Abstract
Purpose of Review
The purpose of this review is to update readers on recent advancements in the use of immune checkpoint inhibitors for the treatment of ovarian, uterine, and cervical cancers.
Recent Findings
Immunotherapy has emerged as a novel therapeutic paradigm in the treatment of gynecologic malignancies. Currently, immune checkpoint inhibitors are approved for use across five solid malignancies, with recent approval of pembrolizumab in patients with MMR-deficient, recurrent, solid tumors in a disease site agnostic fashion. Phase 3 clinical trials are being conducted in the gynecologic cancer arena to determine if checkpoint inhibition will improve oncologic outcomes. Positive signals have been identified in ovarian cancer cohorts, both as single agents and in combination with other agents. It is anticipated that immunotherapy will be effective in MMR-deficient endometrial cancers, and trials are in development to explore these agents in the front line. Furthermore, the HPV-driven biology of cervical cancer suggests that immune checkpoint inhibition may lead to clinical benefit.
Summary
Immune checkpoint inhibitors represent a dynamic and exciting opportunity for patients with limited therapeutic options. We eagerly await the results of ongoing phase 3 clinical trials that will inform practice patterns. In addition, emphasizing translational end-points informing patient selection and response is critical.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
On May 23, 2017, in a landmark decision, the U. S. Food and Drug Administration (FDA) granted accelerated approval for pembrolizumab use in microsatellite instability-high (MSI-high) or mismatch repair (MMR)-deficient recurrent cancers. This represented the first disease site agnostic drug approval that was a biomarker dependent in solid tumors. It is anticipated that this paradigm shift in cancer treatment will continue to catalyze the investigation of novel therapeutics across disease sites that are based on molecular markers.
Given the limited oncologic gains to date with traditional cytotoxic chemotherapy, numerous clinical trials in the gynecologic cancer arena are testing targeted biologic agents alone and in combination with cytotoxic drugs. Blocking or disrupting tumor angiogenesis, enhancing the anti-tumor immune response, inhibiting proliferative pathways, and modulating DNA repair activity to cause synthetic lethality represent a handful of the targets that are currently being explored.
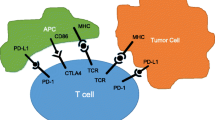
Pembrolizumab, a humanized monoclonal antibody to the PD-1 receptor, belongs to a class of relatively new cancer therapies termed immune checkpoint inhibitors, which function by increasing the body’s immune response against cancer cells. This is accomplished by “inhibiting the inhibitors” that work to suppress the immune response to foreign cancer-related neoantigens. Checkpoint inhibitors, as a class, exert their immune-related effects through regulation of the T cell response. Activated T cells express receptors that receive stimulatory and inhibitory signals and respond to the sum of these signals. PD-1 and CTLA-4 ligands favor T cell inactivation when they bind to their respective receptors. Blockade of this signaling pathway results in T cell stimulation, as demonstrated by anti-PD-1 blocking monoclonal antibodies.
Using in vitro model systems, robust T cell responses to tumor antigens are easily elicited. However, in previous trials examining traditional cytotoxic therapy across solid malignancies, translational investigation failed to identify a robust immune-mediated response. Through a significant amount of both basic science and translational research, it became evident that tumors acquired a resistance to immune destruction. Investigations into the mechanisms of immune evasion revealed complex signaling mechanisms involving, amongst others, the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and its ligand PD-L1 that work to inhibit T cell activation. Programmed cell death protein ligand 1 (PD-L1) is expressed on numerous carcinomas including the lung, ovary, colon, bladder, breast, and cervix. Thus, tumors may exploit this pathway of immune modulation to escape destruction by the immune system facilitating continued proliferation and metastases [1, 2].
In addition to the PD-1/PD-L1 pathway, immune response attenuation involves alternate inhibitory molecules, including CTLA-4. For activation, T cells require costimulatory signals in addition to antigen. Cluster of differentiation 28 (also known as CD28) is a receptor on T cells that binds to the B7 ligand to provide this stimulatory signal. CTLA-4 binds B7 with higher affinity than CD28, but results in T cell inhibition, suspending T cell proliferation and reducing pro-inflammatory cytokine secretion. Expression of CTLA-4 has been shown to inhibit T cell proliferation in vitro, while blockade enhances T cell proliferation [3].
Ovarian Cancer
In 2017, there will be an estimated 22,440 new cases of ovarian cancer in the USA, with approximately 14,080 deaths. The 5-year survival rate of ovarian cancer has increased only modestly from 36% in 1975–1977 to 46% in 2005–2011 [4]. These limited survival gains are attributed to advanced stage at the time of diagnosis due to lack of an effective screening algorithm as well as the development of chemotherapy resistant clones. Following front-line combinatorial platinum-based chemotherapy, the durable response rate to additional lines of treatment in patients with recurrent ovarian cancer remains below 30% [5]. Given this unmet clinical need, investigators have turned their attention to novel approaches, including immunotherapies, examining checkpoint inhibitors as single agents or in multi-agent regimens.
Host immunogenicity against tumor cells is not a novel concept, as observations made as early as the nineteenth century noted tumor regression in the setting of infection. Attempts at “immunotherapy” using vaccination techniques of tumor extracts and various toxins were unsuccessful and were thus eventually overshadowed by other more effective therapeutic modalities [6]. Contemporary research revisiting immunotherapy has identified encouraging results in various cancer types, including ovarian cancer.
In 1991, Ma et al. first reported a survival benefit with the presence of tumor-infiltrating lymphocytes (TILs) in patients with advanced stage ovarian cancer. These TILs are detected in the tumor and tumor stroma and recognize tumor cells as immunogenic/foreign resulting in an immune-mediated response [7•, 8]. Zhang et al. noted a 38% five-year survival in 102 patients whose primary tumors contained T cells, compared to only 4.5% five-year survival in 72 patients whose tumors did not have T cells in their tumor islets, indicating an oncologic advantage conferred by the host immune response [9]. Most recently, a meta-analysis of 21 eligible studies supported the prognostic value of the presence of TILs in patients with advanced stage ovarian cancer [8].
Currently, there are multiple clinical trials that are active or in development examining various immune checkpoint inhibitors in combination with chemotherapy, with other biologic agents, epigenetic modulators, or with vaccines. These agents are being investigated in parallel in the front-line setting (both adjuvant and neoadjuvant approaches), in the recurrent platinum sensitive settings as well as in patients with platinum-resistant or platinum refractory disease (Table 1).
In the primary setting, the majority of patients with ovarian cancer undergo cytoreductive surgery (CRS) followed by six cycles of adjuvant chemotherapy with the cytotoxic agents carboplatin and paclitaxel. Underlying the high response rate of up to 80% to front-line therapy is the almost equally high recurrence rate and the eventual development of platinum resistance. Identifying a successful first-line regimen that effectively delays or prevents recurrence would significantly impact patient outcomes and represent an oncologic milestone in the treatment of advanced stage ovarian cancer.
The use of cytotoxic chemotherapy in combination with checkpoint inhibition in the front-line setting may promote immunogenic cell death and activation of the immune response to residual viable tumor cells, enhancing the anti-tumor activity of checkpoint inhibitors. Multiple reports have demonstrated increased serum inflammatory cytokines, pro-inflammatory changes in tumor microenvironment, and induction of tumor-specific immune responses after exposure to chemotherapy [10]. Following exposure to cytotoxic chemotherapy, it is hypothesized that checkpoint inhibitors may play a role in reversing the subsequent immune tolerance observed in the tumor microenvironments. JAVELIN OVARIAN 100, an active phase III clinical trial, is looking to examine the utility of avelumab, a human monoclonal antibody to the PD-L1 receptor, in combination with chemotherapy in patients with advanced stage ovarian, fallopian tube, or primary peritoneal cancer. The trial is designed to test the combination regimen followed by placebo, in addition to a maintenance approach (NCT02580058). The increased antigenicity of the tumor cells after exposure to chemotherapy along with stimulation of the immune response is hypothesized to translate into improved survival outcomes.
Traditionally, the use of neoadjuvant chemotherapy (NACT) in patients with ovarian cancer was reserved for patients with unresectable disease or those who were deemed unfit to undergo primary cytoreduction. However, following the publication of the Vergote and CHORUS studies, which concluded the non-inferiority of NACT to primary debulking [11], there has been a gradual increase in the utilization of NACT in the USA. Data from the National Cancer Data Base that captures 70% of newly diagnosed malignancies in the USA revealed a significant increase in the frequency of NACT for advanced stage ovarian cancer (IIIC-IV) from 2004 to 2013, increasing from 8.6 to 22.6% (p < 0.001) [12].
In the context above, new clinical trials are specifically examining the efficacy of checkpoint inhibition in the neoadjuvant setting. Pembrolizumab, in combination with carboplatin and paclitaxel, is being assessed in a single-arm phase II study (NCT02834975). The ability to conduct these “window of opportunity trials” provides the additive benefit of tissue samples before and after exposure to immune checkpoint inhibitors. This study design may help identify biomarkers predictive of response or resistance in future trials and may inform novel approaches.
Additionally, in some cases, surgical resection may leave patients with residual disease implants > 1 cm in size, portending a poor prognosis. The role of immune checkpoint inhibitors in the suboptimally cytoreduced ovarian cancer population is being examined. In a single-arm, phase II non-randomized clinical trial, patients with measurable disease after maximal effort cytoreductive surgery will be treated with pembrolizumab, carboplatin, and, weekly, paclitaxel, followed by 12 months of pembrolizumab maintenance (NCT02766582). The primary end-point for this study will be progression-free survival (PFS).
In an effort to expand the therapeutic role of checkpoint inhibition, novel combinations are being examined in patients with solid tumors, including metastatic, recurrent ovarian cancer. In one such study, pembrolizumab is being combined with pexidartinib (PLX3397), an inhibitor of colony stimulating factor 1 (anti-CSF1). The biologic rational of this approach rests on the proposed mechanism of action of pexidartinib. The presence of tumor-associated macrophages (TAMs) is associated with tumor growth and chemotherapy resistance. In addition, the presence of myeloid-derived suppressor cells (MDSCs) inhibits anti-tumor immunity and is hypothesized to lead to anti-PD-1 resistance. The use of pexidartinib is anticipated to antagonize these immune-inhibitory signals via CSF1 signaling. Pexidartinib previously received FDA breakthrough designation in October 2015 for the treatment of unresectable tenosynovial giant cell tumors, based on a small phase I study showing an impressive 52.2% ORR (95% CI 32–73%) in patients without alternate therapeutic options [13]. The confirmatory phase III clinical trial, ENLIVEN, is currently open to enrollment (NCT02371369).
Given the established importance of immunogenicity and neoantigens in cancer immunotherapy, investigators are looking to enhance responses using vaccine administration, prior to treatment with immune modulators. One such study combines the DPX-Survivac vaccine, metronomic low-dose cyclophosphamide, and pembrolizumab. This novel vaccine targets survivin, a promising tumor-associated antigen (TAA). In prior phase I/Ib studies, the vaccine was highly immunogenic when combined with cyclophosphamide in patients with ovarian cancer, inducing the stimulation of a survivin-specific cytotoxic T cell immune responses. This phase 2 clinical trial is expected to enroll 42 subjects, with the primary objective being ORR. The same agent is also being studied in combination with an investigational oral indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, epacadostat, and cyclophosphamide in patients with recurrent platinum-sensitive or platinum-resistant ovarian cancer. IDO1 inhibitors have emerged as exciting novel agents when administered in combination with checkpoint inhibitors. Indoleamine 2,3-dioxygenase 1 is an intracellular enzyme that catabolizes tryptophan resulting in an immunosuppressive microenvironment and tolerance. Essentially, tryptophan degradation and depletion result in amino acid starvation of T cells, inhibition of T cell proliferation, and differentiation of naïve CD4+ T cells into Tregs [14]. At the 2017 ASCO annual meeting, the combination of pembrolizumab and epacadostat in patients with NSCLC, bladder cancer, head and neck cancer, and renal cell carcinoma resulted in a significant improvement in ORR when compared to single agent checkpoint inhibition in historical controls.
In an alternate attempt to increase tumor immunity, poly (ADP-ribose) polymerase (PARP) inhibitors are being studied in combination with checkpoint inhibitors. The Keynote-162 study of niraparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, with pembrolizumab, is investigating the safety and efficacy of using an inhibitor of high fidelity DNA repair, with an immune-modulating agent. PARP enzymes normally respond to DNA damage by recognizing single-strand breaks and effecting DNA base excisional repair. Inhibiting PARP can induce “synthetic lethality” in BRCA-deficient tumor cells, which can no longer correct DNA damage without accumulating lethal mutations. This impaired DNA repair machinery likely results in more antigenic epitopes for immune recognition of tumor. The safety and feasibility of combining checkpoint inhibition with PARP inhibition was recently described by Lee et al., in a phase 1 study testing durvalumab and olaparib in patients with gynecologic cancer. Between June 2015 and May 2016, 19 patients with heavily pretreated recurrent ovarian cancer were enrolled. No dose limiting toxicities were reported on the durvalumab + olaparib combination. In the entire cohort (N = 26), the ORR was 17%, with an 83% disease control rate [15••].
In addition to immune checkpoint inhibition, the utility of adoptive T cell therapy in patients with ovarian cancer is currently being examined. The ACTIVATE trial, not yet open to accrual, involves a personalized, targeted method of combined modality immune stimulation. After administration of chemotherapy, patients are given allografted tumor-infiltrating lymphocytes (TILs) and low-dose interleukin 2, followed by pembrolizumab. First, lymphocytes are harvested from an individual’s tumor, expanded ex vivo in culture, then infused along with the stimulating growth factor interleukin 2 to encourage lymphocyte proliferation. This is followed by pembrolizumab monotherapy in an effort to block the PD-1 inhibitory signal, allowing for an enhanced immune response (NCT03158935).
By definition, patients with platinum refractory ovarian cancer experience disease progression while on first-line platinum-based chemotherapy and have limited treatment options. Given the genetic instability of ovarian cancer, and the lack of identifiable driver mutations, several studies in refractory disease employ a multi-targeted approach. One approach currently under study is examining a lymphocyte-activating antibody varlilumab, with nivolumab, a fully human IgG4 monoclonal anti-PD-1 antibody, in an effort to increase the immune response (NCT02335918). Varlilumab (developed by Celldex Therapeutics) is a fully human monoclonal antibody that targets CD27, which is pivotal for lymphocyte activation. Varlilumab acts as an agonist anti-CD27 antibody, activating T cells, with reduced collateral immune activation, and thus a proposed parallel reduction in side effect profile. In pivotal preclinical studies, the activation of T cells via T cell receptor binding and varlilumab results in multiple cell divisions and the secretion of pro-inflammatory cytokines [16].
Early data regarding the efficacy of avelumab, a human IgG1 monoclonal antibody that binds to PD-L1, in patients with platinum-resistant recurrent ovarian cancer was reported as part of the phase Ib JAVELIN solid tumor trial (NCT02718417). Patients with recurrent or refractory ovarian cancer were treated with avelumab 10 mg/kg intravenously every 2 weeks until disease progression, unacceptable toxicity, or withdrawal. A total of 124 patients were treated, with an objective response rate (ORR) of 9.7% (95% CI 5.1–16.3%) and a disease control rate of 54%. Median progression-free survival (PFS) was 11.3 weeks, and median overall survival (OS) was 10.8 months in this pretreated, unselected patient cohort [17].
Alternate studies aim to augment the effect of avelumab, a fully humanized monoclonal antibody to PD-L1, using etinostat, a histone deacetylase inhibitor. In tumor tissue, etinostat alters gene expression and may induce growth arrest, differentiation, and apoptosis. Recently, it has also been shown to act on the host immune system as an epigenetic modulator of the immune response. Preclinical studies have supported the observation in clinical trials that less immunogenic tumor types have a modest response rate to checkpoint inhibition alone. In a mouse xenograft model, tumors with lower mutational load expressed fewer antigens that could bind MHC–I. Etinostat, through DNA hypomethylation, altered the expression of myeloid-derived suppressor cells (MDSCs) that have potent immunosuppressive function. In a mouse xenograft model, the addition of etinostat at non-cytotoxic levels to checkpoint inhibitors eradicated tumors that had little response to either agent alone [18].
The current standard of care for the treatment of patients with platinum-resistant disease recurrence is chemotherapy plus the anti-angiogenic agent bevacizumab. This is based on the results of the AURELIA study, the first randomized phase III trial to demonstrate an improved PFS with combination over single-agent therapy. Best response rates in this population of cancer patients remain from 15 to 20% with single agents, and the median overall survival is approximately 12 months [5]. In order to better understand the clinical utility of immune checkpoint inhibitors in this same population, a phase II trial of the anti-PD-1 antibody, nivolumab, was conducted in patients with platinum-resistant recurrent ovarian cancer. A total of 20 patients were enrolled, on trial, and the majority (55%) had four or more prior lines of treatment. The best overall response rate was 15%, with a disease control rate of 45%. There were two patients who experienced a durable complete response, one with serous and the other clear cell histology. In this small exploratory study, the median progression-free survival was 3.5 months with median overall survival of 20 months [19•].
These exciting preliminary results helped catalyze the continued development of checkpoint inhibition in patients with this difficult to treat disease. Studies pairing checkpoint inhibitors with both cytotoxic agents and other biologic agents are ongoing. Pembrolizumab is being combined to the cytotoxic doublet of gemcitabine and cisplatin in one trial and to carboplatin alone in another. A larger five-arm trial combining two checkpoint inhibitors targeting PD-1 and its ligand, PD-L1, with the biologic bevacizumab with or without acetylsalicylic acid is ongoing. Also, in the recurrent setting is an NRG-sponsored trial examining two checkpoint inhibitors, nivolumab with or without ipilimumab, a CTLA-4 inhibitor.
Results from expansion cohorts in ongoing basket trials have also shown promise. The Keynote-028 phase Ib multi-cohort trial was designed to evaluate the safety and efficacy of pembrolizumab in patients with advanced solid tumors expressing PD-L1. Keynote-028 results showed an objective response rate of 11.5% in the ovarian cancer cohort, where 26 patients with advanced ovarian cancer were treated with pembrolizumab alone. There was 1 complete response, 2 partial responses, and a 23% stable disease rate (NCT02054806).
Endometrial Cancer
In 2017, approximately 61,380 women were diagnosed with endometrial cancer, with 10,9200 deaths [4]. Unfortunately, despite early symptoms and disease detection, the incidence of uterine cancer, as well as the mortality rate in the USA, continues to rise. The 5-year survival rate declined from 87% in 1975–1977 to 83% in 2005–2011 [20]. Increases in the prevalence of obesity, an aging population, the lack of effective treatment for advanced or recurrent disease, and other yet to be identified factors are thought to contribute to the rising incidence and mortality. In the context above, the recent FDA approval of single-agent pembrolizumab in patients with MMR-deficient recurrent endometrial cancer represented an oncologic milestone. Prior to this approval, which was unique given the disease site agnostic label, the treatment of patients with recurrent endometrial cancer following systemic chemotherapy was limited to hormonal agents, or cytotoxic drugs with limited efficacy.
The rationale for use of immunomodulatory agents in patients with endometrial cancer was less developed than that seen in ovarian cancer cohorts. Specifically, much less was known about the prognostic implications of the presence or absence of TILs in endometrial cancers than in ovarian cancers. Furthermore, the localization and functionality of these cells in patients with endometrial cancer were not well understood [21,22,23]. Nevertheless, given the excitement and promise surrounding immunotherapy and limited options available to these patients, clinical trials exploring checkpoint inhibitors in patients with recurrent endometrial cancer were designed.
Early immunohistochemical studies on endometrial cancer specimens of various histologies have detailed PD-1 and PD-L1 expression levels surpassing those seen in ovarian and cervical cancers, suggesting a potential role for checkpoint inhibitors in this disease setting [24,25,26](Table 2).
In a pivotal clinical trial that ushered in the continued examination of checkpoint inhibitors in patients with endometrial cancer, Le et al. conducted a phase 2 trial of single-agent pembrolizumab, in patients with mismatch repair (MMR)-deficient, MSI-high, progressive metastatic carcinoma [27••]. This trial was designed to test the hypothesis that MMR-deficient, microsatellite unstable-high tumors are more responsive to PD-1 blockade than MMR-proficient tumors, due to the high somatic mutational load, resulting in neoantigen formation and a more prominent lymphocytic infiltrate. As predicted, the two cohorts with MMR-deficient, MSI-high cancers (one with colorectal cancer patients and the other with non-colorectal cancer patients, including two patients with recurrent, previously treated endometrial cancer) had significantly higher objective response rates by immune-related response criteria and by Response Evaluation Criteria in Solid Tumors (RECIST). The MSI-high cohorts also experienced a significantly better immune-related PFS and disease control rate at 20 weeks by RECIST.
Building upon this, A.N. Fader et al. presented an expanded cohort of MMR-deficient, recurrent or persistent, endometrial cancer patients treated with single-agent pembrolizumab [10]. All ten patients received at least one prior line of systemic chemotherapy and up to four previous regimens. The authors reported an overall response rate of 70% (95% CI 21%–86%, n = 7), with two complete responses (CR) and five partial responses (PR). The disease control rate, or “clinical benefit” rate (CR + PR + stable disease), was 80% (n = 8). The 12-month overall survival (OS) rate was 89%, and the median OS was not yet reached. Importantly, MSI status was determined using standard-of-care MMR IHC testing for MLH1, MSH2, MSH6, and PMS2. Patients lacking expression of DNA mismatch repair proteins were classified as MSI-high, consistent with prior studies reporting concordance rates great than 90% between MMR IHC and MSI PCR.
Most recently, following review of pooled data from five uncontrolled, open-label, multi-cohort, multi-center, single-arm trials, single-agent pembrolizumab was approved for the treatment of MMR-deficient (MSI-high) solid tumors that progressed following prior therapy, with no alternative treatment options. Across all five trials, the efficacy analysis showed an ORR of 39.6% (95% CI 31.7–47.9) with a complete response rate of 7.4% and a partial response rate of 32.2%. At the time of data cutoff, median duration of response had not yet been reached (range 1.6+ to 22.7+ months), with 78% of responding patients having responses of 6 months or longer. Of the 149 subjects in the pooled analysis, 14 had recurrent endometrial cancer, with a reported ORR of 36% (DOR range 4.2+, 17.3+), surpassing historical controls in this pretreated patient population.
In an effort to identify molecular predictors of response, and to better define the endometrial cancer immune landscape, Goodfellow et al. confirmed MSI-high status in 28.4% (296 of 1043 EC specimens) of endometrial cancer tumors examined as part of NRG/GOG0210 [28]. Expanding on this assessment, McMeekin et al. examined the clinicopathologic significance of MMR defects in a cohort of endometrioid endometrial cancers (NRG/GOG0210) [29•]. Within this population, MMR defects were identified in 71 (42%) of 168 subjects with stage 3 or 4 disease.
Howitt et al. additionally tested the hypothesis that microsatellite unstable endometrial cancers would exhibit more tumor-specific neoantigens, resulting in increased tumor-infiltrating lymphocytes and a compensatory upregulation of immune checkpoints [30]. Microsatellite unstable tumors exhibited higher numbers of CD3+ and CD8+ tumor-infiltrating lymphocytes, providing a molecular rational for the efficacy of pembrolizumab in this patient population. Furthermore, PD-1 was overexpressed in tumor-infiltrating lymphocytes and peritumoral lymphocytes of microsatellite unstable tumors. This combination of increased mutational load, tumor-infiltrating lymphocytes, and high PD-1/PD-L1 expression suggests that endometrial cancer is an ideal target for immunotherapeutic interventions. An ongoing trial of single-agent pembrolizumab requires that patients have an ultramutated or hypermutated (MMR-deficient) phenotype NCT02899793.
Efficacy results have also been reported from basket trial studies including the endometrial cancer cohort of Keynote-028 [31]. Mismatch repair (MMR) status was not required for patients to be enrolled and treated on Keynote-028, but rather PD-L1 expression defined as at least 1% membranous staining by centralized IHC testing. Of 75 total patients screened, 36 had PD-L1-positive tumors, and 24 were enrolled on trial. In this subgroup of patients with heavily pretreated, PD-L1-positive endometrial cancer, 13% achieved a confirmed partial response (95% CI, 2.8%–33.6%). An additional three patients (13%) achieved stable disease, with median duration of 24.6 weeks. Thirteen patients (54.2%) experienced a treatment-related adverse event, with fatigue, pruritus, pyrexia, and decreased appetite occurring in at least 10% of patients. Importantly, in the three documents partial response, one patient was found to have a polymerase E (POLE) mutation, one had MMR-proficient (non-MSI-high status) disease, and one had unknown MSI status, suggesting efficacy even in the MMR-proficient population [31].
In an effort to expand eligibility and elicit response in the MMR-proficient (microsatellite stable) endometrial cancer patients, representing the majority of subjects, combinatorial approaches using cytotoxic chemotherapy are being explored. In a single institution, phase II study, pembrolizumab in combination with carboplatin and paclitaxel is being examined in patients with unresectable or widely metastatic disease. The rationale behind such an approach parallels that explored in the ovarian cancer arena, namely, capitalizing on the immune stimulatory properties of cytotoxic chemotherapy.
Dual immunomodulation, using a CTLA-4 inhibitor, ipilimumab, with the PD-1 inhibitor nivolumab, may further enhance anti-tumor immune responses. Several studies in the advanced, recurrent, or metastatic setting are using anti-PD-L1 and anti-PD-1 together as a means of immune stimulation. This doublet is also under investigation in endometrial cancer with high-risk histology such as FIGO grade 3 endometrioid, serous, clear cell or mixed high grade endometrial cancer, as well as a subgroup of cancers harboring MSS, MSI-H, or POLE-mutations (NCT02919572). It is hypothesized that these more highly mutated tumors may elicit a stronger immune response, making them better candidates for immune therapy.
Another approach to targeted therapy exploits inhibitors of cell surface receptors on T cells whose downstream effectors lead to proliferation and activation of immune response. INCB050465, an inhibitor of PI3kinase-delta, is one such receptor that stimulates the activity of T cells. T cell activation and proliferation signals result in the release of cytokines that activate downstream effectors such as Janus kinase (JAK/STAT) signal transducers. JAK overexpression or dysregulation has been shown to stimulate cell proliferation and migration, as well as differentiation and apoptosis [32]. JAK inhibition combined with pembrolizumab is an alternate dual-modality targeted therapy being investigated in endometrial cancer.
A phase I/II study is recruiting patients with metastatic endometrial cancer and other solid malignancies to evaluate the safety and efficacy of the agonist anti-GITR (glucocorticoid-induced tumor necrosis factor receptor-related protein) antibody paired with either anti-PD-1 or anti-CTLA-4, as well as the triplet combination given together. INCAGNO1876, a humanized IgG1 monoclonal antibody against GITR, developed by Incyte Corporation, augments the T cell response by abrogating Treg function and simultaneous activation of effector T cells. This approach utilizes stimulatory and inhibitory antibodies simultaneously in an attempt to illicit a greater anti-tumor immune response.
Cervical Cancer
Representing the least common of the three principle gynecologic malignancies in the USA, it is anticipated that there will be 12,820 new cases of cervical cancer in 2017, with 4210 deaths. The tremendous reduction in cervical cancer incidence is attributed to improved screening and vaccination. However, despite vaccination, screening, and efforts at early detection, a subset of patients are diagnosed with advanced stage disease, or develop recurrence following front-line therapy [4, 20].
Depending on clinical International Federation of Gynecology and Obstetrics (FIGO) stage, cervical cancer may be managed with surgery alone or multi-modal therapy involving radiation and cytotoxic chemotherapy. In the advanced stage or recurrent setting, systemic therapy is required and is associated with a much poorer prognosis.
The results of Gynecologic Oncology Group (GOG) 240 represented a paradigm shift in the management of metastatic or recurrent cervical cancer, ushering in a new era of targeted therapies in this disease setting. GOG 240 demonstrated an overall survival advantage with the addition of the biologic, bevacizumab, to this standard chemotherapy doublet backbone (17 vs. 13.3 months; (hazard ratio for death 0.71; 98% CI 0.54–0.95; p = 0.004 in a one-sided test) [33]. In an effort to expand on these survival gains, investigators are exploring the utility of immunotherapy in patients with cervical cancer.
The unique etiology of cervical cancer, resulting from HPV-virus-induced mutations, provides a rationale for immunotherapy in this disease setting. Viral oncogenes E6 and E7 bind to and interfere with the biological function of p53 and Rb tumor suppressor proteins, respectively, leading to accumulation of mutations and eventual cervical dysplasia and carcinoma [34]. The presence of viral DNA may enhance the antigenicity of this tumor type, as it is recognized as foreign by the immune system. Concurrent cytotoxic chemotherapy may further contribute to antigen unmasking. Addition of checkpoint inhibitors to stimulate the immune response could elicit a synergistic effect of this combined modality therapy [6].
HPV oncoproteins E6 and E7 are potential targets for immunotherapy in cervical cancer. Recent evidence has demonstrated recurrent neoantigens from mutations in known oncogenic driver genes in cervical cancer tumors [35]. Therapeutic vaccines, including Lm-LLO-E6 that targets the E6 oncoprotein, are under development and could potentiate the efficacy of checkpoint blockade in HPV-infected cancers [36].
In the cervical cancer cohort of Keynote-028, pembrolizumab demonstrated modest activity. Eligible subjects were required to have unresectable or metastatic disease and to have failed prior systemic therapy. Over 60% of patients had metastatic disease. Of 23 evaluable patients, the overall response rate was 12.5% (95% CI 2.7–32.4%), with a median duration of response of 19.3 weeks (range, 16.3–29.7+ weeks) and median time to response of 8 weeks [37]. The stable disease rate was 12.5%, and in those responding, the median duration of response approached 20 weeks.
Results from Keynote-158, a study of pembrolizumab monotherapy in advanced squamous cell cervical cancer, were recently presented at the 2017 ASCO annual meeting, reporting a 17% overall response rate (95% CI 8–31%), independent of PD-L1 status (2017 ASCO Annual Meeting-abstract 5514) [38]. Amongst the first 47 patients enrolled on trial, there were 3 confirmed and 5 unconfirmed responses. Eighty-seven percent of patients had PD-L1-positive tumors, and the ORR was independent of PD-L1 status. In the entire cohort of 82 subjects, the ORR was 12% (95% CI 6–21%), with 3 complete responses and 7 partial responses. The median time to response was 2.1 months, and all ten responses were ongoing at the time of data cutoff. With respect to safety, 51% of patients experienced any grade treatment-related adverse event and 10% grade 3 or 4 (AST/ALT increase and pyrexia). This preliminary data suggests interesting single agent activity in patients with previously pretreated advanced stage squamous cell cervical cancer.
The CheckMate 358 trial in virus-associated tumors compares monotherapy with nivolumab to doublets of nivolumab and the anti-CTLA-4 ipilimumab, the lymphocyte activation gene-3 (LAG-3) which binds to and blocks an immunosuppressive receptor on tumor-infiltrating lymphocytes, or daratumumab, a human monoclonal antibody agonist to CD38 on T cells that serves a stimulatory role for anti-tumor immune response. Data regarding the metastatic monotherapy cohort (treated with single-agent nivolumab 240 mg every 2 weeks) was presented at the 2017 ASCO annual meeting. A total of 24 patients were evaluable, 19 of whom had recurrent or metastatic cervical cancer. The reported ORR was 26.3% (95% CI 9.1–51.2%), with a disease control rate of 68.4%. The median duration of response was not reached (0.9–5.8+). In this small cohort, the median PFS was 5.5 months (3.5—not reached), and the 6-month OS rate was 87.1%.
An ongoing phase I clinical trial, NCT01711515, has completed accrual and is evaluating the administration of ipilimumab, the CTLA-4-inhibiting antibody, after chemoradiation therapy in patients with stage IB2-IIA or IIIB-IVA cervical cancer (Table 3). Radiation is known to stimulate an anti-tumor immune response through several mechanisms, including the release of tumor antigens, facilitation of tumor antigen uptake and presentation by dendritic cells, and the induction of pro-inflammatory cytokines to mediate T cell recruitment. Administration of systemic checkpoint inhibitors after localized radiation has demonstrated clinical responses in mouse models and in a clinical trial of patients with prostate cancer [39].
Importantly, investigators are looking to identify how to best sequence radiation and checkpoint inhibition. In a phase II study (NCT02635360), patients will be randomized to receive cisplatin and brachytherapy with concurrent pembrolizumab, or to receive pembrolizumab after this standard chemoradiation regimen. It is thought that the timing of therapy may have differential effects based on disease stage and tumor location.
Toxicity
Exploiting the host immune system for anti-tumor therapy affects self-tolerance to some degree and therefore does not avoid systemic as well as target-related toxicities. Autoimmune-like side effects have been noted in all of the studied checkpoint inhibitors, of varying severity.
Dose-related toxicities have been observed with the CTLA-4 inhibitor ipilimumab. In a large phase II ipilimumab monotherapy study in melanoma, at a dose of 10 mg/kg, immune-related grade 3–4 adverse events occurred in 22%. The adverse events observed with the PD-1 inhibitor, nivolumab, do not appear to be dose related (5–11.7% with grade 3–4). In a phase II trial of the PD-1-inhibitor pembrolizumab, 12% of patients experienced grade 3 or 4 adverse events.
Furthermore, it appears that the side effect profiles may differ depending on the specific checkpoint target: CTLA-4, PD-1, and PD-L1. The adverse events associated with PD-1/PD-L1 antibodies appear to be less frequent and less severe than for those noted with CTLA-4-targeted antibodies. Unlike traditional cytotoxic chemotherapy regimens, immune-related adverse events are usually inflammatory in nature and include constitutional (pyrexia, fatigue related to cytokine release), as well as organ-specific dermatologic, gastrointestinal, hematologic, neurologic, endocrine, renal, and pulmonary effects [40, 41]. This emphasizes the importance of provider and patient education as patients begin to more frequently receive these novel agents.
Common skin toxicity in patients exposed to checkpoint inhibitors includes maculopapular rash and pruritus. Rare and more severe conditions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have occurred, as well as oral mucositis and gingivitis. Diarrhea and colitis are the most common gastrointestinal symptoms, which can be severe if not treated early. Severe colitis has occurred in patients treated with ipilimumab (6–14%), but in less than 1% of those treated with PD-1/PD-L1 targeting antibodies. Additionally, endocrinopathies are more often seen with anti-CTLA-4 antibodies and may involve the thyroid, adrenal, and pituitary glands. Hepatitis has been attributed to anti-CTLA-4 therapy, but abnormal AST/ALT levels have been seen with all of the checkpoint inhibitors. Anti-CTLA-4 and PD-1 antibodies have caused pneumonitis, with a typical diffuse lymphocytic infiltrate on biopsy and brushings.
Rarer neurologic toxicities include encephalitis, Guillain-Barre syndrome, and a myasthenia gravis-like syndrome with ipilimumab treatment or PD-1 blockade. Hematologic toxicities are also infrequent, but include thrombocytopenia and leukopenia. Sarcoid-like granulomatous reactions have been reported with CTLA-4 antibodies.
Combining checkpoint inhibitors with other novel agents may increase efficacy, but this will have to be balanced against potential toxicity and impacts on patient reported outcomes including quality of life. The most important to the management of the toxicities associated with use of immune checkpoint inhibitors is early identification and rapid, aggressive treatment with corticosteroids or other immune suppressants [40, 42].
Conclusions
Despite the promise identified in early clinical trials, the use of immune checkpoint inhibition in patients with gynecologic cancer is at its infancy. In conjunction with active clinical assessment of efficacy, clinical trialists are performing critical translational research in an effort to both better predict response and try and identify ways to convert non-responders to responders.
Across solid tumor types, identifying subgroups expressing specific immune profiles more likely to respond to immune modulation will be important as well as selecting appropriate cohorts of patients likely to respond to active immune therapy. Defining patients who require ex vivo expansion and adoptive T cell therapy versus those likely to benefit from checkpoint blockade will be important as the field of immunotherapy matures.
Ultimately, combinatorial approaches may be required, and in a cohort of patients with limited therapeutic options, meaningful clinical advances are critical.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Sumire V, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms †. Mol Cell Biol. 2005;25(21):9543–53. https://doi.org/10.1128/MCB.25.21.9543-9553.2005.
Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–96.
Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science [Internet]. 1996;271(5256):1734–6. (80) Available from:. https://doi.org/10.1126/science.271.5256.1734.
Siegel RL, Miller KD, Jemal A. Cancer Statistics , 2017;67(1):7–30.
Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. https://doi.org/10.1200/JCO.2013.51.4489.
Eskander RN, Tewari KS. Immunotherapy: an evolving paradigm in the treatment of advanced cervical cancer. Clin Ther [Internet]. 2015;37(1):20–38. Available from:. https://doi.org/10.1016/j.clinthera.2014.11.010.
• Ding M, Mei-jiao G. Immune effect of tumor-infiltrating lymphocytes and its relation to the survival rate of patients with ovarian malignancies. J Tongji Med Univ [internet]. 1991;11(4):235–9. https://doi.org/10.1007/BF02888158. One of the earliest studies suggesting the prognostic importance of tumor-infiltrating lymphocytes and outcomes in ovarian cancer
Li J, Wang J, Chen R, Bai Y, Lu X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget. 2017;8(9):15621–31. https://doi.org/10.18632/oncotarget.14919.
Makrigiannakis A, Gray H. Intratumoral T cells, recurrence, and survival in epithelial ovarian. Cancer. 2003:203–13.
Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol [Internet]. 2011;8(3):151–60. Available from:. https://doi.org/10.1038/nrclinonc.2010.223.
Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med [Internet]. 2010;363(10):943–53. Available from:. https://doi.org/10.1056/NEJMoa0908806.
Melamed A, Hinchcliff EM, Clemmer JT, Bregar AJ, Uppal S, Bostock I, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol [Internet]. 2016;143(2):236–40. Available from:. https://doi.org/10.1016/j.ygyno.2016.09.002.
Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med [Internet]. 2015;373(5):428–37. Available from:. https://doi.org/10.1056/NEJMoa1411366.
Jochems C, Fantini M, Fernando RI, Kwilas AR, Donahue RN, Lepone LM, et al. The IDO1 selective inhibitor epacadostat enhances dendritic cell immunogenicity and lytic ability of tumor antigen-specific T cells. Oncotarget [Internet]. 2014;7(25):37762–72. Available from: http://www.oncotarget.com/abstract/9326
•• Lee J, Cimino-mathews A, Peer CJ, Zimmer A, Lipkowitz S, Annunziata CM, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (ADP-ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1–3 inhibitor cediranib in women’s cancers: a dose-escalation, phase I study. 2017; One of the first trials describing safety and preliminary efficacy of combination checkpoint inhibition and PARP inhibition.
Sundarapandiyan, K, Zhao, B, Vitale, L, O’Neill, T, Ramakrishna, V., Marsh, H, Keler T. Characterization of the response of human T cells to an agonistic anti-CD27 mAb. In: AACR. 2012.
Disis ML, Patel MR, Pant S, Hamilton EP, Lockhart AC, Kelly K, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: safety and clinical activity. J Clin Oncol [Internet]. 2016;34(15_suppl):5533. https://doi.org/10.1200/JCO.2016.34.15_suppl.5533.
Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of. PNAS. 2014;111(32):1–6.
• Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):11–3. Early trial detailing efficacy of checkpoint inhibition in ovarian cancer as a single agent.
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66(1):7–30. https://doi.org/10.3322/caac.21332.
Longoria TC, Eskander RN. Immunotherapy in endometrial cancer—an evolving therapeutic paradigm. Gynecol Oncol Res Pract [Internet]. 2015;11(2). Available from: doi: https://doi.org/10.1186/s40661-015-0020-3
Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T Lymphocytes as a prognostic factor of survival in endometrial carcinoma. 2004;10:4450–4456.
Ambros RA, Kurman RI. Combined assessment of vascular and myometrial invasion as a model to predict prognosis in stage I endometrioid adenocarcinoma of the uterine corpus. Cancer. 1992;69(6):1424–31. https://doi.org/10.1002/1097-0142(19920315)69:6<1424::AID-CNCR2820690620>3.0.CO;2-5.
Makker V, Filiaci VL, Chen L, Darus CJ, James E, Sutton G, et al. Phase II evaluation of dalantercept, a soluble recombinant activin receptor-like kinase 1 (ALK-1) receptor fusion protein, for the treatment of recurrent or persistent endometrial cancer: an NRG oncology/gynecologic oncology group study 0229N. Gynecol Oncol. 2015;138(1):24–9. https://doi.org/10.1016/j.ygyno.2015.04.006.
Alvarez EA, Brady WE, Walker JL, Rotmensch J, Zhou XC, Kendrick JE, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol [Internet]. 2017;129(1):22–7. Available from:. https://doi.org/10.1016/j.ygyno.2012.12.022.
Powell MA, Sill MW, Goodfellow PJ, Doris M, Lankes HA, Leslie KK, et al. A phase II trial of brivanib in recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2014;135(1):38–43. https://doi.org/10.1016/j.ygyno.2014.07.083.
•• Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med [Internet]. 2015;372(26):2509–20. https://doi.org/10.1056/NEJMoa1500596. Clinical trial that catalyzed the study of checkpoint inhibitors in MMR-deficient endometrial cancer.
Goodfellow PJ, Billingsley CC, Lankes HA, Ali S, Cohn DE, Broaddus RJ, et al. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: an NRG Oncology and Gynecologic Oncology Group study. J Clin Oncol. 2015;33(36):4301–8. https://doi.org/10.1200/JCO.2015.63.9518.
• McMeekin DS, Tritchler DL, Cohn DE, Mutch DG, Lankes HA, Geller MA, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol [Internet]. 2016;34(25):3062–8. https://doi.org/10.1200/JCO.2016.67.8722. Study describing the frequency of MMR deficiency in endometrial cancer specimens.
Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of pd-2 and pd-l1. JAMA Oncol. 2015;1(9):1319–23. https://doi.org/10.1001/jamaoncol.2015.2151.
Ott PA, Bang Y, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol [internet]. 2017;JCO2017725952. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28489510.
Chikuma S, Kanamori M, Mise-Omata S, Yoshimura A. Suppressors of cytokine signaling: potential immune checkpoint molecules for cancer immunotherapy. Cancer Sci. 2017;108(4):574–80. https://doi.org/10.1111/cas.13194.
Tewari KS, Sill MW, Long HJ, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med [Internet]. 2014;370(8):734–43. Available from:. https://doi.org/10.1056/NEJMoa1309748.
Yim E, Ph D, Park J, Ph D. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. 2005;37(6):319–24.
Roszik, J, Qin, Y, Ekmekcioglu, S, Forget, M-A, Hwu, P, Grimm, E A., Jazaeri AA. The neoantigen landscape and immune regulators in cervical cancer. In: 2017 ASCO Annual Meeting. J Clin Oncol; 2017.
Lin, P Lin, Yen Y, Lee H. A combination of anti-PD-L1 mAb plus LM-LLO-E6 vaccine to supprss tumor growth and metastasis in HPV-infected cancers. In: 2017 ASCO Annual Meeting 2017.
Frenel, J-S, Le Tourneau, C, O’Neill, BH, Ott, PA, Piha-Paul, SA, Goomez-Roca, CA, Van Brummelen, EV, Rugo, HS, Thomas, S, Saraf, S, Chen, M, Varga A. Pembrolizumab in patients with advanced cervical squamous cell cancer: preliminary results from the phase IB Keynote-028 study. In: ASCO. 2016.
Schellens, Jan H.M., M Aurelien, Zeigenfuss, S, Ding, J, K P Scott, Chung HC. Pembrolizumab for previously treated advanced cervical squamous cell cancer: preliminary results from the phase 2 KEYNOTE-158 study. In: 2017 ASCO Annual Meeting. J Clin Oncol; 2017.
Zamarin D, Postow MA. Pharmacology & therapeutics immune checkpoint modulation: rational design of combination strategies. Pharmacol Ther [Internet]. 2015;150:23–32. Available from:. https://doi.org/10.1016/j.pharmthera.2015.01.003.
Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–91. https://doi.org/10.1093/annonc/mdv383.
Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN solid tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol [Internet]. 2017;18(5):587–98. Available from:. https://doi.org/10.1016/S1470-2045(17)30239-5.
Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33(18):2092–9. https://doi.org/10.1200/JCO.2014.60.0379.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflicts of interest as they relate to the content of this manuscript. Ramez N. Eskander declares compensation for speaking on behalf of Genentech, AZ Oncology, and CLOVIS.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Gynecologic Oncology
Rights and permissions
About this article
Cite this article
Anderson, K., Eskander, R.N. Immune Checkpoint Inhibition in the Treatment of Gynecologic Cancer. Curr Obstet Gynecol Rep 7, 6–19 (2018). https://doi.org/10.1007/s13669-018-0231-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-018-0231-9