Author.
Visser SN, Zablotsky B, Holbrook JR, Danielson ML, Bitsko RH. Diagnostic experiences of children with attention-deficit/hyperactivity disorder. Natl Health Stat Rep. 2015;81:1–7.
Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484–500. https://doi.org/10.1097/CHI.0b013e31819c23d0.
Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56(3):345–65. https://doi.org/10.1111/jcpp.12381.
Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994–1001. https://doi.org/10.1542/peds.2014-3482.
Wolraich ML, McKeown RE, Visser SN, Bard D, Cuffe S, Neas B, et al. The prevalence of ADHD: its diagnosis and treatment in four school districts across two states. J Atten Disord. 2014;18(7):563–75. https://doi.org/10.1177/1087054712453169.
Rowland AS, Skipper BJ, Umbach DM, Rabiner DL, Campbell RA, Naftel AJ, et al. The prevalence of ADHD in a population-based sample. J Atten Disord. 2015;19(9):741–54. https://doi.org/10.1177/1087054713513799.
•• Cortese S. The association between ADHD and obesity: intriguing, progressively more investigated, but still puzzling. Brain Sci. 2019;9(10):256. https://doi.org/10.3390/brainsci9100256Most recent review of literature describing the association between obesity and ADHD.
Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Peñalver C, Rohde LA, Faraone SV. Association between ADHD and obesity: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(1):34–43. https://doi.org/10.1176/appi.ajp.2015.15020266.
Nigg JT, Johnstone JM, Musser ED, Long HG, Willoughby MT, Shannon J. Attention-deficit/hyperactivity disorder (ADHD) and being overweight/obesity: New data and meta-analysis. Clin Psychol Rev. 2016;43:67–79. https://doi.org/10.1016/j.cpr.2015.11.005.
Khalife N, Kantomaa M, Glover V, Tammelin T, Laitinen J, Ebeling H, et al. Childhood attention-deficit/hyperactivity disorder symptoms are risk factors for obesity and physical inactivity in adolescence. J Am Acad Child Adolesc Psychiatry. 2014;53(4):425–36. https://doi.org/10.1016/j.jaac.2014.01.009.
Aguirre Castaneda RL, Kumar S, Voigt RG, Leibson CL, Barbaresi WJ, Weaver AL, et al. Childhood attention-deficit/hyperactivity disorder, sex, and obesity: a longitudinal population-based study. Mayo Clin Proc. 2016;3:61. https://doi.org/10.1016/j.mayocp.2015.09.017.
Bowling AB, Tiemeier HW, Jaddoe VWV, Barker ED, Jansen PW. ADHD symptoms and body composition changes in childhood: a longitudinal study evaluating directionality of associations. Pediatr Obes. 2018:567–75. https://doi.org/10.1111/ijpo.12288.
Seymour KE, Reinblatt SP, Benson L, Carnell S. Overlapping neurobehavioral circuits in ADHD, obesity, and binge eating: evidence from neuroimaging research. CNS Spectr. 2015;20(4):401–11. https://doi.org/10.1017/S1092852915000383.
Martins-Silva T, Vaz JDS, Hutz MH, Salatino-Oliveira A, Genro JP, Hartwig FP, et al. Assessing causality in the association between attention-deficit/hyperactivity disorder and obesity: a Mendelian randomization study. Int J Obes. 2019;43(12):2500–8. https://doi.org/10.1038/s41366-019-0346-8.
Cortese S, Angriman M, Comencini E, Vincenzi B, Maffeis C. Association between inflammatory cytokines and ADHD symptoms in children and adolescents with obesity: a pilot study. Psychiatry Res. 2019;278:7–11. https://doi.org/10.1016/j.psychres.2019.05.030.
Nousen EK, Franco JG, Sullivan EL. Unraveling the mechanisms responsible for the comorbidity between metabolic syndrome and mental health disorders. Neuroendocrinology. 2013;98(4):254–66. https://doi.org/10.1159/000355632.
•• Berthoud HR, Münzberg H, Morrison CD. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology. 2017;152(7):1728–38. https://doi.org/10.1053/j.gastro.2016.12.050New conceptual framework of human energy regulation addressing how hedonic controls interact with homeostatic controls to regulate body weight in a flexible and adaptive manner that takes environmental conditions into account.
Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, et al. Obesity pathogenesis: an endocrine society scientific statement. Endocr Rev. 2017;4:267–96. https://doi.org/10.1210/er.2017-00111.
Roh E, Kim MS. Brain regulation of energy metabolism. Endocrinol Metab (Seoul). 2016;31(4):519–24. https://doi.org/10.3803/EnM.2016.31.4.519.
Verbeken S, Braet C, Goossens L, van der Oord S. Executive function training with game elements for obese children: a novel treatment to enhance self-regulatory abilities for weight-control. Behav Res Ther. 2013;51(6):290–9. https://doi.org/10.1016/j.brat.2013.02.006.
Davis C, Levitan RD, Muglia P, Bewell C, Kennedy JL. Decision-making deficits and overeating: a risk model for obesity. Obes Res. 2004;12(6):929–35. https://doi.org/10.1038/oby.2004.113.
Yang Y, Shields GS, Guo C, Liu Y. Executive function performance in obesity and overweight individuals: a meta-analysis and review. Neurosci Biobehav Rev. 2018;84:225–44. https://doi.org/10.1016/j.neubiorev.2017.11.020.
Laurent JS, Watts R, Adise S, Allgaier N, Chaarani B, Garavan H, et al. Associations among body mass index, cortical thickness, and executive function in children. JAMA Pediatr. 2019;174(2):170–7. https://doi.org/10.1001/jamapediatrics.2019.4708.
Heinitz S, Reinhardt M, Piaggi P, Weise CM, Diaz E, Stinson EJ, et al. Neuromodulation directed at the prefrontal cortex of subjects with obesity reduces snack food intake and hunger in a randomized trial. Am J Clin Nutr. 2017;106(6):1347–57. https://doi.org/10.3945/ajcn.117.158089.
Münzberg H, Qualls-Creekmore E, Yu S, Morrison CD, Berthoud HR. Hedonics act in unison with the homeostatic system to unconsciously control body weight. Front Nutr. 2016;3:6. https://doi.org/10.3389/fnut.2016.00006.
Brooks SJ, Cedernaes J, Schiöth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One. 2013;8(4):e60393. https://doi.org/10.1371/journal.pone.0060393.
Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes. 2010;34(10):1494–500. https://doi.org/10.1038/ijo.2010.84.
Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev. 2013;37(9 Pt A):2047–58. https://doi.org/10.1016/j.neubiorev.2012.12.001.
Blum K, Thanos PK, Gold MS. Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol. 2014;5:919. Published 2014 Sep 17. https://doi.org/10.3389/fpsyg.2014.00919.
Zhang Y, Liu J, Yao J, Ji G, Qian L, Wang J, et al. Obesity: pathophysiology and intervention. Nutrients. 2014;6(11):5153–83. https://doi.org/10.3390/nu6115153.
Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. 2017;10(6):679–89. https://doi.org/10.1242/dmm.026609.
Cortese S. The neurobiology and genetics of attention-deficit/hyperactivity disorder (ADHD): what every clinician should know. Eur J Paediatr Neurol. 2012;16(5):422–33. https://doi.org/10.1016/j.ejpn.2012.01.009.
•• Koss KJ, Gunnar MR. Annual research review: early adversity, the hypothalamic-pituitary-adrenocortical axis, and child psychopathology. J Child Psychol Psychiatry. 2018;59(4):327–46. https://doi.org/10.1111/jcpp.12784Review of neurobiology of hypothalamic-pituitary-adrenocortical (HPA) axis and relationship between early adversity-HPA axis activity and HPA axis activity-psychopathology. Also, discusses the role of regulatory mechanisms and sensitive periods in development.
Koss KJ, Mliner SB, Donzella B, Gunnar MR. Early adversity, hypocortisolism, and behavior problems at school entry: a study of internationally adopted children. Psychoneuroendocrinology. 2016;66:31–8. https://doi.org/10.1016/j.psyneuen.2015.12.018.
Cherkasova MV, Hechtman L. Neuroimaging in attention-deficit hyperactivity disorder: beyond the frontostriatal circuitry. Can J Psychiatr. 2009;54(10):651–64. https://doi.org/10.1177/070674370905401002.
Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67(5):728–34. https://doi.org/10.1016/j.neuron.2010.08.040.
Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(5):540–9. https://doi.org/10.1001/archpsyc.63.5.540.
Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362(9397):1699–707. https://doi.org/10.1016/S0140-6736(03)14842-8.
Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, et al. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168(2):143–51. https://doi.org/10.1176/appi.ajp.2010.10030385.
Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(7):795–807. https://doi.org/10.1001/archpsyc.63.7.795.
Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, et al. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167(4):397–408. https://doi.org/10.1176/appi.ajp.2009.09030398.
Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47(10):1051–62. https://doi.org/10.1111/j.1469-7610.2006.01671.x.
Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104(49):19649–54. https://doi.org/10.1073/pnas.0707741104.
Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF 3rd, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164(4):647–55. https://doi.org/10.1176/ajp.2007.164.4.647.
Clerkin SM, Schulz KP, Berwid OG, Fan J, Newcorn JH, Tang CY, et al. Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remitted ADHD. Am J Psychiatry. 2013;170(9):1011–9. https://doi.org/10.1176/appi.ajp.2013.12070880.
Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74(8):599–606. https://doi.org/10.1016/j.biopsych.2013.04.007.
Francx W, Oldehinkel M, Oosterlaan J, Heslenfeld D, Hartman CA, Hoekstra PJ, et al. The executive control network and symptomatic improvement in attention-deficit/hyperactivity disorder. Cortex. 2015;73:62–72. https://doi.org/10.1016/j.cortex.2015.08.012.
Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. J Child Psychol Psychiatry. 2008;49(9):958–66. https://doi.org/10.1111/j.1469-7610.2008.01926.x.
Rajendran K, Rindskopf D, O'Neill S, Marks DJ, Nomura Y, Halperin JM. Neuropsychological functioning and severity of ADHD in early childhood: a four-year cross-lagged study. J Abnorm Psychol. 2013;122(4):1179–88. https://doi.org/10.1037/a0034237.
Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e145–57. https://doi.org/10.1016/j.biopsych.2011.02.036.
Prince J. Catecholamine dysfunction in attention-deficit/hyperactivity disorder: an update. J Clin Psychopharmacol. 2008;28(3 Suppl 2):S39–45. https://doi.org/10.1097/JCP.0b013e318174f92a.
Patte KA, Davis CA, Levitan RD, Kaplan AS, Carter-Major J, Kennedy JL. A behavioral genetic model of the mechanisms underlying the link between obesity and symptoms of ADHD. J Atten Disord. 2016;1087054715618793:1425–36. https://doi.org/10.1177/1087054715618793.
Lundahl A, Nelson TD. Attention deficit hyperactivity disorder symptomatology and pediatric obesity: psychopathology or sleep deprivation? J Health Psychol. 2016 Jun;21(6):1055–65. https://doi.org/10.1177/1359105314544991.
Hakim F, Kheirandish-Gozal L, Gozal D. Obesity and altered sleep: a pathway to metabolic derangements in children? Semin Pediatr Neurol. 2015;22(2):77–85. https://doi.org/10.1016/j.spen.2015.04.006.
• Fang CT, Chen VC, Ma HT, Chao HH, Ho MC, Gossop M. Attentional bias, "cool" and "hot" executive functions in obese patients: roles of body mass index, binge eating, and eating style. J Clin Psychopharmacol. 2019;39(2):145–52. https://doi.org/10.1097/JCP.0000000000001016Study examined "cool" (inhibitory control and mental flexibility) and "hot" (affective decision making) executive functions (EFs) in relation to body mass index, binge-eating tendency, and eating styles.
Lindberg L, Hagman E, Danielsson P, Marcus C, Persson M. Anxiety and depression in children and adolescents with obesity: a nationwide study in Sweden. BMC Med. 2020;18(1):30. https://doi.org/10.1186/s12916-020-1498-z.
Chihara Y, Akamizu T, Azuma M, Murase K, Harada Y, Tanizawa K, et al. Among metabolic factors, significance of fasting and postprandial increases in acyl and desacyl ghrelin and the acyl/desacyl ratio in obstructive sleep apnea before and after treatment. J Clin Sleep Med. 2015;11(8):895–905. https://doi.org/10.5664/jcsm.4942.
Gileles-Hillel A, Kheirandish-Gozal L, Gozal D. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat Rev Endocrinol. 2016;12(5):290–8. https://doi.org/10.1038/nrendo.2016.22.
Tankersley CG, O'Donnell C, Daood MJ, Watchko JF, Mitzner W, Schwartz A, et al. Leptin attenuates respiratory complications associated with the obese phenotype. J Appl Physiol (1985). 1998;85(6):2261–9. https://doi.org/10.1152/jappl.1998.85.6.2261.
Aygun AD, Gungor S, Ustundag B, Gurgoze MK, Sen Y. Proinflammatory cytokines and leptin are increased in serum of prepubertal obese children. Mediat Inflamm. 2005;2005(3):180–3. https://doi.org/10.1155/MI.2005.180.
Halbower AC, Mahone EM. Neuropsychological morbidity linked to childhood sleep-disordered breathing. Sleep Med Rev. 2006;10(2):97–107. https://doi.org/10.1016/j.smrv.2005.10.002.
Vgontzas AN, Bixler EO, Chrousos GP. Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Ann N Y Acad Sci. 2006;1083:329–44.
Turkoglu S, Cetin FH. The relationship between chronotype and obesity in children and adolescent with attention deficit hyperactivity disorder. Chronobiol Int. 2019;36:1138–47.
Matherne CE, Tanofsky-Kraff M, Altschul AM, Shank LM, Schvey NA, Brady SM, et al. A preliminary examination of loss of control eating disorder (LOC-ED) in middle childhood. Eat Behav. 2015;18:57–61. https://doi.org/10.1016/j.eatbeh.2015.04.001.
Kalarchian MA, Marcus MD. Psychiatric comorbidity of childhood obesity. Int Rev Psychiatry. 2012;24(3):241–6. https://doi.org/10.3109/09540261.2012.678818.
Furlong TM, Jayaweera HK, Balleine BW, Corbit LH. Binge-like consumption of a palatable food accelerates habitual control of behavior and is dependent on activation of the dorsolateral striatum. J Neurosci. 2014;14:5012–22. https://doi.org/10.1523/JNEUROSCI.3707-13.2014.
• Puhl RM, Himmelstein MS, Pearl RL. Weight stigma as a psychosocial contributor to obesity. Am Psychol. 2020;75(2):274–89. https://doi.org/10.1037/amp0000538Overview of recent evidence examining links between weight stigma and weight-related behaviors and health including health consequences and implications for treatment.
Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–5. https://doi.org/10.1126/science.1171203.
•• Stern A, Agnew-Blais JC, Danese A, Fisher HL, Matthews T, Polanczyk GV, et al. Associations between ADHD and emotional problems from childhood to young adulthood: a longitudinal genetically sensitive study. J Child Psychol Psychiatry. 2020. https://doi.org/10.1111/jcpp.13217Review of developmental associations between ADHD and childhood emotional problems; examined the genetic and environmental contributions.
Overgaard KR, Aase H, Torgersen S, Zeiner P. Co-occurrence of ADHD and anxiety in preschool children. J Atten Disord. 2016;20(7):573–80. https://doi.org/10.1177/1087054712463063.
Jarrett MA, Wolff JC, Davis TE 3rd, Cowart MJ, Ollendick TH. Characteristics of children with ADHD and anxiety. J Atten Disord. 2016;20(7):636–44. https://doi.org/10.1177/1087054712452914.
Özcan Ö, Arslan M, Güngör S, Yüksel T, Selimoğlu MA. Plasma leptin, adiponectin, neuropeptide Y levels in drug naive children with ADHD. J Atten Disord. 2018;22(9):896–900. https://doi.org/10.1177/1087054715587095.
• Barker ED, Ing A, Biondo F, Jia T, Pingault JB, Du Rietz E, et al. Do ADHD-impulsivity and BMI have shared polygenic and neural correlates? Mol Psychiatry. 2019. https://doi.org/10.1038/s41380-019-0444-yExamined the neural and polygenic correlates between obesity and ADHD; found a common neural substrate that may account for shared genetic underpinnings of ADHD and obesity along with the manifestation of their (observable) phenotypic association.
Hanc T, Dmitrzak-Weglarz M, Borkowska A, Wolanczyk T, Pytlinska N, Rybakowski F, et al. Overweight in boys with ADHD is related to candidate genes and not to deficits in cognitive functions. J Atten Disord. 2018;22:1158–72.
Albayrak Ö, Pütter C, Volckmar AL, Cichon S, Hoffmann P, Nöthen MM, et al. Psychiatric GWAS Consortium: ADHD Subgroup. Common obesity risk alleles in childhood attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(4):295–305. https://doi.org/10.1002/ajmg.b.32144.
Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20(12):1185–92. https://doi.org/10.1093/sleep/20.12.1185.
Guerdjikova AI, Blom TJ, Mori N, Matthews A, Cummings T, Casuto LL, et al. Lisdexamfetamine in pediatric binge eating disorder: a retrospective chart review. Clin Neuropharmacol. 2019;42(6):214–6. https://doi.org/10.1097/WNF.0000000000000367.
Fleck DE, Eliassen JC, Guerdjikova AI, Mori N, Williams S, Blom TJ, et al. Effect of lisdexamfetamine on emotional network brain dysfunction in binge eating disorder. Psychiatry Res Neuroimaging. 2019;286:53–9. https://doi.org/10.1016/j.pscychresns.2019.03.003.
Guerdjikova AI, Mori N, Casuto LS, McElroy SL. Novel pharmacologic treatment in acute binge eating disorder - role of lisdexamfetamine. Neuropsychiatr Dis Treat. 2016;12:833–41. https://doi.org/10.2147/NDT.S80881.
Srivastava G, O'Hara V, Browne N. Use of lisdexamfetamine to treat obesity in an adolescent with severe obesity and binge eating. Children (Basel). 2019;6(2):22. https://doi.org/10.3390/children6020022.
Sheinbein DH, Stein RI, Hayes JF, Brown ML, Balantekin KN, Conlon RPK, et al. Factors associated with depression and anxiety symptoms among children seeking treatment for obesity: A social-ecological approach. Pediatr Obes. 2019;14(8):e12518. https://doi.org/10.1111/ijpo.12518.
Eiraldi RB, Mautone JA, Power TJ. Strategies for implementing evidence-based psychosocial interventions for children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2012;21(1):145-x. https://doi.org/10.1016/j.chc.2011.08.012.
Wolraich ML, Hagan JF Jr, Allan C, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents [published correction appears in Pediatrics. 2020 Mar;145(3):]. Pediatrics. 2019;144(4):e20192528. https://doi.org/10.1542/peds.2019-2528.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Childhood Obesity
Rights and permissions
About this article
Cite this article
O’Hara, V.M., Curran, J.L. & Browne, N.T. The Co-occurrence of Pediatric Obesity and ADHD: an Understanding of Shared Pathophysiology and Implications for Collaborative Management. Curr Obes Rep 9, 451–461 (2020). https://doi.org/10.1007/s13679-020-00410-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-020-00410-0