Abstract
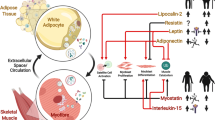
The crosstalk between adipose tissue and skeletal muscle has gained considerable interest, since this process, specifically in obesity, substantially drives the pathogenesis of muscle insulin resistance. In this review, we discuss novel concepts and targets of this bidirectional organ communication system. This includes adipo-myokines like apelin and FGF21, inflammasomes, autophagy, and microRNAs (miRNAs). Literature analysis shows that the crosstalk between fat and muscle involves both extracellular molecules and intracellular organelles. We conclude that integration of these multiple crosstalk elements into one network will be required to better understand this process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological crosstalk occurs between different organs either directly in a paracrine way at tissue interfaces or via biological fluids in an endocrine way. Endocrine organs such as adipose tissue and skeletal muscle express and subsequently release a wide range of proteins and other molecules into the circulation, thereby participating in a multidirectional crosstalk. Secretion of the so-called adipokines or myokines by adipose tissue or skeletal muscle, respectively, plays a crucial role in the regulation of multiple organs or tissues in terms of energy homeostasis [1]. Based on an intensive search of literature on novel concepts and targets for crosstalk between adipose tissue and skeletal muscle from the last 3 years, we selected four innovative topics for this review: 1. While adipokines and myokines are known to be important endocrine mediators in the field of metabolic homeostasis, recent advances have been made to identify common molecules to be released by both tissues, the so-called adipo-myokines. These molecules may play a yet underestimated role in the bidirectional crosstalk between adipose tissue and muscle. 2. Inflammasomes as specialized organelles may contribute to an endocrine crosstalk between adipose tissue and skeletal muscle in a way that is still incompletely understood. 3. Very recently, autophagy has been recognized as an additional mediator of organ crosstalk. 4. In addition to metabolites and proteins, circulating microRNAs have been associated with regulation of metabolic homeostasis, representing a further level of organ communication.
Adipo-myokines—Mediators of Bidirectional Crosstalk
Skeletal muscle cells and adipocytes secrete a variety of metabolically active proteins called myokines or adipokines, respectively. Besides, a new term referred to as adipo-myokines has been introduced. Adipo-myokines are expressed and released from both skeletal muscle and adipose tissue. Secreted adipo-myokines can affect the organ itself in a paracrine or autocrine manner. Additionally, they have endocrine effects, contributing to a bidirectional crosstalk. Gene expression of adipo-myokines is shifted in obesity or after exercise, thus affecting the circulating pool and the crosstalk between adipose tissue and skeletal muscle.
Besides the well-characterized adipo-myokines such as IL-6 and IL-15, several factors have been identified as adipo-myokines in recent years, including follistatin-related protein 1 (Fstl-1), plasminogen activator inhibitor 1 (PAI1), myostatin, and many more [2]. In particular, the adipo-myokines apelin and fibroblast growth factor 21 (FGF21) gained considerable interest and will be presented in detail here.
Apelin
Apelin is a peptide binding to the apelin receptor (APJ), a G-protein-coupled receptor [3]. APJ expression is ubiquitously including skeletal muscle and adipose tissue [4], so that both tissues display possible targets. Apelin has been suggested to have cardio-protective impact [5] and is an important player in energy metabolism with anti-obesity and anti-diabetic properties [6]. Until recently, apelin has been described as an adipokine expressed and released from adipocytes [7]. Apelin expression in adipose tissue as well as circulating levels of apelin are elevated in obesity [8]. Furthermore, protein expression of apelin in adipocytes and the circulation have been reported to be increased in mice associated with hyperinsulinemia [8]. In addition, messenger RNA (mRNA) expression of apelin was induced by insulin in murine 3T3-L1 adipocytes [9]. Injection of apelin lowered blood glucose levels in mice [10] and stimulated glucose uptake in adipose tissue [11]. Amplified glucose uptake was also observed in human adipose tissue [12]. Besides, treatment of apelin diminished triglyceride amounts in adipose tissue in vivo [10] and decreased lipid storage in 3T3-L1 preadipocytes [13]. In summary, these findings disclose the fundamental role of apelin in glucose and lipid metabolism.
Recently, apelin was identified as a myokine, expressed and secreted from human myotubes [14•]. Apelin expression is upregulated due to exercise in obese subjects, and apelin mRNA expression in muscle is positively related to improved insulin sensitivity. In addition, apelin improved glucose uptake in C2C12 myotubes and skeletal muscle insulin sensitivity in mice [11]. These results were supported by observations from apelin-deficient mice, which had reduced insulin sensitivity and were hyperinsulinemic. Indeed, administration of apelin to these mice led to improved insulin sensitivity [15]. Disruption of apelin also had a negative impact on glucose uptake and Akt-phosphorylation in skeletal muscle [15]. Furthermore, apelin is suggested to promote fatty acid oxidation in skeletal muscle [16]. These data indicate that circulating apelin, derived from muscle and adipose tissue, has beneficial effects on lipid and glucose metabolism in both tissues.
FGF21
FGF21 belongs to the fibroblast growth factor (FGF) family which contains 22 members in humans. FGF members play an important role in the regulation of whole-body metabolism and development. They are expressed ubiquitously in almost every tissue and act in a paracrine as well as endocrine manner. Endocrine FGFs, like FGF15/19, FGF21, and FGF23, signal through high-affinity FGF receptors (FGFR), depending on α-klotho or β-klotho as a co-receptor [17]. FGF21 in particular requires the co-receptor β-klotho [18] to bind and fully activate FGFR.
FGF21 is mainly expressed and secreted by the liver [19, 20], but also skeletal muscle cells [21–23] and adipocytes [24] have been reported as sources for FGF21. Moreover, these tissues display suitable targets for FGF21 action since they express β-klotho and FGFR [25, 26].
FGF21 serum levels are upregulated in subjects with impaired glucose tolerance, type 2 diabetic patients, and obese nondiabetic subjects [27]. Furthermore, enhanced FGF21 serum levels correlate with high expression of FGF21 in subcutaneous adipose tissue [28], implicating that adipose tissue is a relevant source. FGF21 reduces lipolysis in vivo [29] and in vitro [30], potentially interfering with growth hormone (GH)-mediated lipolysis [29]. Moreover, FGF21 stimulates glucose uptake in human adipocytes [31, 32] in relation to FGF21-induced GLUT1 expression [31]. Thus, based on these data and in line with animal studies [33, 34], FGF21 arises as a potential factor to improve insulin sensitivity in adipose tissue, indicating that elevated levels of FGF21 in obesity could be a compensatory response or resistance to FGF21.
FGF21 has also been described as an exercise-regulated myokine, expressed and secreted by skeletal muscle cells, although at lower levels compared to adipose tissue and liver. In young healthy women, levels of FGF21 in serum were elevated after long-term exercise over 2 weeks, but not enhanced after acute exercise [35]. Controversially, in healthy male subjects, acute exercise raised circulating levels of FGF21 [36]. Another study consisting of nondiabetic obese women showed even reduced FGF21 plasma levels after a 12-month exercise program [37]. Furthermore, Kim et al. observed increased serum FGF21 after a single bout of acute exercise in healthy men and mice. Nevertheless, increase in FGF21 expression could not be detected in WAT or skeletal muscle but was elevated in liver of mice after acute exercise. However, in these studies, the potential source of circulating FGF21 was not determined. It has been suggested that FGF21 plays a special role in mediating the stress response from skeletal muscle to adipose tissue by modulating lipolysis and gene expression in adipose tissue [38]. An adipokine upregulated by FGF21 is adiponectin, potentially mediating the positive metabolic effects of FGF21 on whole-body glucose metabolism and insulin sensitivity [38, 39•]. Functions of FGF21 in skeletal muscle itself might be the induction of insulin-stimulated glucose uptake in a paracrine manner [19] and the protection from insulin resistance [39•, 40].
FGF21 acts in a paracrine fashion by regulating metabolism in tissues of its origin such as liver, adipose tissue, and skeletal muscle. Furthermore, it operates as an endocrine factor released in the circulation and arises as an important player in the regulation of energy homeostasis and whole-body glucose and lipid metabolism. Although its impact on tissues is still not completely understood, this factor might be of interest as a potential therapeutic agent to treat metabolic diseases.
Inflammasomes—Critical Players in Adipose Tissue Crosstalk
Specialized organelles called inflammasomes, especially NLR family, pyrin domain-containing 3 (NLRP3) inflammasomes, have gained much attention over the last years in research on metabolic diseases. This organelle is able to sense a wide range of exogenous and endogenous danger signals via pattern recognition receptors in order to translate these signals into activation of caspase 1 and subsequent production of IL-1β from pro-IL-1β. Inflammasomes form on demand by assembly of multiple proteins into a multiprotein complex containing one or more cofactors such as PYD and CARD domain-containing protein (PYCARD, ASC) and caspase 1. Originally, inflammasomes were only described to be present in immune cells as part of the innate immune response. Today, inflammasomes have been described in various cell types including adipocytes, hepatocytes, and pancreatic β-cells. Inflammasomes are major players in regulating adipose tissue inflammation as they act as cellular sensors for obesity-associated activators of caspase-1 such as palmitate and ceramide. Subsequently, inflammasomes act as regulators of inflammation and associated effects on insulin signaling in other organs including skeletal muscle. Inflammasomes are activated in adipose tissue of obese mouse models [41] as well as in obese humans [42]. In the latter study in humans, induction of inflammasomes in adipose tissue is clearly associated with insulin resistance and impaired glucose metabolism in coincidence with a T cell shift towards T helper cells of the pro-inflammatory TH1 type. Accordingly, weight loss achieved either by caloric restriction or by exercise reduces inflammasome activity [43]. NLRP3 inflammasome levels also positively correlate with glycemia in type 2 diabetic patients after weight loss induced either by caloric restriction or by exercise [43].
Within adipose tissue, inflammasomes are also important regulators of adipocyte function and insulin sensitivity, which has been demonstrated first in caspase 1 and NLRP3 knockout mice [41, 44]. Lacking inflammasome activity in these models protects from insulin resistance. Inflammasomes are also critical for the induction of obesity [44]. Inflammasomes are crosstalk mediators, a concept that has not been proven directly for the adipose tissue and muscle crosstalk but for a crosstalk between adipose tissue and liver. Activation of inflammasomes, specifically in macrophages, contributes to the development of insulin resistance in hepatocytes in vitro [45]. Similarly, selective activation of inflammasomes in adipocytes also leads to impaired insulin signaling in hepatocytes [46]. A functional link between adipose tissue inflammation in obesity and insulin resistance in skeletal muscle can be deduced from activation of inflammasomes specifically in immune cells within adipose tissue which leads to deteriorated insulin tolerance in mice [45]. Indirect evidence comes from inflammasome-deficient mice that have significantly improved insulin signaling in skeletal muscle, adipose tissue and liver in diet-induced obesity demonstrating that all major insulin target tissues are equally involved.
Less is known about the role of inflammasomes in skeletal muscle. Nevertheless, inflammation in skeletal muscle can also result in production of IL-1β [47]. After induction of IL-1β by injury, this cytokine stimulates myoblast proliferation [48]. In myotubes, IL-1β stimulates catabolism [49]. Furthermore, inflammation in skeletal muscle induces amyloid precursor protein (APP) and subsequent intracellular aggregation of β-amyloid in parallel to IL-1β induction [50]. This is an interesting observation, as inflammasome activation is also related to Alzheimer disease where aggregation of β-amyloid causes tissue degeneration, which may be translated to skeletal muscle dysfunction. In addition, IL-1β can also be induced by exercise in vitro [51]. In the light of IL-1β and inflammasomes being also activated in skeletal muscle, therapeutic approaches to modulate inflammasome activation in metabolic diseases have been tested. Blocking of IL-1β signaling by anakinra improves glycemia in type 2 diabetes [52], but does not alter skeletal muscle gene expression [53]. It is currently discussed that IL-1β and inflammasomes are not playing the major role in skeletal muscle in the context of metabolic diseases related to obesity. However, data obtained in animal models clearly reveal a novel metabolic function of the inflammasomes in adipose tissue. Overall, these data suggest that pharmacological modulation of inflammasome activation in obese patients or patients with type 2 diabetes could be a strategy to improve the metabolic function of adipose tissue. This might then contribute to recover insulin sensitivity in skeletal muscle, adding a novel level of organ crosstalk.
Autophagy—a New Element in Organ Crosstalk
Autophagy, from Greek “self-eating,” describes a highly conserved catabolic process of degrading and recycling of misfolded or damaged proteins and organelles in lysosomes. Autophagy is an enduring process, regulated for example by amino acid and glucose availability in the basal state [54, 55•], but can also be modulated by diverse factors such as starvation or other kinds of stress [56]. Under these circumstances, the assembly of phagosomes is strongly induced [57, 58] to provide energy and metabolic substrates to the cell. Three different kinds of autophagy, namely chaperone-mediated autophagy, micro-autophagy and macro-autophagy, can be distinguished. However, macro-autophagy is mostly referred to “autophagy” and will be the subject of this section. As a complicated process, autophagy is separated into several steps including the formation of the so-called autophagosome surrounding the target substrate, fusion with the lysosome, degradation, and amino and fatty acid generation. These steps are closely regulated by a group of autophagy-related (Atg) proteins.
Currently, autophagy has gained a lot of interest in the field of energy homeostasis and the pathophysiology of the metabolic syndrome [59]. Impaired autophagy has differential effects as it can on one hand deteriorate metabolic control [45, 60] while it can also lead to improved insulin sensitivity together with beneficial effects in the context of obesity and type 2 diabetes [55•]. In the obese state, autophagy in macrophages is impaired which is accompanied by defective mitophagy and increased IL-1β production by activated NLRP3 inflammasommes [45]. As a result of autophagy-induced IL-1β release, insulin resistance develops in adipose tissue and skeletal muscle. Furthermore, another study shows that inhibition of autophagy leads to enhanced levels of pro-inflammatory cytokines such as IL-6, IL-1β, and IL-8 in adipose tissue explants of mice and humans [61]. Thus, autophagy might control the release of pro-inflammatory cytokines and therefore contribute to the prevention of inflammation. Recently published data also show beneficial effects of autophagy, namely adiponectin-stimulating skeletal muscle autophagy to reduce high-fat diet (HFD)-induced insulin resistance [62].
Interesting insight into the role of autophagy in skeletal muscle and adipocytes on whole-body glucose and lipid metabolism has been obtained by tissue-specific depletion of Atgs in mice. Autophagy deficiency due to skeletal muscle-specific deletion of Atg7 contributes to the improvement of diet-induced insulin resistance and obesity [55•]. Interestingly, impaired autophagy is mechanistically linked to mitochondrial dysfunction and subsequent FGF21 release. Endogenous FGF21 entering the circulation can then act as a mediator of crosstalk between different kinds of tissues by modulating the lipid metabolism. Furthermore, mice with disruption of Atg7 in skeletal muscle show increased β-oxidation and energy expenditure through thermogenic uncoupling. Additionally, the specific knockout of Atg7 in adipose tissue of mice has protective function regarding HFD-induced obesity and glucose tolerance [63]. In detail, impairment of autophagy through Atg7 ablation causes decreased white fat mass, smaller adipocyte size, and higher mitochondrial content potentially coupled to increased energy expenditure in adipose tissue. These animal data are partially contradictory to data obtained in vivo in obese humans and rodents illustrating the need of additional mouse model such as inducible tissue-specific knockouts to clarify the role of autophagy at different disease states.
Taken together, these findings indicate that autophagy deficiency in specific organs affects whole-body metabolism in various ways also involving endocrine mediators. The importance of autophagy is still unclear and has to be further elucidated to benefit from autophagy-related crosstalking mediators, such as FGF21, to prevent and treat obesity and metabolic diseases such as type 2 diabetes.
microRNAs—an Additional Level of Crosstalk
In addition to adipokines and myokines, microRNAs (miRNAs) as a class of small noncoding RNAs increase the complexity of crosstalk between organs in terms of metabolic control [64]. Per definition, miRNAs are 20–22 nucleotides long, single-stranded noncoding RNAs that regulate gene expression at the level of target messenger RNAs (mRNAs). miRNAs bind by base-pairing with complementary sequences within target mRNAs resulting in silencing of target mRNAs by cleavage, destabilization, and less-efficient translation. Today, nearly 2000 different miRNAs are known within the human genome with a constant increase in their number [65]. miRNAs are not only regulated by various stimuli including metabolites, adipokines, and myokines [66], but they also regulate how organs communicate via released factors and thereby modulate metabolic control. Thus, miRNAs are participating in tissue crosstalk in multiple ways, first representing crosstalk molecules themselves altering gene expression in distant organs, second regulating expression of other crosstalk molecules, and third being regulated by other crosstalk molecules.
Both adipose tissue and skeletal muscle release various miRNAs [67]. Although, the overall profile of expressed miRNAs is different between adipose tissue and skeletal muscle, there is also a substantial overlap of common miRNAs for both tissues. Several miRNAs were associated with hypoglycemia in skeletal muscle such as miR-222, miR-27a, miR-195, miR-103, and miR-10b. For miR-222 and miR-27a, regulation by hypoglycemia is also observed in adipocytes as a response to increased glucose levels, which favors the idea of a crosstalk between skeletal muscle and adipose tissue via miRNA in relation to metabolic dysfunction.
Obesity alters miRNA expression in adipose tissue mostly affecting adipogenesis [68]. Several miRNAs have been described to be regulated in the state of insulin resistance and inflammation in adipose tissue as for example miRNA 143 [69], miRNA 223 [70], and miRNA 93 [71•]. One interesting study revealed that the cluster of miR-126/miR-193b/miR-92a control monocyte chemotactic protein-1 (MCP-1) production by transcription factors, indicating that miRNAs can contribute to adipose tissue inflammation and may be important for the development of insulin resistance and potentially type 2 diabetes. miRNA 378 is increased in obesity and type 2 diabetes in mice and reduces adiponectin expression, which might be related to insulin resistance [72]. Another feature of miRNAs in adipose tissue is the fact that miRNA processing in general and several single miRNAs alter distribution and determination of white and brown adipocytes. Ablation of dicer, the enzyme that catalyzes the final maturation of all miRNAs, in adipose tissue of mice leads to partial dystrophy and a whitening of brown adipose tissue demonstrating the importance of miRNAs in adipose tissue development and determination [73]. As for single miRNAs, miRNA 133 is able to induce browning of skeletal muscle precursors in vitro and to increase browning in vivo [74, 75].
miRNA expression is also altered in skeletal muscle in relation to insulin resistance [76]. Unsaturated fatty acids potentially released from adipose tissue upregulate miRNA 29a which then negatively interferes with insulin signaling [77]. Another miRNA, namely miRNA 106b, induces insulin resistance via mitochondrial dysfunction in skeletal muscle cells [78]. This miRNA is also significantly increased in skeletal muscle of patients with type 2 diabetes [76]. Diminished miRNA expression in general in skeletal muscle is observed in aging [79] and might also be related to age-induced metabolic disturbances, a topic that should be further pursued in the future.
Although many miRNAs are described to be involved in adipose tissue inflammation and insulin resistance as well as in skeletal muscle insulin resistance, so far only single miRs have been identified to be real crosstalk molecules. This is the case for miRNA 130b and miRNA 2 [80, 81•]. miRNA 130b might even be of interest for therapeutic use as its delivery by microvesicle to fat cells decreases lipid deposition via the transcription factor PPAR gamma [82]. As only a few miRNAs are already identified as crosstalk molecules, there is a huge potential of identifying novel miRNAs together with their regulators and targets for diagnostic and therapeutic intervention which is not limited to metabolic disease.
Conclusion
Extensive research during the last 20 years has substantially promoted our understanding of how different organs communicate within the body and how this complex process impacts on metabolic homeostasis in both health and disease. Due to its prominent secretory activity, adipose tissue is now considered as a major player in organ crosstalk. However, skeletal muscle also acts as an endocrine organ, and a highly complex multidirectional crosstalk scenario is operating in humans. Classically, protein molecules (adipokines, myokines) released from these tissues are considered to mediate the communication between different organs, but recent evidence points to a much more complex structural hierarchy of organ crosstalk. Thus, intracellular organelles like inflammasomes and autophagosomes have been recognized to regulate organ communication, albeit in an indirect and yet incompletely understood way. Whereas the inflammasome is most important for adipose tissue crosstalk function, autophagy appears to influence the fat/muscle crosstalk in both tissues. Finally, circulating miRNAs constitute an additional element of cellular communication and extend the complexity of this process. The future challenge will be to integrate the different levels of organ crosstalk into one network, to potentially identify new targets of metabolic homeostasis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Trayhurn P, Drevon CA, Eckel J. Secreted proteins from adipose tissue and skeletal muscle—adipokines, myokines and adipose/muscle cross-talk. Arch Physiol Biochem. 2011;117(2):47–56.
Raschke S, Eckel J. Adipo-myokines: two sides of the same coin—mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013;2013:320724.
Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–6.
Hosoya M, Kawamata Y, Fukusumi S, et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem. 2000;275(28):21061–7.
Zhang BH, Guo CX, Wang HX, et al. Cardioprotective effects of adipokine apelin on myocardial infarction. Heart Vessels. 2014;29(5):679–89.
Catalan V, Gomez-Ambrosi J, Rodriguez A, et al. Increased circulating and visceral adipose tissue expression levels of YKL-40 in obesity-associated type 2 diabetes are related to inflammation: impact of conventional weight loss and gastric bypass. J Clin Endocrinol Metab. 2011;96(1):200–9.
Castan-Laurell I, Boucher J, Dray C, et al. Apelin, a novel adipokine over-produced in obesity: friend or foe? Mol Cell Endocrinol. 2005;245(1–2):7–9.
Boucher J, Masri B, Daviaud D, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146(4):1764–71.
Wei L, Hou X, Tatemoto K. Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul Pept. 2005;132(1–3):27–32.
Higuchi K, Masaki T, Gotoh K, et al. Apelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology. 2007;148(6):2690–7.
Dray C, Knauf C, Daviaud D, et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008;8(5):437–45.
Attane C, Daviaud D, Dray C, et al. Apelin stimulates glucose uptake but not lipolysis in human adipose tissue ex vivo. J Mol Endocrinol. 2011;46(1):21–8.
Guo M, Chen F, Lin T, et al. Apelin-13 decreases lipid storage in hypertrophic adipocytes in vitro through the upregulation of AQP7 expression by the PI3K signaling pathway. Med Sci Monit. 2014;20:1345–52.
Besse-Patin A, Montastier E, Vinel C, et al. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes (Lond). 2014;38(5):707–13. The data of this work identify apelin as a new exercise-regulated myokine in humans, involved in the improvement of whole-body insulin sensitivity of obese subjects.
Yue P, Jin H, Aillaud M, et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298(1):E59–67.
Attane C, Foussal C, Le GS, et al. Apelin treatment increases complete fatty acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes. 2012;61(2):310–20.
Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16(2):107–37.
Suzuki M, Uehara Y, Motomura-Matsuzaka K, et al. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol. 2008;22(4):1006–14.
Mashili FL, Austin RL, Deshmukh AS, et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev. 2011;27(3):286–97.
Nishimura T, Nakatake Y, Konishi M, et al. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492(1):203–6.
Izumiya Y, Bina HA, Ouchi N, et al. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008;582(27):3805–10.
Hojman P, Pedersen M, Nielsen AR, et al. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes. 2009;58(12):2797–801.
Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–65.
Muise ES, Azzolina B, Kuo DW, et al. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol. 2008;74(2):403–12.
Fon TK, Bookout AL, Ding X, et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24(10):2050–64.
Keipert S, Ost M, Johann K, et al. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am J Physiol Endocrinol Metab. 2014;306(5):E469–82.
Chavez AO, Molina-Carrion M, Abdul-Ghani MA, et al. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32(8):1542–6.
Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57(5):1246–53.
Chen W, Hoo RL, Konishi M, et al. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem. 2011;286(40):34559–66.
Arner P, Pettersson A, Mitchell PJ, et al. FGF21 attenuates lipolysis in human adipocytes—a possible link to improved insulin sensitivity. FEBS Lett. 2008;582(12):1725–30.
Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–35.
Lee DV, Li D, Yan Q, et al. Fibroblast growth factor 21 improves insulin sensitivity and synergizes with insulin in human adipose stem cell-derived (hASC) adipocytes. PLoS One. 2014;9(11):e111767.
Murata Y, Nishio K, Mochiyama T, et al. Fgf21 impairs adipocyte insulin sensitivity in mice fed a low-carbohydrate, high-fat ketogenic diet. PLoS One. 2013;8(7):e69330.
Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58(1):250–9.
Cuevas-Ramos D, Almeda-Valdes P, Meza-Arana CE, et al. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One. 2012;7(5):e38022.
Kim KH, Kim SH, Min YK, et al. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One. 2013;8(5):e63517.
Yang SJ, Hong HC, Choi HY, et al. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-A levels and arterial stiffness in obese women. Clin Endocrinol (Oxf). 2011;75(4):464–9.
Luo Y, McKeehan WL. Stressed liver and muscle call on adipocytes with FGF21. Front Endocrinol (Lausanne). 2013;4:194.
Lin Z, Tian H, Lam KS, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17(5):779–89. This study describes adipose tissue-derived FGF21 as an inducer of adiponectin with glucose-lowering and insulin-sensitizing impact, coupling adipose tissue to liver and skeletal muscle.
Lee MS, Choi SE, Ha ES, et al. Fibroblast growth factor-21 protects human skeletal muscle myotubes from palmitate-induced insulin resistance by inhibiting stress kinase and NF-kappaB. Metabolism. 2012;61(8):1142–51.
Stienstra R, Joosten LA, Koenen T, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12(6):593–605.
Goossens GH, Blaak EE, Theunissen R, et al. Expression of NLRP3 inflammasome and T cell population markers in adipose tissue are associated with insulin resistance and impaired glucose metabolism in humans. Mol Immunol. 2012;50(3):142–9.
Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–88.
Stienstra R, van Diepen JA, Tack CJ, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108(37):15324–9.
Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12(5):408–15.
Nov O, Kohl A, Lewis EC, et al. Interleukin-1beta may mediate insulin resistance in liver-derived cells in response to adipocyte inflammation. Endocrinology. 2010;151(9):4247–56.
Rawat R, Cohen TV, Ampong B, et al. Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am J Pathol. 2010;176(6):2891–900.
Otis JS, Niccoli S, Hawdon N, et al. Pro-inflammatory mediation of myoblast proliferation. PLoS One. 2014;9(3):e92363.
Li W, Moylan JS, Chambers MA, et al. Interleukin-1 stimulates catabolism in C2C12 myotubes. Am J Physiol Cell Physiol. 2009;297(3):C706–14.
Schmidt J, Barthel K, Wrede A, et al. Interrelation of inflammation and APP in sIBM: IL-1 beta induces accumulation of beta-amyloid in skeletal muscle. Brain. 2008;131(Pt 5):1228–40.
Scheler M, Irmler M, Lehr S, et al. Cytokine response of primary human myotubes in an in vitro exercise model. Am J Physiol Cell Physiol. 2013;305(8):C877–86.
Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–26.
Berchtold LA, Larsen CM, A V, et al. IL-1 receptor antagonism and muscle gene expression in patients with type 2 diabetes. Eur Cytokine Netw. 2009;20(2):81–7.
Galluzzi L, Pietrocola F, Levine B, et al. Metabolic control of autophagy. Cell. 2014;159(6):1263–76.
Kim KH, Jeong YT, Oh H, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19(1):83–92. This study provides an insight into the complicated role of autophagy regarding the regulation of energy homeostasis by use of an autophagy-deficient mouse model. They also highlight the impact of autophagy-induced FGF21 as a mediator of inter-organ crosstalk.
Lum JJ, Bauer DE, Kong M, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120(2):237–48.
Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12.
Deter RL, De DC. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967;33(2):437–49.
Kim KH, Lee MS. Autophagy as a crosstalk mediator of metabolic organs in regulation of energy metabolism. Rev Endocr Metab Disord. 2014;15(1):11–20.
Yang L, Li P, Fu S, et al. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11(6):467–78.
Jansen HJ, Van Essen P, Koenen T, et al. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology. 2012;153(12):5866–74.
Liu Y, Palanivel R, Rai E, et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes. 2015;64(1):36–48.
Zhang Y, Goldman S, Baerga R, et al. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci U S A. 2009;106(47):19860–5.
Dumortier O, Hinault C, Van OE. MicroRNAs and metabolism crosstalk in energy homeostasis. Cell Metab. 2013;18(3):312–24.
Friedlander MR, Lizano E, Houben AJ, et al. Evidence for the biogenesis of more than 1,000 novel human microRNAs. Genome Biol. 2014;15(4):R57.
Zhu L, Shi C, Ji C, et al. FFAs and adipokine-mediated regulation of hsa-miR-143 expression in human adipocytes. Mol Biol Rep. 2013;40(10):5669–75.
Herrera BM, Lockstone HE, Taylor JM, et al. Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes. Diabetologia. 2010;53(6):1099–109.
Hilton C, Neville MJ, Karpe F. MicroRNAs in adipose tissue: their role in adipogenesis and obesity. Int J Obes (Lond). 2013;37(3):325–32.
Takanabe R, Ono K, Abe Y, et al. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochem Biophys Res Commun. 2008;376(4):728–32.
Zhuang G, Meng C, Guo X, et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125(23):2892–903.
Chen YH, Heneidi S, Lee JM, et al. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes. 2013;62(7):2278–86. This manuscript presents a detailed mechanistic study on how a single miRNA can affect insulin sensitivity in polycystic ovary syndrome.
Ishida M, Shimabukuro M, Yagi S, et al. MicroRNA-378 regulates adiponectin expression in adipose tissue: a new plausible mechanism. PLoS One. 2014;9(11):e111537.
Mori MA, Thomou T, Boucher J, et al. Altered miRNA processing disrupts brown/white adipocyte determination and associates with lipodystrophy. J Clin Invest. 2014;124(8):3339–51.
Yin H, Pasut A, Soleimani VD, et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013;17(2):210–24.
Liu W, Bi P, Shan T, et al. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 2013;9(7). e1003626.
Gallagher IJ, Scheele C, Keller P, et al. Integration of microRNA changes in vivo identifies novel molecular features of muscle insulin resistance in type 2 diabetes. Genome Med. 2010;2(2):9.
Yang WM, Jeong HJ, Park SY, et al. Induction of miR-29a by saturated fatty acids impairs insulin signaling and glucose uptake through translational repression of IRS-1 in myocytes. FEBS Lett. 2014;588(13):2170–6.
Zhang Y, Yang L, Gao YF, et al. MicroRNA-106b induces mitochondrial dysfunction and insulin resistance in C2C12 myotubes by targeting mitofusin-2. Mol Cell Endocrinol. 2013;381(1–2):230–40.
Rivas DA, Lessard SJ, Rice NP. et al. Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J. 2014.
He A, Zhu L, Gupta N, et al. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol. 2007;21(11):2785–94.
Wang YC, Li Y, Wang XY, et al. Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia. 2013;56(10):2275–85. This manuscript describes evidence for an endocrine mediation between adipose tissue and skeletal muscle via miRNAs.
Pan S, Yang X, Jia Y, et al. Microvesicle-shuttled miR-130b reduces fat deposition in recipient primary cultured porcine adipocytes by inhibiting PPAR-g expression. J Cell Physiol. 2014;229(5):631–9.
Acknowledgments
This work was supported by the Ministeriumfür Wissenschaft und Forschung des Landes Nordrhein-Westfalen (Ministry of Science and Research of the State of North Rhine-Westphalia) and the Bundesministeriumfür Gesundheit (Federal Ministry of Health).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Ira Indrakusuma, Henrike Sell, and Jürgen Eckel declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Metabolism
Rights and permissions
About this article
Cite this article
Indrakusuma, I., Sell, H. & Eckel, J. Novel Mediators of Adipose Tissue and Muscle Crosstalk. Curr Obes Rep 4, 411–417 (2015). https://doi.org/10.1007/s13679-015-0174-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-015-0174-7