Abstract
Purpose of Review
Skin cancer is one of the leading causes of skin disease burden worldwide. This systematic review provides an overview of the United States (US) and global melanoma and non-melanoma skin cancer (NMSC) epidemiologic studies published in the last 5 years.
Recent Findings
The incidence of melanoma and NMSC continues to rise both in the US and worldwide. The highest global age-standardized incidence rates (ASIRs) for melanoma were Australasia, North America, Eastern Europe, Western Europe, and Central Europe. Various results were reported for the mortality of skin cancer; however, overall, the mortality was higher in men than women and remained relatively stable. Notably, NMSC caused more deaths globally than melanoma, albeit at a slower rate.
Summary
Epidemiologic studies on skin cancer provide the vital information needed to implement effective preventive efforts. Increased global skin cancer burden necessitates continued surveillance and production of accurate, high-quality NMSC and melanoma epidemiologic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin cancer is one of the leading causes of dermatologic disease burden worldwide [1]. According to recent American Cancer Society data, skin cancer is the most commonly diagnosed cancer in the United States (US) [2]. The pathogenesis for both melanoma and non-melanoma skin cancer (NMSC) is multifactorial and involves many environmental and behavioral risk factors. The most well-known and studied risk factor for the development of skin cancer is UV radiation [3]. For this reason, preventative efforts have focused extensively on reducing UV exposure and skin cancer screening. Despite these efforts, the incidence of skin cancer continues to rise in both the US and worldwide. While UV exposure remains an important risk factor, recent epidemiologic studies indicate that other factors may also play a role in the increasing incidence. In the US alone, it is estimated that 4.9 million adults were treated for skin cancer each year between 2007 and 2011 [3]. It is well known that melanoma leads to higher mortality rates than NMSC; however, while NMSC metastasizes less readily than melanoma, both can still lead to significant burden of disease as evidenced by increasing incidence rates as well as increased mortality. While NMSC is the most common type of skin cancer, accurate incidence and mortality values are difficult to estimate because it is not mandatory to report these cases in some cancer registries [2]. Herein, we provide a review of US and global NMSC and melanoma epidemiologic studies published in the last 5 years for the incidence, mortality, and burden of skin cancer.
Methods
A literature search was performed using PubMed, Embase, and Google Scholar databases in March of 2020 for articles published between January 2015 and March 2020. MeSH search terms for PubMed were “skin cancer AND epidemiologic burden NOT review” which yielded 190 results. In Embase, the following search terms, by title only, were used: “skin cancer OR melanoma OR non melanoma skin cancer AND burden.” Search terms for Google Scholar, by title only, were “skin cancer AND epidemiology OR incidence.” In total, the above search criteria yielded 431 results. After duplicates were removed, 14 were excluded for being abstract-only, and 267 were excluded because they were not written in English, not pertinent towards skin cancer, or not focused on the epidemiology, incidence, or prevalence of skin cancer. Review articles were also excluded. As a result, 29 articles were used for the purpose of this systematic review.
Literature Search and Description of Select Studies
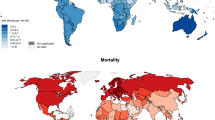
Of the included articles, 17 were from non-US countries, 9 were US based, and 3 consisted of global data. Of which, 6 were from the UK or Europe. Further, there were 5 epidemiologic studies from Asia, 4 from Oceania, 2 from South America, 1 from non-US North America, and 1 from Africa that were produced in the last 5 years. A detailed report of article selection can be found in Fig. 1. Further, the top 30 melanoma age-standardized incidence rate (ASIR) for countries worldwide is characterized in Fig. Fig. 2. Below, select epidemiologic studies outline the highest incidence or mortality rates, epidemiologic data from underserved areas, or results of various public health initiatives. Comprehensive NMSC and melanoma incidence and mortality data collected can be found in Tables 1 and 2.
Top 30 Melanoma ASIR Rates Worldwide [4]. *Data exhibited in the image is pulled from the Global Cancer Observatory, which is owned by the International Agency for Research on Cancer. Incidence data is pulled from population-based cancer registries. While these registries may cover whole countries, they often only include data in smaller, subnational regions—especially urban areas. Thus, data may not be fully representative of a given country
Epidemiologic Burden of Non-melanoma Skin Cancer
NMSC Incidence
According to the World Cancer Research Fund, in 2018, NMSC was estimated to be the fifth most common cancer in men and women worldwide [34]. In a 2017 Global Burden of Disease (GBD), there were 7.7 million (31.4% of all incident cancer cases) NMSC incident cancer cases worldwide [7••]. More specifically, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) accounted for 5.9 million and 1.8 million of all cases, respectively. Further, the ASIR of NMSC increased from 2016 to 2017 from 18.6 → 77.9 (per 100,000) for females and 29.1 → 122.1 (per 100,000) for males globally [7, 34]. Alternatively, GLOBOCAN 2018 reports that the NMSC ASIR for the world is 10.1 (per 100,000). Further stratified, the world NMSC ASIR for males and females is 13.9 and 7.0, respectively. This review found that NMSC ASIR ranged from 1531 to 10.1 [11, 35].
In a retrospective US-based population study, Rogers et al. estimated totals of new skin cancer diagnoses and affected individuals in the US. The data presented in this study indicates that the incidence rates of NMSC continue to dramatically rise, with a 35% increase from 1992 to 2012 [7••]. Historically, BCC has had a higher incidence when compared to SCC with a ratio of 4:1. However, according to Rogers et al., the age-adjusted incidence ratio of BCC to SCC was 1, signifying a shift towards increased SCC [28]. This is an important result to consider given that SCC is more likely to metastasize than BCC.
Across all 29 studies, Australia had the highest NMSC ASIR (1531 per 100,000 person years). Of Australian states, Queensland had the highest NMSC ASIR, reportedly 2679 (per 100,000 person years). It is well documented that Australia historically has the highest NMSC incidence rates in the world. In a 3-year Australian Medicare study, 6.6% of all patients had a NMSC excised. More specifically, the ASIR for BCC and SCC was 770 (men 899; women 656) and 271 (men 341; women 209), respectively. With respect to multiplicity, 47% of all individuals who had a NMSC excised had ≥ 2 NMSC excised. Notably, this study excluded lesions that were treated destructively, resulting in a more conservative incidence value. The values for all NMSC incidences can be found in Table 1.
NMSC Mortality
According to the GBD Cancer Collaboration 2017 data, NMSC accounted for 65,000 global deaths (33,000 males and 29,000 females). Additionally, the age-standardized mortality rate (ASMR) for both males and females was 1.3 (per 100,000) and 0.5 (per 100,000), respectively [7••]. Interestingly, these numbers did not change from the prior 2016 GBD data. In total, NMSC accounted for 1.3 million disability-adjusted life years (DALYs). Of these, years of life lost (YLL) accounted for 97% of all DALYs. With respect to other types of cancer, NMSC is ranked no. 27 in YLL [7••].
NMSC caused more overall deaths than melanoma (65,000 NMSC deaths vs. 62,000 melanoma deaths) worldwide, according to GBD, and GLOBOCAN reported similar numbers (65,200 NMSC deaths vs 60,700 melanoma deaths) [11••]. Melanoma is traditionally thought to be more dangerous than NMSC, as demonstrated by Dunn et al. (NMSC ASMR 1.0 vs melanoma ASMR 3.0), according to US data [28]. While this may be true in developed countries that have greater access to healthcare, GBD and GLOBOCAN demonstrate that this may not be true globally, as regions with less healthcare access are more likely to present with delayed diagnosis and advance stage disease [36].
In this review, the NMSC ASMR ranged from 1.0 to 0.255 [6, 30, 31]. However, mortality trends were not as clear as NMSC incidence rates. While incidence rates demonstrate an increasing trend globally, mortality rates lack a cohesive global trend. Instead, it shows locoregional variation, with overall global mortality remaining stable. For example, Nishi et al. reports that the crude NMSC mortality rate in Japan has increased 2-fold over 15 years. Alternatively, Leiter et al. reports that the ASMR has decreased in men in Schleswig-Holstein federal state, decreasing from 0.45 to 0.31 (per 100,000) between 1999 and 2001 and 2010 and 2012, respectively, a population that has traditionally experienced higher mortality rates than women [6]. Similarly, rates in the federal state of Saarland decreased from 18.7 to 6.3 and 13.9 to 5.4 in males and females over a 40-year period [6]. It is thought this decrease is the result of skin cancer screening, earlier detection and treatment, and enhanced community awareness of NMSC [6]. However, similar decreases in mortality rates are not seen at national levels. Table 2 outlines other NMSC mortality-related studies.
Epidemiologic Burden of Melanoma
Melanoma Incidence
Globally, there were 309,000 incidence cases (157,000 male and 152,000 female) of melanoma, per 2017 GBD data. The ASIR for both males and females was 4.2 and 3.6 (per 100,000) [7••]. Interestingly, these rates decreased for men and increased for women from the 2016 GBD data (4.8 and 3.5 per 100,000 for males and females, respectively) [34]. According to GLOBOCAN, total melanoma ASIR is 3.1. For males and females, the ASIR is 3.5 and 2.9 (per 100,000), respectively [11••]. This review found that melanoma incidence rates ranged from 72 to 0.9 (per 100,000). In an additional GBD study by Karimkhani et al., the highest global ASIRs were Australasia (54.12), North America (21.08), Eastern Europe (7.83), Western Europe (15.67), and Central Europe (8.36), according to the 2015 GBD data [26••].
Melanoma is the fifth most common cancer for men and the seventh most common cancer for women in the US [2]. Guy et al. investigated the incidence and mortality trends and projections of melanoma and found that US melanoma incidence rates doubled from 1982 to 2011. In 2011, the melanoma incidence rate was 19.7 per 100,000, and in 2015, the incidence of melanoma in North America was 21 per 100,000 [3, 26]. In the absence of new interventions, Guy et al. predicted that there will be 112,000 new cases of melanoma in 2030 [22•].
Watson et al. investigated the burden and incidence rates of melanoma in non-Hispanic Caucasians aged 15–49 years old in the US in 2012. This study reported that the incidence of melanoma in non-Hispanic Caucasian women was 7.8 per 100,000 which was almost double that of men in the same age group [21]. Although data from children appear to correlate with previous studies (showing a higher incidence of melanoma in individuals with a higher socioeconomic status (SES)), this does not appear to be the case in adults living in non-metropolitan areas. In a study by Azhar et al., 57% of non-metropolitan melanoma cases were in higher poverty districts [22•]. Previous studies have demonstrated that the incidence of melanoma is higher as age increases. While true, the most common skin cancer in children is melanoma, followed by BCC and SCC [28]. In a study assessing the incidence and survival of melanoma in children and young adults, melanoma was more common in young adults aged 20–24 (63.5%) than in adolescents aged 10–19 (31.8%) and children aged 0–9 (4.7%) [28]. Those with middle or high SES also had a higher incidence of melanoma [28].
In a 2015 population-based study of Victoria, Australia, by Curchin et al., it was found that the ASIR of melanoma in situ and invasive melanoma was 54.5 and 46.0, respectively. This study also concluded that rates of melanoma in situ are increasing faster than those of invasive melanoma [19]. Similarly, a New Zealand–based study found that the ASIR of melanoma was 69.0 and 55.2 for males and females. For melanoma in situ and invasive melanoma, the male/female rates were 27.6/20.9 and 41.4/34.3, respectively [37]. In Queensland, Australia, the highest melanoma ASIR of any country was reported to be 72 over a 4-year period. Similar to other Oceania-based studies, the rate of melanoma in situ is increasing [16]. The authors of all three studies suspect this increase in melanoma in situ is due to public health initiatives, as melanomas are being caught before they become invasive.
In Germany, between 2008 and 2011, melanoma ASIR remained relatively stable from 17.7 to 17.9. This study also found that the mortality rate remained stable (range of 1.5–1.7 between 2004 and 2013) despite implementation of nationwide screening in 2008 [38]. Alternatively, Aitken et al. documented a series of Australian public health initiatives that demonstrated that skin cancer–related deaths decreased in all populations, with the exception of men > 60 years of age. These initiatives focused on youth populations, which highlights the importance of educating younger generations’ perspectives on skin cancer risk factors [17]. Summaries of other incidence-related studies can be found in Table 1.
Melanoma Mortality
According to 2017 GBD data, there were 62,000 melanoma deaths (33,000 males and 29,000 females) worldwide. The respective ASMR for males and females was 0.9 and 0.7 [7••]. Interestingly, these rates decreased from 1.1 and 0.8 for males and females, according to the 2016 GBD data [34]. Further, according to GLOBOCAN, total melanoma ASMR is 0.6. For males and females, the ASMR is 0.8 and 0.5, respectively. In this review, reported melanoma ASMRs ranged from 3.0 to 0.28—the highest of which was reported by Mokdad et al. [7, 21, 30, 31]. This study extracted 13,400 de-identified skin cancer–related death records from the National Center for Health Statistics [30•]. Mokdad et al. found that melanoma was the 17th leading cause of death out of 29 cancers studied and caused 10,100 total deaths in this cohort. Additionally, melanoma resulted in 225,700 YLL in this cohort, ranking 16th out of 29 cancers [30•]. According to 2015 GBD data, Australasia (5.63), North America (2.30), Eastern Europe (2.27), Western Europe (2.07), and Central Europe (2.08) had the greatest ASMRs. Further, global age-standardized DALY rates were 27 in males and 19 in females. This study found that in New Zealand, Australia, Europe, the elderly and male populations maintain the greatest burden worldwide. The authors speculate this is due to high UV exposure, relatively fair-skinned population, and behavioral emphasis on tanning [26••].
In the US, melanoma is responsible for the most skin cancer–related deaths (9000 each year), accounting for 41% of all skin-related deaths [37]. The overall ASMR for melanoma in the US for 2011 was 2.7 (per 100,000) with a higher death rate among non-Hispanic Caucasians (3.4 per 100,000) based on data from a large database [22•]. Watson et al. reported that the rate of deaths due to melanoma increased with age and was also higher in men than in women in all age groups [21]. Interestingly, thin melanomas ranging from 0.01 to 1 mm had the highest incidence among both men and women with 9.5 and 13.8 (per 100,000) respectively; however, men had higher incidences of thicker melanomas when compared with females [21]. Dunn et al. reported that adolescents and young adults had poorer survival than children [28]. Additionally, young adults, adolescents, and children with low SES had worse 1–3- and 5-year survival rates as compared with those in middle-low, middle, and high SES—despite the increased incidence related to children of higher SES [28]. The remainder of worldwide melanoma mortality data can be found in Table 2.
NMSC and Melanoma Concomitant Burden
In renal transplant patients (RTR), NMSC makes up nearly 50% of all transplant-related malignancies. A single-center, Brazilian-based study stratified NMSC in RTR patients and found that of 343 diagnosed NMSCs, 40.5% were invasive SCC, 30.0% were SCC in situ, and 29.5% were BCC. Notably, NMSC in solid organ transplant patients was found to be more aggressive when compared with individuals who had not received a transplant [16].
Individuals with higher leptin levels have an increased rate of metastasis in melanoma.. Additionally, the progression of melanoma worsens if diabetes mellitus (DM) is also present. Oba et al. determined that 87.5% of studied individuals who suffer from DM developed progressive melanoma (e.g., local recurrence, metastasis) in a population of patients with stage I or II primary cutaneous melanoma [39]. Additionally, the incidence of NMSC in gastric bypass patients was 0.7 per 1000 patients while those who did not receive surgery nor reduced their body mass index (BMI) had an incidence of 1.2 per 1000 patients; this was a statistically significant difference (p = 0.047) [40].
With respect to alopecia areata, Mostahhimi et al. found that individuals with alopecia areata (AA) had significantly decreased incidences of NMSC. Compared with controls (7%), 4.5% of individuals with AA developed NMSC. Likewise, only 1.2% of individuals with AA developed melanoma compared with controls (1.8%), although this difference was not significant. Skin cancer incidences in AA patients were not reported in this study [41].
Alternatively, melanoma incidence in vitiligo patients (5.17 per 10,000) was significantly higher when compared with controls (1.64 per 100,000) [42]. Likewise, the incidence of NMSC in individuals with vitiligo (18.26 per 100,000) compared with controls (13.32 per 100,000) was also higher, although this was not a significant difference [42].
High-Risk Populations and Literature Disparities
Several US studies have demonstrated skin cancer incidence disproportions exist among gender, geographic locations, and socioeconomic status (SES). In the US, melanoma appears to have a higher incidence in women; however, the mortality is higher among men [21]. Men were also found to have higher incidences in melanoma with thickness > 1.00 mm [21]. With a higher death rate than females and a higher incidence of thick melanomas, this may indicate that men may experience delayed diagnosis. Interestingly, another study determined that more deaths resulted from T1 melanomas as compared with T4 melanomas [32]. They attribute this finding to previous studies’ postulation about thin melanomas having more aggressive characteristics, higher Clark level, and location, found on head and neck, to explain poor prognosis [32]. What these facts demonstrate is the need for earlier detection and the need for increased preventative efforts directed towards men.
Historically, higher SES has been associated with higher incidences of melanoma, likely due to increased access to healthcare and increased screening. US studies in children from the past 5 years highlight this fact; however, in adults living in non-metropolitan areas, higher incidences of melanoma were seen in lower SES communities [28, 29]. Likewise, there is a higher incidence of NMSC seen in the uninsured adult population. Sturgeon et al. showed that the incidence of NMSC in the last 5 years was higher in communities with low SES, finding that uninsured patients in the 50–64 age range had an incidence of 2.8% of NMSC, which was higher than the national age independent average of 0.65% in 2006 and 1.05% in 2012 (p < 0.01) [14]. Further, incidence and mortality rates are likely under-reported for specific subpopulations, such as young women, Hispanics, and gay men [43].
Compared with the US, there is limited data regarding the epidemiologic burden of skin cancer worldwide. Among international studies, there is disparity among the extent and representation of data produced. With the data provided, however, the incidence of both melanoma and NMSC is increasing, despite nationwide public health and educational initiatives in first-world countries [38]. According to GBD, this is largely due to a change in population age structure and population growth, with change due to age and cause-specific incidence rates being a minor cause [34]. However, among US and worldwide subpopulations, there may be additional factors that contribute to skin cancer burden. Notably, the non-US studies included in this review were primarily from first-world countries. This implicit bias, apparent within the locations and populations of these studies, may be representative of access to research funding and disparities in academia and education.
There is a significant lack of incidence, mortality, and DALY skin cancer data from Asia, South Africa, and South America. Regardless of the propensity of each population to develop skin cancer, it is important to characterize skin cancer burden because rates of skin cancer may be underestimated, especially in minority groups or populations with darker skin, as skin cancer in these populations tend to present atypically or in advanced stages [44]. While US studies sought to stratify skin cancer burden in US subpopulations (e.g., children, homeless, uninsured, high socioeconomic status), there was a lack of data of similar subpopulations globally. Similarly, there was no global data that analyzed associations of skin cancer and concomitant conditions, such as renal transplant recipients or obese patients.
Skin Cancer Registries
A common postulated cause for the increased incidence of skin cancer globally is increased reporting and more thorough registries that document BCC, SCC, and melanoma incidence and mortality rates. To provide reliable epidemiological data, thorough and accurate skin cancer registries are necessary. While there are a variety of countries that require reporting of melanoma (e.g., US, Australia, Germany, Switzerland, New Zealand, Canada), it is not necessary to report NMSC in the US, Belgium, and others [9, 22, 38, 45,46,47,48,49,50,51]. Callens et al. report that there are few registries that comprehensively collect data on NMSC. The authors state that there are often discrepancies among incidence rates due to variations in data capture, recording, and processing [9]. According to recent epidemiologic data, however, NMSC causes more annual deaths than melanoma worldwide, creating an argument for NMSC becoming a reportable cancer [7••].
While the majority of databases were claims or electronic medical record observational data, some databases documented skin cancer rates with respect to histologic grading and confirmation. Though these were few in number, these databases afford the added benefit of how advanced a skin cancer is when it was excised. These details have the potential to guide future public health initiatives by allowing for more accurate comparison at both national and international levels. Providing information on the histological grade and the degree of thickness will also provide vital information on the time at which the skin cancer was diagnosed.
Epidemiology and Public Health Initiatives
This review highlights the increasing burden of NMSC and melanoma in US and global populations. To reduce global burden, skin cancer prevention is of the utmost importance. Currently, however, the US Preventive Services Task Force (USPSTF) determined that there is insufficient evidence to provide a recommendation for regular skin cancer screening, based on a 2016 systematic review [49]. However, these recommendations are not the result of a lack of skin cancer burden. Rather, they are the result of a paucity of high-quality, long-term epidemiologic randomized control trials to elucidate the efficacy of screening. Current USPSTF guidelines, therefore, highlight areas for future investigative pathways.
In a successful pilot study conducted in Schleswig-Holstein, Germany, Katalinic et al. reported a 50% decrease in melanoma mortality in individuals > 35 years old over a 10-year period. However, when transitioned to the national level, the results did not translate due to loss of patient follow up and lack of quality skin checks. Queensland, Australia, historically has had some of the highest rates of skin cancer in the world. Following a series of public health initiatives, including “Slip (on a shirt), Slop (on sunscreen), Slap (on a hat)” (1980s), SunSmart campaign (1990s), and “No Hat, No Play,” skin cancer mortality rates fell in all demographic groups except in men > 60 years of age. Despite an overall increase in incidence rates, this study found that incidence rates stabilized in individuals younger than 35 years of age, a demographic estimated to account for 20% of cancer diagnoses. These campaigns focused on adolescent populations, ultimately resulting in a generational shift of decreased skin cancer incidence [47]. Future public health initiatives such as sun safety campaigns and school programs aimed at enforcing sun protection for outdoor activities, which have shown to be effective in Australia, may improve burden if implemented in all countries. Further, it is essential to evaluate the effectiveness of public health programs and guide future efforts.
Skin cancer screening is a form of secondary prevention. In addition to catching skin cancers early, preventing development of skin cancer needs to become a priority, considering that at least half of skin cancers may be preventable [50]. There are a multitude of documented carcinogenic exposures that are related to the development of skin cancer, including indoor tanning, excessive exposure to midday sun, lack of utilization of shade, lack of protective clothing, and minimal sunscreen use [51]. Personal factors, such as skin type, history of sunburns, and family history of skin cancer, contribute to the risk of developing skin cancer. Thus, necessary skin cancer prevention strategies are not universal, and more extensive epidemiologic studies are necessary to develop personalized prevention plans based on individualized risk factors. Furthermore, adolescent UV exposure (prior to age 20) accounts for almost 40–50% of total UV exposure by age 60 [52]. Therefore, adolescent populations should be considered for the focus of future public outreach efforts to negate adolescent UV exposure [43].
Conclusion
NMSC and melanoma maintain significant burden globally. In each country evaluated, incidence rates are increasing, and the efficacy of public health initiatives varies. In recent years, NMSC killed more individuals than melanoma, albeit at a lower rate. However, incidence and mortality numbers are limited on a global scale, with studies primarily being produced from first-world countries. Countries that have implemented secondary prevention public health initiatives have seen varied results. Moving forward, a shift towards primary prevention, such as educating youth populations, may be a solution to minimize skin cancer burden. Thus, continued surveillance and production of accurate, high-quality epidemiologic data is essential to characterize the evolution of skin cancer burden.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014 Jun;134(6):1527–34. https://doi.org/10.1038/jid.2013.446.
Cancer Facts & Figures [Internet]. Cancer. 2019 [cited 2020 Mar 23]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed 23 Mar 2020.
Guy GP, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002−2006 and 2007−2011. Am J Prev Med. 2015;48(2):183–7. https://doi.org/10.1016/j.amepre.2014.08.036.
Skin cancer statistics [Internet]. World Cancer Research Fund. 2018 [cited 2020 Apr 27]. Available from: https://www.wcrf.org/dietandcancer/cancer-trends/skin-cancer-statistics
Pandeya N, Olsen CM, Whiteman DC. The incidence and multiplicity rates of keratinocyte cancers in Australia. Med J Aust. 2017;207(8):339–43. https://doi.org/10.5694/mja17.00284.
Leiter U, Keim U, Eigentler T, Katalinic A, Holleczek B, Martus P, et al. Incidence, mortality, and trends of nonmelanoma skin cancer in Germany. J Invest Dermatol. 2017;137(9):1860–7. https://doi.org/10.1016/j.jid.2017.04.020.
Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5(12):1749–68. https://doi.org/10.1001/jamaoncol.2019.2996Large Global Burden of Disease Study that found that melanoma was ranked no. 25 and NMSC was no. 27 in years of life lost (YLL) due to cancer. In addition, the aging and growing population has led to a 33% increase in NMSC cases, from 5.8 to 7.7 million (2007 to 2017).
Rudolph C, Schnoor M, Eisemann N, Katalinic A. Incidence trends of nonmelanoma skin cancer in Germany from 1998 to 2010. J Dtsch Dermatol Ges J Ger Soc Dermatol JDDG. 2015;13(8):788–97. https://doi.org/10.1111/ddg.12690.
Callens J, Eycken LV, Henau K, Garmyn M. Epidemiology of basal and squamous cell carcinoma in Belgium: the need for a uniform and compulsory registration. J Eur Acad Dermatol Venereol. 2016;30(11):1912–8. https://doi.org/10.1111/jdv.13703.
Umezono Y, Sato Y, Noto M, Yamada K, Noguchi N, Hasunuma N, et al. Incidence rate of cutaneous squamous cell carcinoma is rapidly increasing in Akita prefecture: urgent alert for super-aged society. J Dermatol. 2019;46(3):259–62. https://doi.org/10.1111/1346-8138.14759.
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53. https://doi.org/10.1002/ijc.31937Large global study that found that NMSC was the most common cancer in North America, and Australia/New Zealand, and the 2nd most common cancer in all of the Americas, Northern Europe, and Western Europe in males. It also reported that NMSC was the 2nd most common cancer in all of the America, North America, Western Europe, and Australia/New Zealand in females.
Şuteu O, Blaga ML, Nicula F, Şuteu P, Coza O, Achimaş-Cadariu P, et al. Incidence trends and survival of skin melanoma and squamous cell carcinoma in Cluj County, Romania. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP. 2017;26 Joining forces for better cancer registration in Europe:S176–82. https://doi.org/10.1097/CEJ.0000000000000382.
Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151(10):1081. https://doi.org/10.1001/jamadermatol.2015.1187Large US-based study that found that a 35% increase in NMSC incidence from 1992 to 2012. Additionally, this study reports that BCC to SCC is 1:1, whereas previous studies demonstrate a 4:1 ratio.
Sturgeon A, Pate DA, Patel R, Snodgrass K, Tarbox M, Prabhu F, et al. Incidence of non-melanoma skin cancer in the uninsured. J Health Care Poor Underserved. 2017;28(4):1327–32. https://doi.org/10.1353/hpu.2017.0117.
Egeler SA, Huang A, Johnson AR, Ibrahim A, Bucknor A, Peymani A, et al. Regional incidence of and reconstructive management patterns in melanoma and nonmelanoma skin cancer of the head and neck: a 3-year analysis in the inpatient setting. J Plast Reconstr Aesthetic Surg. 2020;73(3):507–15. https://doi.org/10.1016/j.bjps.2019.10.017.
Hayashida MZ, Fernandes VMC, Fernandes DR de M, Ogawa MM, Tomimori J. Epidemiology and clinical evolution of non-melanoma skin cancer in renal transplant recipients: a single-center experience in São Paulo, Brazil. Int J Dermatol. 2015;54(10):e383–8. https://doi.org/10.1111/ijd.12632.
Aitken JF, Youlden DR, Baade PD, Soyer HP, Green AC, Smithers BM. Generational shift in melanoma incidence and mortality in Queensland, Australia, 1995–2014. Int J Cancer. 2018;142(8):1528–35. https://doi.org/10.1002/ijc.31141.
Elwood JM, Kim SJ-H, Ip KH-K, Oakley A, Rademaker M. In situ and invasive melanoma in a high-risk, New Zealand, population: a population-based study. Australas J Dermatol. 2019;60(1):38–44. https://doi.org/10.1111/ajd.12884.
Curchin DJ, Forward E, Dickison P, Harris VR, McCormack CJ, Smith SD. The acceleration of melanoma in situ: a population-based study of melanoma incidence trends from Victoria, Australia, 1985–2015. J Am Acad Dermatol. 2019;80(6):1791–3. https://doi.org/10.1016/j.jaad.2018.12.067.
Minini R, Rohrmann S, Braun R, Korol D, Dehler S. Incidence trends and clinical-pathological characteristics of invasive cutaneous melanoma from 1980 to 2010 in the Canton of Zurich, Switzerland. Melanoma Res. 2017;27(2):145–51. https://doi.org/10.1097/CMR.0000000000000312.
Watson M, Geller AC, Tucker MA, Guy GP, Weinstock MA. Melanoma burden and recent trends among non-Hispanic whites aged 15-49years, United States. Prev Med. 2016;91:294–8. https://doi.org/10.1016/j.ypmed.2016.08.032.
Guy GP, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC, et al. Vital signs: melanoma incidence and mortality trends and projections - United States, 1982–2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591–6 Large database that found that the average number of adults treated annually for NMSC a melanoma increase from 3.4 million to 4.9 million between 2002–2006 and 2007–2011. Additionally, the annual cost to treat skin cancer increased by 126.2%.
Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. Documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112(38):629–34. https://doi.org/10.3238/arztebl.2015.0629.
Steglich RB, Coelho KM d PA, Cardoso S, Gaertner MH d CN, Cestari TF, Franco SC. Epidemiological and histopathological aspects of primary cutaneous melanoma in residents of Joinville, 2003-2014. An Bras Dermatol. 2018;93(1):45–53. https://doi.org/10.1590/abd1806-4841.20185497.
Ghazawi FM, Cyr J, Darwich R, Le M, Rahme E, Moreau L, et al. Cutaneous malignant melanoma incidence and mortality trends in Canada: a comprehensive population-based study. J Am Acad Dermatol. 2019;80(2):448–59. https://doi.org/10.1016/j.jaad.2018.07.041.
Karimkhani C, Green AC, Nijsten T, Weinstock MA, Dellavalle RP, Naghavi M, et al. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol. 2017;177(1):134–40. https://doi.org/10.1111/bjd.15510Large global burden of disease study that demonstrated that Australasia, North America, Eastern Europe, Western Europe, and Central Europe had the highest ASIR, ASMR, and DALYs for melanoma across the world.
Wu Y, Wang Y, Wang L, Yin P, Lin Y, Zhou M. Burden of melanoma in China, 1990-2017: findings from the 2017 global burden of disease study. Int J Cancer. 2019;147:692–701. https://doi.org/10.1002/ijc.32764.
Dunn EC, Moore KJ, Miao F, Kirsner RS, Koru-Sengul T. Survival of children and young adults with skin cancer: analysis of a population-based Florida cancer registry: 1981-2013. Pediatr Dermatol. 2018;35(5):597–601. https://doi.org/10.1111/pde.13588.
Azhar AF, Faheem S. Comparison of melanoma incidence in metropolitan areas versus nonmetropolitan areas in the state of Texas stratified by poverty classification. Proc (Baylor Univ Med Cent). 2019;32(3):345–7. https://doi.org/10.1080/08998280.2019.1593725.
Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, Stubbs RW, Bertozzi-Villa A, Morozoff C, et al. Trends and patterns of disparities in cancer mortality among US counties, 1980–2014. JAMA. 2017;317(4):388–406. https://doi.org/10.1001/jama.2016.20324Large US study that found that NMSC was the 24th leading cause of death out of 29 cancers, that NMSC caused 3300 total deaths in the cohort, and NMSC resulted in 53,900 YLL in the cohort.
Nishi M. Epidemiology of skin Cancer in Japan. J Tumor. 2016;4(2):369–73.
Landow SM, Gjelsvik A, Weinstock MA. Mortality burden and prognosis of thin melanomas overall and by subcategory of thickness, SEER registry data, 1992-2013. J Am Acad Dermatol. 2017;76(2):258–63. https://doi.org/10.1016/j.jaad.2016.10.018.
Wright CY, Kapwata T, Singh E, Green AC, Baade P, Kellett P, et al. Trends in melanoma mortality in the population groups of South Africa. Dermatol Basel Switz. 2019;235(5):396–9. https://doi.org/10.1159/000500663.
Fitzmaurice C, Akinyemiju TF, Lami FHA, Alam T, Alizadeh-Navaei R, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4(11):1553–68. https://doi.org/10.1001/jamaoncol.2018.2706.
Pandeya N, Olsen CM, Whiteman DC. The incidence and multiplicity rates of keratinocyte cancers in Australia. Med J Aust. 2017;207(8):339–43. https://doi.org/10.5694/mja17.00284Large study that reported the highest ASIR of any skin cancer in this systematic review.
Gupta AK, Bharadwaj M, Mehrotra R. Skin cancer concerns in people of color: risk factors and prevention. Asian Pac J Cancer Prev APJCP. 2016;17(12):5257–64. https://doi.org/10.22034/APJCP.2016.17.12.5257.
Lim HW, Collins SAB, Resneck JS, Bolognia JL, Hodge JA, Rohrer TA, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958–972.e2. https://doi.org/10.1016/j.jaad.2016.12.043.
Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. Documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112(38):629–34. https://doi.org/10.3238/arztebl.2015.0629.
Oba J, Wei W, Gershenwald J, Johnson M, Wyatt C, Ellerhorst J, et al. Elevated serum leptin levels are associated with an increased risk of sentinel lymph node metastasis in cutaneous melanoma. Medicine (Baltimore) [Internet]. 2016;95(11) [cited 2020 Mar 26];Available from: insights.ovid.com. https://doi.org/10.1097/MD.0000000000003073.
Taube M, Peltonen M, Sjöholm K, Anveden Å, Andersson-Assarsson JC, Jacobson P, et al. Association of bariatric surgery with skin cancer incidence in adults with obesity: a nonrandomized controlled trial. JAMA Dermatol. 2020;156(1):38–43. https://doi.org/10.1001/jamadermatol.2019.3240.
Mostaghimi A, Qureshi S, Joyce C, Guo Y, Huang KP. Reduced incidence of skin cancer in patients with alopecia areata: a retrospective cohort study. Cancer Epidemiol. 2016;41:129–31. https://doi.org/10.1016/j.canep.2016.02.009.
Kim HS, Kim HJ, Hong ES, Kim KB, Lee JD, Kang TU, et al. The incidence and survival of melanoma and nonmelanoma skin cancer in patients with vitiligo: a nationwide population-based matched cohort study in Korea. Br J Dermatol. 2020;182(4):907–15. https://doi.org/10.1111/bjd.18247.
Linos E, Katz KA, Colditz GA. Skin cancer—the importance of prevention. JAMA Intern Med. 2016;176(10):1435–6. https://doi.org/10.1001/jamainternmed.2016.5008.
Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21(4):170–7, 206; quiz 178. https://doi.org/10.1016/j.jaad.2005.08.063.
Wu Y, Wang Y, Wang L, Yin P, Lin Y, Zhou M. Burden of melanoma in China, 1990–2017: findings from the 2017 global burden of disease study. Int J Cancer. 2019;147:692–701. https://doi.org/10.1002/ijc.32764.
Elwood JM, Kim SJ-H, Ip KH-K, Oakley A, Rademaker M. In situ and invasive melanoma in a high-risk, New Zealand, population: a population-based study. Australas J Dermatol. 2019;60(1):38–44. https://doi.org/10.1111/ajd.12884.
Aitken JF, Youlden DR, Baade PD, Soyer HP, Green AC, Smithers BM. Generational shift in melanoma incidence and mortality in Queensland, Australia, 1995–2014. Int J Cancer. 2018;142(8):1528–35. https://doi.org/10.1002/ijc.31141.
Minini R, Rohrmann S, Braun R, Korol D, Dehler S. Incidence trends and clinical-pathological characteristics of invasive cutaneous melanoma from 1980 to 2010 in the Canton of Zurich, Switzerland. Melanoma Res. 2017;27(2):145–51. https://doi.org/10.1097/CMR.0000000000000312.
Wernli KJ, Henrikson NB, Morrison CC, Nguyen M, Pocobelli G, Blasi PR. Screening for skin cancer in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316(4):436–47. https://doi.org/10.1001/jama.2016.5415.
Song M, Giovannucci E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among white adults in the United States. JAMA Oncol. 2016;2(9):1154–61. https://doi.org/10.1001/jamaoncol.2016.0843.
WHO | Individual protection against UV [Internet]. [cited 2020 Apr 25]. Available from: https://www.who.int/uv/faq/protect/en/index2.html
Green AC, Wallingford SC, McBride P. Childhood exposure to ultraviolet radiation and harmful skin effects: epidemiological evidence. Prog Biophys Mol Biol. 2011;107(3):349–55. https://doi.org/10.1016/j.pbiomolbio.2011.08.010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
CR—Salary funded by Pfizer Independent Grants for Learning and Change (PI: RP Dellavalle): Inflammatory and Immune-mediated Skin Disease Fellowship.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Co-first authors Chandler W. Rundle and Michelle Militello
This article is part of the Topical Collection on Skin Cancer
Rights and permissions
About this article
Cite this article
Rundle, C.W., Militello, M., Barber, C. et al. Epidemiologic Burden of Skin Cancer in the US and Worldwide. Curr Derm Rep 9, 309–322 (2020). https://doi.org/10.1007/s13671-020-00311-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-020-00311-4