Abstract
Purpose of Review
The purpose of this review was to determine the most frequently utilized outcome measures in clinical trials and to review the scoring criteria, advantages, disadvantages, and application in research and clinical settings, as well as provide an overview of the published guidelines on the use of outcome measures in clinical practice.
Recent Findings
The most frequently utilized physician-reported outcome measurement instruments in clinical trials from 2018 to 2019 were Physician Global Assessment (PGA), Psoriasis Area Severity Index (PASI), and body surface area. The most frequently utilized patient-reported outcome measurement instruments were Dermatology Quality of Life Index, EQ-5D-5L, and patient global assessment.
Summary
In research, further standardization of outcome measures may allow for more useful comparison of different treatment modalities. In clinical practice, although, the PASI provides a detailed measurement of disease severity, and the PGA score has the advantage of being more clinically useful to physicians and patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriasis affects 2% of the population worldwide [1]. While there are several types of psoriasis, plaque psoriasis—which accounts for 90% of cases—is characterized by sharply demarcated erythematous plaques with silvery scales with a predilection for the extensor surfaces of elbows and knees, scalp, and nails [2]. The characteristics of the lesions—the redness, thickness, and scaliness, along with the extent of involvement—lend themselves to objective measures of disease severity.
Objective outcome measures can serve as a standardized way of comparing disease severity before and after treatment in clinical practice and research. Reliability, the extent to which a measurement instrument yields the same result on repeated use, and validity, the ability of an instrument to measure what it is designed to measure, are key qualities for useful outcome measures. Ease of use, sensitivity to change, and clinical relevance are other important considerations. These tools should be easily calculated and interpreted by both physicians and patients. These outcome measurements can either assess lesion-disease severity reported by the physician or measure quality of life reported by the patient. However, these measures used in trials may not be practical in clinical practice.
Over the past decade, there has been a dramatic increase in the treatment modalities available to patients with plaque psoriasis; however, comparison is difficult when clinical trials are presenting their data using different outcome measures and assessment tools. There have been more than 44 different scoring systems used in clinical trials from 1997 to 2000 [3]. The aim of this paper is to review the most frequently utilized psoriasis outcome measures used in clinical trials in 2018–2019. This review will cover the scoring criteria, advantages, disadvantages, and application in research and clinical settings and provide an overview of the published guidelines on the use of outcome measures in clinical practice.
Methods
The National Institute of Health’s clinicaltrials.gov was reviewed, and 38 trials met the following inclusion criteria: phase III, phase IV, plaque psoriasis, starting date July 15, 2018, to ending date July 15, 2019. Measurement instruments that were present in three clinical trials or fewer were excluded. Published guidelines from National Psoriasis Foundation (NPF), Medicare Merit–based Incentive plan, and Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) were reviewed.
Results
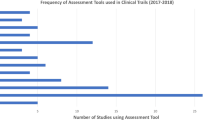
A total of 31 phase III and 7 phase IV clinical trials were reviewed, and the frequency of use for each outcome measure was summed (Fig. 1). The most common patient-reported outcome measure was Dermatology Quality of Life Index (DLQI) used in 20/38 trials (52%) (Table 1). The most common physician-reported measure of disease severity was Physician Global Assessment (PGA) which was used in 30/38 trials (79%) (Table 2). The National Psoriasis Foundation (NPF) endorses the use body surface area (BSA) as an outcome measurement in clinical practice. Medicare Merit–based Incentive plan (MIPS) endorses a combination of PGA, Psoriasis Area Severity Index (PASI), DLQI, and/or BSA. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) endorses any measure that fully assesses the patient’s disease and incorporated patient-reported outcomes (Table 3).
Physician-Reported Measurements
Five-Point Physician’s Global Assessment
Physicians’ Global Assessment, also known as Psoriasis Global Assessment or Investigator’s Global Assessment (IGA), is the most frequently used physician-reported outcome measure [12]. It has been used in clinical trials in a wide variety of different specialties and is not isolated to psoriasis [11]. It is a five-point scale used to assess severity of disease as a whole. Disease is categorized as 0 (clear), 1 (minimal), 2 (mild), 3 (moderate), 4 (marked), and 5 (severe) [6]. There are two forms of the PGA: dynamic and static. The dynamic form is used to rate improvement relative to baseline severity, while the static form is used to measure clinical severity at a single time and does not depend on a baseline assessment [11]. A 6-point scale has also been used in clinical trials with a score of 6 corresponding to a “very severe” category; however, the FDA now recommends using the 5-point scale for clinical trials on psoriasis [11]. This measure most closely resembles the assessment that physicians use when seeing patients in clinical practice. It has good validity and tends to correlate with BSA and PASI disease severity [12, 21]. However, it is less objective compared with other outcome measures and does not discriminate small changes in disease severity [4].
Psoriasis Area Severity Index
PASI is a popular research tool used in assessing severe psoriasis [12]. It is considered the gold standard in assessing psoriasis severity and monitoring treatment response in clinical trials. It is rarely utilized in clinical practice due to difficulties in scale interpretation and complicated calculations. The intensity of the erythema, desquamation, and induration are rated on a 5-point scale with 0 indicating no involvement, 1 (slight), 2 (moderate), 3 (severe), and 4 (very severe). Additionally, percentage involvement of the head, arms, trunk, and legs is captured. Scores vary from 0 to 72 with higher scores indicating severe disease. Clinical improvement is calculated by percentage change from baseline [10]. For example, a PASI-75, frequently used as an endpoint in clinical trials, demonstrates a 75% improvement in score from baseline [4]. PASI has high validity and reliability in trained individuals, although there is variation between experienced and novice scorers [12].
Although a popular outcome measure, the PASI has several disadvantages. The score is not sensitive to small changes in the surface area [13]. It does not evaluate disease in all body areas—hand, feet, genital, and nails—which are commonly involved in psoriasis [11]. Scoring may be affected by changes in temperature, humidity, and application of emollients, which can reduce the amount of erythema and scale [10]. Quality of life and other comorbidities associated with psoriasis are not evaluated. Higher score may not represent disease severity. For example, two patients may present with a PASI score of 12; one patient may have localized disease with erythematous and scaly plaques, while the second patient may have extensive body involvement of flat non-scaly lesions. Clinical trials have incorporated a minimum BSA of 10% to define severe psoriasis due to this limitation of the PASI [13].
Body Surface Area
BSA measures the percentage of skin surface area affected by lesion. The percentage can be determined by either “rules of nine” method or by area determined by the patient’s hand size. Rule of nine assumes that a total BSA is composed of 9% for the head, 18% for the anterior trunk, 18% for the posterior trunk, 9% for the anterior leg, and 9% for the posterior leg [14]. The second method is by using the patient’s closed fist as a reference. Previously, the fist represented 1% BSA; however, it actually represents 0.7% [15, 16]. Both methods of measuring BSA tend to overestimate the extent of psoriatic involvement. BSA is quick and easy to determine and is commonly used in clinical practice. It does not adequately define the severity of lesions and just takes into account the extent of involvement. It has lower levels of validation and high variability compared with PASI and PGA [21].
Patient-Reported Measurements
Dermatology Life Quality Index
DLQI is a patient-reported outcome measure used for more than 33 different chronic skin conditions worldwide [8]. It quantifies the impact psoriasis has on patients’ lives and is an important endpoint to consider in both clinical trials and clinical practice. Quality of life measures are important as the physical appearance of lesions may not demonstrate the full impact of disease on the patient [13]. There is not a clear positive correlation between the extent of disease involvement and degree of psychological distress [22]. The DLQI is composed of ten questions covering six domains: symptoms, embarrassment, daily activities, leisure, work and school, personal relationships, and hassle with psoriasis treatment with each item scored on a four-point scale of 0 (not at all), 1 (a little), 2 (a lot), and 3 (very much) [4]. For several questions, a “not relevant” option may be selected, which will be scored as 0 for that question. This can lead to a lower total average of the DLQI score which may falsely demonstrate a lower quality of life burden [9]. DLQI is widely used and has good evidence for strong validity and reliability [8]. It is popular in both clinical practice and trials since it is a self-administered, easy, and user-friendly questionnaire that can be completed on average in about 2 min [7].
EQ-5D-5L
EQ-5D-5L is a patient-reported questionnaire that assesses five general aspects of health: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The patients are able to self-report status as 1 (no problem), 2 (slight problem), 3 (moderate problem), 4 (severe problem), and 5 (extreme problem). The second part of the questionnaire consists of a self-rated health status on a vertical axis from 0 to 100 with end points labeled as “worst imaginable health status” to “best imaginable health status.” It provides patients an opportunity to report quality of life on both a number scale and visual scale. However, the mood category of this measurement is limited to just anxiety and depression and does not factor other emotions that patients may feel such as anger or frustration. It also does not account for social life or relationships [5].
Patient Global Assessment
Patient Global Assessment is comparable to the Physician’s Global Assessment with a five-point scale with 0 (clear), 1 (minimal), 2 (mild), 3 (moderate), 4 (severe), and 5 (very severe); however, the score is reported by the patient. It is simple to use but offers the same advantages and disadvantages described in the Physician’s Global Assessment discussed above. It can be assessed day 1 for a baseline score and then repeated at each subsequent encounter.
Discussion
Psoriasis severity is difficult to assess. There is no blood test or imaging study that can provide an objective evaluation of treatment efficacy. Severity is based on the subjective and objective measurement by both physician and patient. These measurements can provide a quantitative value used to compare disease severity and efficacy of treatment over time. It is important that clinical trials are consistent in the measurements they use to truly compare efficacy between different drugs; however, this is difficult when several dozen measurements have been created for plaque psoriasis.
The frequency and specific use of outcome measurements in clinical practice has not yet been quantified. Many of the outcome measurements discussed above are impractical outside of a research setting. Some measurements such as the PASI and DLQI may take a significant amount of time, which is not feasible in a busy clinical practice. They can be complicated to calculate and confusing to apply the calculated score to clinical practice. However, it can be beneficial to have a routine assessment of psoriasis severity and its impact on the patient to be recorded on a regular interval to guide treatment. Since there are so many measurements to choose from, professional societies have published guidelines to help guide dermatologists on which measurements can be most useful in clinical practice.
The National Psoriasis Foundation (NPF) published a consensus statement in 2017 stating that BSA was identified as the most practical and appropriate instrument for use by general dermatologists. Although they do not provide a specific patient-reported measure, the statement states that BSA does not capture location, symptoms, comorbidities, and life quality so it is encouraged to also use a patient-reported outcome measure along with the BSA. These guidelines were published to provide guidance but should be used at the discretion of both the physician and the patient [17•].
The Medicare Merit–based Incentive Payment System (MIPS) endorses a combination of PGA, PASI, DLQI, and BSA in its guideline for successful treatment therapy. At least one of the following must be provided in order to claim successful treatment: PGA score ≤ 2, BSA ≤ 3%, PASI ≤ 3, or DLQI ≤ 5 [18].
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) meeting in 2016, endorsed by Outcome Measures in Rheumatology (OMERACT), established guidelines on which outcome measure to use for psoriasis and psoriatic arthritis. GRAPPA concluded that they do not endorse one specific outcome measure but rather any measure that fully assesses patients’ disease and incorporates patient-reported outcomes [19•].
A 2011 European consensus statement, representing nineteen European countries, recommended the use of PASI together with DLQI to define treatment goals in clinical practice [20].
The three most frequently utilized measurements remained unchanged from last year: the PASI, PGA, and DLQI. However, the PGA is now the most utilized measurement in clinical trials, whereas last year, it was the PASI [23•]. Pascoe et al. has studied the feasibility of implementing PGA for psoriasis in clinical practice. It has been incorporated into billing sheets, which allowed for easy scoring and incorporation it into practice. This resulted in a high adherence from physicians to PGA usage. It required little additional time but yielded rich information about the disease severity. Although not a mandated requirement, implementing an outcome measurement in clinical practice can provide additional pieces of information that can help track patient’s disease improvement between visits [24].
Conclusion
An array of outcome measures have been utilized in clinical trials and have been proposed in different psoriasis guidelines for clinical practice use. Our review determined that the PGA was most frequently used in clinical trials in 2018–2019. Since there are so many measurement instruments, determining which to use can be difficult. Standardizing these tools in research and in clinical practice may improve the utility of outcome measures.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–20. https://doi.org/10.1046/j.1365-2230.2001.00832.x.
Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386(9997):983–94. https://doi.org/10.1016/S0140-6736(14)61909-7.
Naldi L. Scoring and monitoring the severity of psoriasis. What is the preferred method? What is the ideal method? Is PASI passe? Facts and controversies. Clin Dermatol. 2010;28(1):67–72. https://doi.org/10.1016/j.clindermatol.2009.03.001.
Feldman S, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(suppl 2):ii65–i8.
Poór AK, Rencz F, Brodszky V, Gulácsi L, Beretzky Z, Hidvégi B, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L in psoriasis patients. Qual Life Res. 2017;26(12):3409–19.
Berth-Jones J, Grotzinger K, Rainville C, Pham B, Huang J, Daly S, et al. A study examining inter-and intrarater reliability of three scales for measuring severity of psoriasis: Psoriasis Area and Severity Index, Physician’s Global Assessment and Lattice System Physician’s Global Assessment. Br J Dermatol. 2006;155(4):707–13.
Loo W, Diba V, Chawla M, Finlay A. Dermatology life quality index: influence of an illustrated version. Br J Dermatol. 2003;148(2):279–84.
Basra M, Fenech R, Gatt R, Salek M, Finlay A. The dermatology life quality index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159(5):997–1035.
Langenbruch A, Radtke M, Gutknecht M, Augustin M. Does the Dermatology Life Quality Index (DLQI) underestimate the disease-specific burden of psoriasis patients? J Eur Acad Dermatol Venereol. 2019;33(1):123–7.
Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology. 2005;210(3):194–9.
Langley RG, Feldman SR, Nyirady J, van de Kerkhof P, Papavassilis C. The 5-point Investigator’s Global Assessment (IGA) scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatol Treat. 2015;26(1):23–31.
Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician’s global assessment. J Am Acad Dermatol. 2004;51(4):563–9.
Feldman S. A quantitative definition of severe psoriasis for use in clinical trials. J Dermatol Treat. 2004;15(1):27–9.
Po LW. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol. 1999;141(2):185–91.
Long C, Finlay A, Averill R. The rule of hand: 4 hand areas= 2 FTU= 1 g. Arch Dermatol. 1992;128(8):1129–30.
Stern RS, Armstrong RB, Anderson TF, Bickers DR, Lowe NJ, Harber L, et al. Effect of continued ultraviolet B phototherapy on the duration of remission of psoriasis: a randomized study. J Am Acad Dermatol. 1986;15(3):546–52.
• Armstrong AW, Siegel MP, Bagel J, Boh EE, Buell M, Cooper KD, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76(2):290–8 This is a consensus paper that provides guidelines on what outcome measurements to use endrosed by the National Psoriasis Foundation. This is important as it is a well-known professional soceity offereing guidance on this topic.
Golbari NM, Porter ML, Kimball AB. Current guidelines for psoriasis treatment: a work in progress. Cutis. 2018;101(3S):10–2.
• Coates LC, FitzGerald O, Merola JF, Smolen J, van Mens LJ, Bertheussen H, et al. Group for research and assessment of psoriasis and psoriatic arthritis/outcome measures in rheumatology consensus-based recommendations and research agenda for use of composite measures and treatment targets in psoriatic arthritis. Arthritis Rheum. 2018;70(3):345–55 This paper provided consenses from GRAPPA’s meeting to disucss recommendations for use of measurements and treatment targets. This is important as it provides additional guidelines for which outcome measurements to use.
Mrowietz U, Kragballe K, Reich K, Spuls P, Griffiths C, Nast A, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10.
Puzenat E, Bronsard V, Prey S, Gourraud PA, Aractingi S, Bagot M, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24:10–6.
Kirby B, Richards HL, Woo P, Hindle E, Main CJ, Griffiths CE. Physical and psychologic measures are necessary to assess overall psoriasis severity. J Am Acad Dermatol. 2001;45(1):72–6.
• Wechter T, Heath M, Aung-Din D, Sahni D, Cline A, Feldman SR. Current psoriasis efficacy outcome measures in clinical trials. Curr Dermatol Rep. 2018;7(4):261–8 This paper is a review paper in which studied the outcome measurements from 2017–2018. The methods was very similar to the methods used for this paper; however, we incorportated clinical practice guidelines and reviewed measurements for 2018–2019.
Pascoe VL, Enamandram M, Corey KC, Cheng CE, Javorsky EJ, Sung SM, et al. Using the Physician Global Assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol. 2015;151(4):375–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Steven Feldman has received research, speaking, and/or consulting support from a variety of companies including Galderma, GSK/Stiefel, Almirall, Leo Pharma, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Valeant, Abbvie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate, and National Psoriasis Foundation. He is founder and majority owner of www.DrScore.com and founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment.
Payvand Kamrani and Arjun Bashyam have no conflicts to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Psoriasis
Rights and permissions
About this article
Cite this article
Kamrani, P., Bashyam, A.M. & Feldman, S.R. Review of Outcome Measures in Trials and Practice for Psoriasis. Curr Derm Rep 8, 313–318 (2019). https://doi.org/10.1007/s13671-019-00283-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-019-00283-0