Abstract
Purpose of Review
Lentigo maligna (LM) is a form of melanoma in situ and represents a diagnostic and therapeutic challenge due to its poorly defined, irregular borders; propensity for subclinical extension; and location on the background of severely sun-damaged skin. Reflectance confocal microscopy (RCM) is a non-invasive optical imaging tool that allows clinicians to detect LM and assess margin status in vivo.
Recent Findings
RCM is very accurate for diagnosing LM and guiding biopsies, with sensitivities ranging from 93 to 100% and specificities 71–82%. It has also been used pre-operatively to map lesions, intra-operatively for real-time evaluation of surgical margins, and post treatment to monitor for recurrence.
Summary
The advent of newer technologies, including RCM, has advanced our ability to better diagnose and manage LM. RCM offers clinicians the ability to guide biopsies, accurately detect subclinical disease, and readily map LM margins. Future research is aimed at using RCM to allow clinicians to better spare healthy tissue and to reduce disease recurrence without subjecting patients to the lost time, cost, and potential morbidity associated with multiple surgeries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cutaneous melanoma is an increasingly common malignant tumor. The incidence of melanoma has steadily risen in the USA over the past 30 years, and an estimated 161,790 new cases will be diagnosed in 2017. Of these new cases, approximately 74,680 will be non-invasive tumors (or melanoma in situ, MIS) [1]. Since the prognosis of melanoma is most directly correlated to tumor thickness at the time of diagnosis, the ability for clinicians to accurately detect these lesions early while they are still relatively thin is paramount to improving patient survival.
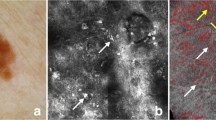
Lentigo maligna (LM) is one such type of MIS that occurs on chronically sun-exposed skin. LM can be surgically cured with minimal risk for disease-related mortality if detected early; however, 5–15% of these lesions may progress to invasive melanoma (lentigo maligna melanoma, LMM) [2, 3]. Clinical diagnosis of LM can be challenging, even for experienced dermatologists, as the lesions of LM occur on the skin with heavy actinic damage and often show overlapping features with benign pigmented lesions such as pigmented actinic keratoses, solar lentigines, seborrheic keratoses, and lichen planus-like keratosis (Fig. 1). Advances in dermoscopy have helped clinicians to better distinguish LM from benign pigmented lesions, but dermoscopy too is limited by its inability to distinguish shared features between benign and malignant pigmented macules, particularly in early, difficult-to-diagnose LMs [4].
Indeed, histologic assessment is the current gold standard for the diagnosis of LM. Biopsies, though, can be complicated by a variety of factors: diffused background melanocytic hyperplasia obscuring the diagnosis, presence of collision lesions, and histologic heterogeneity of LM throughout different areas of the lesion. As a result, multiple “scouting” biopsies are often required to increase diagnostic certainty. Even with the adjunct use of dermoscopy and Wood’s lamp, invasive areas of melanoma and areas of amelanotic melanoma may still be missed. As the number of biopsies obtained increases, so too can patient morbidity and costs of care. These factors are of particular concern in the setting of LM as these lesions tend to occur in cosmetically and functionally sensitive areas of the head and neck and have significant subclinical extension.
Reflectance confocal microscopy (RCM) is a non-invasive optical imaging tool that allows clinicians to detect LM in vivo, without the need for biopsy. RCM takes advantage of interactions between near-infrared laser light and endogenous contrast agents in the skin such as melanin and keratin to provide real-time, non-invasive imaging with a cellular-level resolution comparable to conventional histology. The primary devices on the market at present include the VivaScope 1500 and VivaScope 3000 (Caliber ID, Rochester, NY, USA) (Fig. 2). The VivaScope 1500 has a semi-flexible head that moves freely along the x- and y-axes, making it best suited for imaging the relatively planar surfaces of the trunk, extremities, forehead, and cheeks. The VivaScope 3000 utilizes a more compact handheld device with a 5-mm tip that allows for real-time video imaging over a 750-by-750-μm field of view and ease of access to anatomically difficult areas such as around the eye or behind the ear. Each device permits both static image capture and real-time assessment, as well as the creation of multi-image 3D stacks extending through a section of intact skin. Individual frames may be stitched together to enhance the field of view at a fixed depth. To date, the utility of RCM has been investigated in a wide variety of neoplastic processes, including melanoma, non-melanoma skin cancers, and Merkel cell carcinoma. RCM has also shown great potential in the evaluation of vascular, inflammatory, and infectious dermatologic lesions [5,6,7,8,9,10,11]. This article will review the use of RCM in the margin assessment of LM, specifically highlighting its ability to accurately detect subclinical disease, thereby enabling clinicians to use RCM not only for diagnosis but also for margin assessment and the extent of subclinical spread. As a result, surgical margins can be pre-operatively determined and the site can be longitudinally monitored for recurrence.
Features of LM on RCM
RCM is capable of imaging intact skin to the level of the superficial papillary dermis, corresponding to a maximum depth of about 200 μm. Images appear in horizontal, en face sections with lateral and axial resolutions of 1.25 and 5 μm, respectively. Pellacani et al. were among the first to characterize melanoma using RCM, and later, Guitera et al. proposed an algorithm specifically for the diagnosis of LM [12, 13]. In Guitera’s pivotal study, a total of 64 RCM features were systematically evaluated in a set of 284 clinically equivocal macules of the face. From this data, two major criteria and four minor criteria were adopted to distinguish LM from benign macules. The major criteria, which received a score of +2 points each, include the presence of large round pagetoid cells (larger than surrounding keratinocytes) and non-edged dermal papillae. The minor criteria, scored +1 point each, include the presence of nucleated cells in the dermal papillae, follicular localization of atypical cells, and three or more atypical cells at the dermal-epidermal junction in five 0.5 × 0.5-mm2 images (Fig. 3). An additional minor criterion, scored −1 point, is based on the presence of a benign-appearing broadened honeycomb pattern of the epidermis. A score of ≥2 using these criteria is generally considered positive with a resultant sensitivity of 85% and specificity of 76% in their study series. Of note, the LM score proposed by Guitera cannot reliably be used for the assessment of LM margins, as there may be only mild cytologic atypia and blending of the lesion into background melanocytic hyperplasia [14]. In these areas, RCM operators must be alert for subtle isolated morphologic changes, including atypical pleomorphic cells, pagetoid spread, epidermal disarray, and periadnexal extension.
RCM Performance in Diagnosing LM
The diagnostic utility of RCM for LM has been shown to be quite good. In Guitera’s original study, RCM achieved a sensitivity of 93% and specificity of 82% for diagnosing LM in a test set of 29 LMs and 44 benign macules. RCM was equally effective in diagnosing amelonotic lesions. Alani et al. have additionally reported a case of RCM used in the detection and monitoring of amelonotic LM, a rare but often misdiagnosed entity due to its lack of pigment [15]. Likewise, Menge et al. demonstrated a high degree of concordance between RCM and histopathology using a handheld RCM device. In a series of 63 biopsy sites obtained from 17 patients, handheld RCM showed a sensitivity of 100% and specificity of 71% in detecting LM [16•]. Importantly, this series included cases of recurrent and/or previously treated lesions. These lesions pose significant hurdles in diagnosis for clinicians due to their frequent association with non-specific pigmentation or treatment-induced inflammation. Taken together, these studies support the use of RCM to non-invasively detect LM with an accuracy approaching that of conventional histology when used by experienced dermatologists.
RCM Mapping of LM in Dermatologic Surgery
Delineating margins of LM is challenging because of its horizontal growth and subclinical extension beyond the clinically apparent margins. Moreover, clear demarcation is complicated when lesions occur amidst a field of sun-damaged skin and neighboring solar lentigines as the margin of a LM may trail off into a background of melanocytic hyperplasia. This phenomenon can sometimes be seen clinically and histologically. Hazan et al. have shown that, on average, LM of the head and neck requires a margin of 7 mm to clear surgically [17]. When approaching LM treatment surgically, accurate margin control is paramount to prevent persistent or recurrent disease.
In the setting of dermatologic surgery, RCM has emerged as a robust tool for mapping LM and evaluating lesion margins prior to surgical or non-surgical treatment. Traditional management of LM includes the approximation of surgical margins using a combination of clinical examination, dermoscopy, and Wood’s light examination, then performing surgery based on such. The type of surgical excision, whether staged excision or Mohs micrographic surgery, may need to be repeated because of the degree of subclinical extension inherent to LM. Assessing the margins histologically may also post a challenge due to the high degree of background photodamage and melanocytic hyperplasia that can surround the lesions of LM. In a recent study by Bolshinsky et al., LM was found to be the most likely melanoma subtype to have residual disease following wide local excision (odds ratio = 2.7). Such imprecision can be frustrating for clinicians and patients alike due to the time, cost, and discomfort associated with multiple staged excisions. Chen et al. first reported a case of RCM as an adjunct to Wood’s light and dermoscopy in the pre-operative evaluation of surgical margins of a large LM of the scalp [18]. RCM allowed for a more precise outline of the approximate lesion margins initially determined by dermoscopy and Wood’s light examination. The excised area as determined by RCM was free of tumor at the margins on histopathology.
Several authors have since demonstrated the utility of RCM in the perioperative margin determination of LMs. For example, in a case series of 37 patients with clinically challenging LMs (faintly colored/amelonotic and/or recurrent lesions) done by Guitera et al., RCM altered the management in 27 (73%) patients due to the detection of a subclinical disease extending beyond the standard 5-mm margins initially determined by clinical examination or dermoscopy [19]. Eleven of these 27 patients required a different reconstruction technique than originally planned, while the other 16 were offered radiotherapy or topical treatment because excision and reconstruction of their lesions were considered too challenging. Though these authors only imaged the lesions with RCM in four radial directions, on average, the clinical margins were shown to be 60% smaller compared to RCM findings.
Building on this idea, the current authors image surgical margins continuously using the handheld VivaScope 3000 for pre-, intra-, and post-operative mapping of LM margins. One advantage of intra-operative mapping of LM margins is the ability to rapidly evaluate the periphery of a resected lesion prior to surgical closure. Hibler et al. described a case in which RCM used immediately after excision of 1-cm margins as determined by Wood’s light examination of an LM on the cheek revealed residual tumor at one edge of the lesion [20]. Post-operatively, the rate of recurrence of LM excised by conventional surgery is typically noted to be 10–20% but has been reported to be as high as 31%. In contrast, there are very few instances of LM recurrence observed in the above studies and others in which RCM was used to determine surgical margins. This evidence points to RCM as a potential high-value tool in the surgical management of complex LMs.
Techniques for Margination of LM Using RCM
Authors’ Approach to Mapping Margins
As a result of the aforementioned inherent challenges in defining the edges of LM, the use of margin mapping with RCM is attractive from both a physician and patient perspective. Akin to imaging tumors in advance of treatment pre-operatively in other fields of medicine, knowing the breadth of a LM’s spread on the head and neck allows the physician to counsel the patient regarding treatment, both surgically or non-surgically, and plan the reconstruction.
Herein, the general approach to mapping LM using the handheld VivaScope 3000 is outlined (Fig. 4). First, the clinical lesion margins are delineated using dermoscopy and Wood’s lamp. Some authors opt to measure and identify the standard surgical margins at 5 to 10 mm from the farthest clinical border depending on the location and whether invasion was detected on the initial biopsy. Sequences of confocal sections are captured at the previous biopsy site (if a residual lesion is still present) to serve as a control and assess for features of melanoma. Using the handheld microscope, confocal stacks of images in the z-axis, ranging from the stratum corneum down to the papillary dermis, are captured in each quadrant, often at the 12-, 3-, 6-, and 9-o’clock positions. Earlier studies suggest imaging lesions in four radial directions until no features of LM are observed in any direction [19]. The newer handheld iteration of RCM has overcome these time constraints, allowing for RCM video imaging to be assessed in real time, circumferentially, and in free form along the entire periphery of the surgical margins of each quadrant [21•]. This imaging is performed at the level of the dermoepidermal junction. If previously described features of LM, including large, round pagetoid cells and epidermal disarray, are identified along the surgical margin, RCM is used to interrogate the margins out radially until no further features are identified and this new surgical margin is marked with a surgical marker. The use of video capture is to recreate video mosaics by stitching together sequences of images to recreate a larger field of view [22•]. Additional stacks of images can be captured at suspicious sites within the lesion to assess for perifollicular infiltration suspicious for invasion or at surrounding pigmented macules also suspicious for LM. When the final surgical margins are delineated (Fig. 5), a planned staged surgical excision with circumferential histologic margin assessment takes place along the RCM-outlined margins.
Lentigo maligna map. The blue circle denotes Wood’s lamp margins. The yellow circle is the 5-mm surgical margin aligned with the inner circle of the paper ring. At the 7-o’clock position, the 5-mm surgical margin was deemed positive, so imaging was performed in a radial fashion across the paper ring (2 mm) to a new surgical margin of 7 mm and was negative at this distance. Bx biopsy, MMIS malignant melanoma in situ, + positive features of lentigo maligna, − no features of lentigo maligna
Alternative “Spaghetti Technique”
Multiple surgical techniques have been utilized for LM, including staged excision with complete circumferential margin assessment (MSKCC technique), Mohs micrographic surgery (MMS), and the “spaghetti technique.” The spaghetti technique was described by Gaudy-Marqueste et al. [23], in which thin strips of the peripheral margin surrounding a lesion are first excised and sent for pathologic examination without removing the LM. This is repeated until the minimal tumor-free margins are excised. Subsequently, excision of the central island of LM and reconstruction are performed at the same time.
Champin et al. described their approach combining the spaghetti technique with in vivo RCM to define LM margins more accurately [14]. In their protocol, the surgical margins were outlined using in vivo RCM (VivaScope 3000, Caliber, MAVIG GmbH, Munchen, Germany). Imaging was performed down through the papillary dermis. Margins were considered positive if there was at least one bright, large (>20 μm), round/dendritic cell in the epidermis and considered negative in the absence of these atypical cells.
Marking began at the clinical and dermoscopic margins of LM, and the scope was moved upward until the first malignant cell-free examination. This was performed in a clockwise fashion around the LM by moving either closer or further to the clinical margin in 5-mm increments (the size of the tip of the VivaScope 3000 camera) and by making dots on the skin at the center of the camera “footprint.” The dots were then connected to create one continuous RCM margin clear beyond any atypical cells. Surgical excision took place in accordance with the spaghetti technique as previously described, using a 2-mm-wide double-bladed knife, leaving the bulk of the LM in place. This tissue margin was sent for pathology, and in the case of a positive margin, a new “spaghetti” piece limited to the affected area was resected using the same technique. A limitation of this spaghetti technique is that an invasive disease in the center of the lesion may not be assessed until final excision and repair.
Paper Rings and Radial Approach
A limitation in the use of RCM for margination is the exact pinpoint correlation between imaging cellular structures and their corresponding cutaneous location. In order to improve the registration between the exact area of RCM imaging and the site of planned biopsy or excision, the use of gummed rings or paper rings has been suggested [24, 25]. Commercially available sticky paper rings of varying widths are selected according to lesion size. One advantage of sticky paper rings is their ability to conform to curved surfaces of the skin or to be cut and stuck together to create modified shapes for lesions that are not uniformly circular. Until a fully automated “scan” is developed, the use of these cutaneous markers helps to physically locate the scope to the lesion demarcation. Multiple scenarios exist for the use of these paper ring demarcations.
-
1.
Paper rings are available in a variety of sizes (varying diameter). As such, a paper ring may be chosen such that the lesion fits nicely in the center. Since the width of the ring is a specific measure, imaging around the ring provides a constant millimeter margin and allows the user to follow along the ring for precise imaging registration.
-
2.
If a larger ring is used such that the lesion and a 3-mm margin are within the inner circle of the ring, this boundary can be assessed for features of LM. If features are identified, imaging radially across the paper ring may be performed, providing an additional x-mm margin of tissue (depending on the width of the paper ring). Imaging is captured along the entire circumference of the inner ring, and, when positive, along the edge of the outer ring.
In both applications, markings with a surgical pen are used to create a preliminary map. An advantage of this approach is the consistency of mapping along the paper ring, as the ring appears black on RCM and the edge of the paper is readily identified (Fig. 6). Challenges include LM of atypical shapes when multiple paper rings must be pieced together to create a track to image along and that the rings may slide on the skin when too much oil is applied.
Reflectance confocal microscopy features of lentigo maligna and the paper ring approach. Green arrows large, dendritic, atypical cells with perifollicular infiltration suspicious for lentigo maligna, blue dahed line the edge of the surgical margin as it approaches the paper ring, red asterisk the dark region with white structures is the paper ring with fibers, which does not reflect the light from the microscope
Monitoring LM Using RCM
Another area in which RCM has gained significant traction is in the non-surgical management of LMs. Non-surgical therapies for LM, such as topical imiquimod, may be offered when surgery would be disfiguring or multiple medical co-morbidities are present. Cryotherapy and radiation therapies have also been utilized in these circumstances. However, these therapies are prone to the development of local recurrence, often due to the inadequate treatment of the entire lesion because of incomplete penetrance, incorrect margins, or incomplete eradication of tumor cells over the course of the treatment. As noted above, the local recurrence of LM following definitive therapy can be challenging to diagnose due to non-specific pigmentation, background photodamage, and scarring and inflammation surrounding the initial lesion.
The ability of RCM to directly visualize bright, atypical melanocytes within lesions undoubtedly affords clinicians an added advantage in assessing true tumor margins, monitoring response to treatment, and identifying local recurrence. There have been numerous studies detailing the use of RCM for these purposes [20, 26,27,28]. Importantly, in their study of 98 patients undergoing topical therapy for biopsy-confirmed LM, Guitera et al. demonstrated that the recognition of treatment failure could be more reliably detected with RCM compared to dermoscopy (100% sensitivity and 94% specificity vs. 80% sensitivity and 56% specificity, respectively) [29]. Similarly, Alarcon et al. have shown that there is no significant difference between RCM and conventional histology in accurately assessing tumor clearance following a course of topical imiquimod [30]. Nadaminti et al. also utilized RCM pre- and post-topical imiquimod treatment for a large complex facial LM and showed resolution of such [27], while Kai et al. used RCM to assess the tumor clearance of an area of previous LM following incomplete excision and adjuvant imiquimod use [31]. The ability of RCM to image the same area of the skin over time without biopsy may further enhance its diagnostic range, as dynamic skin changes could help clinicians to detect recurrence earlier and to limit false positives.
Limitations
Although the use of RCM in the management of complex skin lesions such as LM has dramatically expanded in recent years, there still exist several obstacles that may limit its more widespread use in its current state (Table 1). The fields of view for both the static 1500 and handheld 3000 VivaScopes are small relative to the size of the probe. Therefore, there are still areas not amenable to imaging, and for large lesions, imaging may take a considerable amount of time. There is also the initial costs of the devices, ranging in price from £62,300 to £90,224 depending on devices and manufactures [32]. Additionally, the use of the device requires extensive training to properly set up, capture, and interpret RCM images, and images may be time-intensive to acquire, particularly in the hands of inexperienced users. Finally, the technical limitations of the device may not make RCM suitable for use in all patients. For example, lesions occurring on relatively thick skin may not be wholly visualized given the device’s depth limit of about 200 μm. Similarly, the presence of heavy scale on a lesion may obscure RCM images and limit accuracy.
Conclusion
Advances in RCM over the past decade appear to hold tremendous potential in improving the management of LM. The ability of RCM to reliably detect LM in the setting of diffused solar damage, variable lesion pigmentation, and even previously treated skin may help to alleviate the diagnostic uncertainty many clinicians face in the evaluation of clinically equivocal macules of the head and neck. This in turn may lead to fewer biopsies of benign lesions and earlier detection of malignancy. Within the context of treatment of LM, RCM is a useful tool for defining lesion margins and monitoring for recurrence.
As RCM continues to evolve in the hands of the industry, academia, and private practice alike, it is possible that new developments in technology or user education will further enhance the benefits of RCM while minimizing barriers to usage such as those mentioned in the “Limitations” section. For instance, advances in technology may lower production costs of the devices or allow for the addition of new features such as real-time video mosaic generation that could increase image acquisition speed and improve ease of interpretation. One by-product of this goal would be to one day have an automated “scan” capable of imaging complex lesions of varying shapes and sizes over different topographies in order to create a digital map upon which the physician can act. Furthermore, education initiatives driven by experienced users of the devices, such as hands-on workshops or the production of RCM imaging atlases, could increase the accessibility of RCM for new and inexperienced users. Developments such as these represent exciting possibilities in improving the management of LM using RCM, allowing clinicians to better spare healthy tissue and to reduce disease recurrence without subjecting patients to the lost time, cost, and potential morbidity associated with biopsy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2014. Bethesda: National Cancer Institute. https://seer.cancer.gov/csr/1975_2014/ based on November 2016 SEER data submission, posted to the SEER web site 2017. Accessed March 1, 2017.
Weinstock MA, Sober AJ. The risk of progression of lentigo maligna to lentigo maligna melanoma. Br J Dermatol. 1987;116:303–10.
Penneys NS. Microinvasive lentigo maligna melanoma. J Am Acad Dermatol. 1987;17:675–80.
de Carvalho N, Farnetani F, Ciardo S, et al. Reflectance confocal microscopy correlates of dermoscopic patterns of facial lesions help to discriminate lentigo maligna from pigmented nonmelanocytic macules. Br J Dermatol. 2015;173:128–33.
Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017 doi:10.1007/s00432-017-2391-9.
Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside. Lasers Surg Med. 2017;49:7–19.
Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487–96.
Lacarrubba F, Verzi AE, Pippione M, et al. Reflectance confocal microscopy in the diagnosis of vesicobullous disorders: case series with pathologic and cytologic correlation and literature review. Skin Res Technol. 2016;22:479–86.
Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754–63.
Ardigo M, Prow T, Agozzino M, et al. Reflectance confocal microscopy for inflammatory skin diseases. G Ital Dermatol Venereol. 2015;150:565–73.
Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222–48.
Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions—improvement in melanoma diagnostic specificity. J Am Acad Dermatol. 2005;53:979–85.
Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080–91.
Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40:247–56.
Alani A, Ahmad K. Diagnosis of amelanotic lentigo maligna by using in vivo reflectance confocal microscopy. Acta Derm Venereol. 2016;96(3):406–7. doi:10.2340/00015555-2267.
• Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74:1114–20. This study demonstrates the effectiveness of handheld reflectance confocal microscopy in the diagnosis of lentigo maligna.
Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142–8.
Chen CS, Elias M, Busam K, et al. Multimodal in vivo optical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. Br J Dermatol. 2005;153:1031–6.
Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692–8.
Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980–3.
• Hibler BP, Yelamos O, Cordova M, et al. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis. 2017;99:346–52. This case series outlines the utility of reflectance confocal microscopy for managing complex cases of lentigo maligna both surgically and non-surgically.
• Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171(5):1239–41. doi:10.1111/bjd.13050. This manuscript describes the approach for creating confocal video mosaics to increase the field of view. This technique is often applied to create maps of the periphery of lesions in a clockwise fashion.
Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The “spaghetti technique”: an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113–8.
Ali FR, Craythorne EE. Gummed rings as the outer marker of microscopically examined tissue (GROMMETs) as mapping adjuncts to in vivo reflectance confocal microscopy (RCM). J Am Acad Dermatol. 2016;75:e103–4.
Marino ML, Rogers T, Sierra Gil H, et al. Improving lesion localization when imaging with handheld reflectance confocal microscope. Skin Res Technol. 2016;22:519–20.
Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5(5):e543–7. doi:10.1016/j.prro.2015.03.006.
Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177–84.
Erfan N, Kang HY, Cardot-Leccia N, et al. Reflectance confocal microscopy for recurrent lentigo maligna. Dermatol Surg. 2011;37:1519–24.
Guitera P, Haydu LE, Menzies SW, et al. Surveillance for treatment failure of lentigo maligna with dermoscopy and in vivo confocal microscopy: new descriptors. Br J Dermatol. 2014;170:1305–12.
Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49–55.
Kai AC, Richards T, Coleman A, et al. 5 year recurrence rate of lentigo maligna after treatment with imiquimod. Br J Dermatol. 2016;174(1):165–8. doi:10.1111/bjd.14311.
Edwards SJ, Mavranezouli I, Osei-Assibey G, et al. VivaScope® 1500 and 3000 systems for detecting and monitoring skin lesions: a systematic review and economic evaluation. Southampton: NIHR Journals Library; 2016. Health Technology Assessment, No. 20.58. https://www.ncbi.nlm.nih.gov/books/NBK378819/. doi:10.3310/hta20580.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding/Support
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Additional information
This article is part of the Topical Collection on Skin Cancer
Rights and permissions
About this article
Cite this article
Menge, T.D., Hibler, B.P., Cordova, M. et al. Reflectance Confocal Microscopy for Margin Assessment and Management of Lentigo Maligna. Curr Derm Rep 6, 222–229 (2017). https://doi.org/10.1007/s13671-017-0194-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-017-0194-5