Abstract
Purpose of Review
This review highlights lung denervation procedure as a potential bronchoscopic treatment for patients with advanced chronic obstructive pulmonary disease (COPD).
Recent Findings
To date, clinical studies of bronchoscopic TLD for patients with COPD proved feasibility, demonstrated a good safety profile, established a therapeutic dose of energy, and identified a potential effectiveness outcome. The AIRFLOW-2, multicenter, randomized, sham-controlled, double-blinded trial showed fewer respiratory adverse events, including hospitalization for COPD exacerbation in patients treated with TLD.
Summary
TLD of the parasympathetic airway nerves appears to be safe and shows favorable clinical outcomes in patients with advanced COPD. Results of the ongoing clinical trial and long-term follow-up of previously treated patients will provide more information on the efficacy and durability of TLD in the management of this challenging group of patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Chronic obstructive pulmonary obstructive disease (COPD) is associated with high morbidity and mortality [1]. According to the Global Burden of Disease (GBD), COPD is currently the third leading cause of death worldwide [2]. In the USA, COPD kills more than 120,000 individuals each year [3]. COPD is characterized by persistent respiratory symptoms of cough, dyspnea, and limited exercise capacity secondary to peripheral airway obstruction, hyperinflation, and emphysematous parenchyma destruction [4]. Management of COPD includes smoking cessation, influenza and pneumococcal vaccination, supplemental oxygen for severe hypoxemia, pharmacologic treatment guided by the severity of symptoms, and pulmonary rehabilitation. Pharmacologic treatment of stable COPD comprise mainly of inhaled bronchodilators (beta agonists and muscarinic antagonist) alone, in combination, or with an added inhaled corticosteroid [5]. In patients with both COPD and obstructive sleep apnea, the use of continuous positive airway pressure (CPAP) was shown to improve both survival and the risk of hospital admissions. Lung transplantation has been shown to optimize the functional capacity and improve the quality of life in patients with advanced COPD [6, 7]. Lung volume reduction surgery (LVRS) has shown to prolong survival in a selected group of patients with upper lobe predominant emphysema and low exercise capacity after rehabilitation [8]. However, many patients with COPD are not candidates for this surgery because of their multiple comorbidities and the specific inclusion criteria that limits the applicability of LVRS [9].
During the past decade, several techniques of bronchoscopic lung volume reduction (BLVR) emerged as part of severe COPD and emphysema management, to achieving lung volume reduction with different degree of reversibility and whose application is dependent on the emphysema distribution and presence or absence of interlobar collateral ventilation [10]. Targeted lung denervation is another bronchoscopic technique for the treatment of COPD. This technology is under clinical investigation and not yet commercially available. In 2020 Nuvaira® Lung Denervation System has been designated as a Breakthrough Device by the US Food and Drug Administration (FDA) after reviewing the safety data on the first 50 patients enrolled in the AIRFLOW-3 clinical trial [11].
What is Targeted Lung Denervation
TLD therapy is a bronchoscopic treatment delivered via a dual-cooled radio frequency (RF) catheter (Holaira, Minneapolis, MN, USA) (Fig. 1) designed to generate sufficient energy at a limited depth from the inner surface of the main bronchus to ablate the nerves traveling parallel to the airways to the lung (Fig. 2). The RF current travels from the electrodes through the airways and surrounding tissue where the nerves are located on the outer surface of the bronchus. An expandable balloon that has continuously running fluid keeps the electrodes cool, while energy is applied to minimize the damage to the mucosal surface of the bronchi [12••].
The Rationale of Targeted Lung Denervation
Despite all available pharmacologic and non-pharmacologic therapies available for the management of patients with COPD, many patients with persistent symptoms and history of disease exacerbation remain at risk of future respiratory events. Targeted lung denervation (TLD) is a potential treatment for those patients, in which the parasympathetic airway nerves are ablated, aiming to achieve sustained bronchodilation and reduced mucous secretion, simulating the effect of anticholinergic drugs. The ultimate goal of this intervention is to reduce the rate of COPD exacerbation. TLD is designed as a potential treatment for GOLD group D COPD patients, those with FEV1 30–60% predicted, and a history of at least two moderate or one severe exacerbation in the past 12 months, and persistent symptoms based on COPD assessment test (CAT) ≥10 while on optimal pharmacologic treatment.
Description of the Procedure
The bronchoscopy is done, while the patient is under general anesthesia. Initially, an esophageal balloon filled with contrast is inflated to be used to determine the distance between the outer surface of the esophagus and the TLD catheter under fluoroscopic guidance to avoid delivering a RF treatment dose that can damage the esophageal nerve plexus.
The TLD catheter is introduced through a bronchoscope with a working channel of 3.2 mm. Once the balloon was inflated, good contact with the airways is visually confirmed as well as the electrode position using fluoroscopy. The electrodes are activated to treat each main bronchus at four rotational positions to achieve a complete circumferential treatment with up to eight treatments for each patient (Fig. 3).
The technique requires an experienced operator as failing to visualize a good contact of the cooling balloon with the inner wall of the airways can lead to serious side effects including ulceration and bronchial fistula formation.
Summary of Clinical Evidence
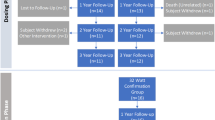
In 2015, Slebos et al. published a pilot study, conducted in South Africa and the Netherlands [12••]. This was the first-in-human study in treating patients with moderate to severe COPD. Twenty-two patients were treated. Twelve patients were in the 20 Watt (W) cohort and ten patients in the 15 W cohort. Patients underwent staged TLD, 30 days apart at 20 or 15 W following baseline assessment off bronchodilators. One year, changes from baseline in the 20 W dose compared to the 15 W dose suggested a larger and more durable improvement in lung function: FEV1 (+11.6%±32.3 vs +0.02%±15.1, p=0.324), exercise capacity, submaximal cycle endurance (+6.8 min±12.8 vs 2.6 min±8.7, p=0.277), and health-related quality of life represented by the St. George’s Respiratory Questionnaire (−11.1 points ±9.1 vs −0.9 points ±8.6, p=0.044). The primary safety endpoint was achieved in 95% of patients, and technical feasibility was 93%. There was no difference in the safety profiles between the two energy doses. The most common side effect was COPD exacerbation in 59% of the patients in the first year. Three of the 20 W cohort patients developed asymptomatic airway wall defects after which the procedure was modified to ensure a more accurate visual assessment of balloon contact before activation, more distal treatment to the main carina, and decreasing the overall power to 15 W. This was a pilot study to prove the concept and assess the safety of the new technology, and it showed a non-significant trend toward improved lung function and quality of life in patients treated with the higher RE energy dose (20 W vs 15 W); however, no placebo group was included.
A post hoc analysis was performed to assess bronchodilator response post-TLD, where an increase in FEV1 following inhalation of 80 μg ipratropium was assessed at 3, 6, and 12 months. A greater bronchodilator response was observed in patients treated with 20 W, with a mean response of 355 ml (43%) that was retained at 12 months follow-up [13]. These findings suggest that TLD and anticholinergic inhaler treatment might have a synergistic effect in the management of patients with COPD. This data also suggests that the vagal denervation of the airways does not result in permanent and complete suppression of the distal parasympathetic nervous system in the airways.
In 2018, Valipour et al. conducted a multicenter, nonrandomized, prospective study to further evaluate the feasibility and long-term safety of TLD when performed in a single procedure [14]. The rationale behind was to reduce the risk of COPD exacerbations induced by bronchoscopy and reduce the repeated risk to anesthesia. This study had similar design and inclusion criteria to the pilot study [12••] with the exception that TLD was delivered at a single power of 15 W to both lungs during a single procedure. Fifteen patients were treated, and the primary safety endpoint of freedom from worsening of COPD was 100%. There were no procedure-related complications. The most common respiratory side effect was COPD exacerbation that occurred in 5 patients at least 3 months post-TLD. Feasibility was 93%, where one patient received treatment in seven of eight expected locations. Two patients had a staged procedure as the proper balloon size was unavailable at the time of the initial procedure. This small cohort was followed up for 3 years, and the low rate of COPD exacerbation was noticed in the 9 patients that did not lose follow-up. This cohort of this study had a significantly lower serious adverse events (SAE) rate than the first-in-human pilot study which could be attributed to the bilateral approach, lower energy level used, and a more distal electrode placement away from the thermally sensitive main carina which could have reduced the airway complications.
Following the first two feasibility studies [12••, 14], a second-generation TLD system was developed to enhance the compatibility of the catheter with a flexible bronchoscope and having a larger electrode to reduce the procedure. In contrast to the first-generation larger catheter of TLD, which required the use of rigid bronchoscopy, the modified catheter can be placed through the working channel of flexible bronchoscopy.
In 2019, Valipour et al. published the AIRFLOW-1 trial that evaluated the safety and the feasibility of this device and tested the optimal RF treatment dose. Forty-six patients were treated. Thirty patients with COPD (FEV1 30–60%) were 1:1 randomized to receive TLD with either 29 or 32 W. Five patients early in the randomization phase developed symptoms of impaired gastric emptying which led to holding the trial. Procedure modification was implemented to avoid injury to the esophageal-vagal plexus that supplies the stomach. An esophageal balloon filled with contrast was used to mark the distance between the RF electrode and the outer surface of the esophagus using fluoroscopy. RF energy was decreased at sites close to the esophagus. After this modification, the incidence of SAEs related to the procedure decreased by more than half in the remaining patients that were in the randomized dosing phase. A confirmatory phase of the trial included sixteen patients treated with 32 W after implementing the new modification, none of them developed any gastrointestinal side effects. This study evaluated 2 higher dose power level compared to the ones used in preclinical work [15,16,17,18,19]; hence, the primary safety outcome was the rate of acute airway side effects. Airway wall effects were observed in 15% of treated patients, with complete recovery at follow-up visits. Feasibility was achieved in 29/30 patients (97%). At 1-year follow-up, improvements in FEV1 of 94.2 ± 228 mL (p = 0.18), FVC 212 ± 497 mL (p = 0.17), SGRQ-C –7.5 ± 10.3 (p = 0.036), and CAT –2.9 ± 6.1 (p = 0.14) were observed in the 32 W group compared to baseline. The 29 W group had changes in FEV1 of 57 ± 82 mL (p = 0.0272), FVC 238 ± 316 mL (p = 0.0188), SGRQ-C –1.9 ± 12.5 (p = 0.6166), and CAT 0.3 ± 7.8 (p = 0.8898) compared to their baseline. There was no statistically significant difference between the two groups. The improvement observed in the quality of life and COPD symptoms scores suggested a dose-response relationship of TLD; however, the authors acknowledged that the study was not powered to assess the superiority of efficacy outcomes between the different dosing groups. There was no control group in this trial.
Later in 2019, Slebos et al. published the results of the AIRFLOW-2 trial [20•]. This was a prospective, multicenter, randomized, sham-controlled, double-blind study in patients with COPD with similar inclusion criteria as prior studies [12••, 14, 21] This was a small study but appropriately randomized 1:1, with a sham ablation procedure in the patients assigned to the control group. Both the patients and the study assessment team were blinded. Eighty-two patients were included, and 41 patients received treatment in the TLD group. The primary endpoint was the difference between the two groups in the rate of respiratory events between 3 and 6.5 months after treatment. The TLD group experienced significantly fewer respiratory adverse events, 32% (13/41) compared to the sham group 71% (29/41) (p = 0.008). The most common respiratory events in both groups were COPD exacerbation and worsening dyspnea. Between 0 and 12.5 months post TLD treatment, there were no differences in the rate of respiratory adverse events between the two groups (83% vs 90%; p = 0.52). The time to first moderate to severe COPD exacerbation was not statistically different between the two groups over 12.5 months of follow-up; however, the risk of COPD exacerbation requiring hospitalization was significantly lower in the TLD group than in the sham group (hazard ratio, 0.35; 95% confidence interval, 0.13–0.99; p = 0.039) over the same period.
The longer follow-up period of this study was important to confirm safety, especially the low number of transient gastrointestinal side effects that were not statistically different between the two groups. Surprisingly, the sham bronchoscopy group experienced more than twice as many adverse respiratory events compared with the TLD group. It is important to note that the present study did not require a COPD exacerbation history for inclusion, and a possible explanation for this outcome could be that the relatively small sham bronchoscopy group experienced an unexpected higher number of respiratory events. Also, for the entire 12.5 months assessment period, there was no difference in respiratory adverse events between the two groups.
In summary, TLD is a novel therapeutic concept that targeted denervation of the parasympathetic airway nerves is safe and can improve the clinical outcomes in patients with COPD. A phase 3 randomized controlled trial (AIRFLOW-3) [22] is currently ongoing, and a history of recent COPD exacerbation is part of the inclusion criteria. This trial should be appropriately powered and could potentially provide more information on the efficacy of TLD in the management of this group of patients. Outcome data of longer follow-up on previously treated patients will provide more information on the durability of the effect of TLD.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. https://doi.org/10.1016/S2213-2600(17)30293-X.
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. https://doi.org/10.1016/S0140-6736(12)61728-0.
Mortality in the United States, 2016 - PubMed. https://pubmed.ncbi.nlm.nih.gov/29319473/. Accessed 23 June 2020.
Casanova C, Cote C, De Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591–7. https://doi.org/10.1164/rccm.200407-867OC.
Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020.
Global Initiative for Chronic Obstructive Lung Disease Global Initiative for Chronic Obstructive Lung Disease pocket guide to copd diagnosis, management, and prevention a guide for health care professionals. 2017.
Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–31. https://doi.org/10.1164/rccm.200912-1869OC.
Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–73. https://doi.org/10.1056/NEJMoa030287.
Decker MR, Leverson GE, Jaoude WA, Maloney JD. Lung volume reduction surgery since the National Emphysema Treatment Trial: study of Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg. 2014;148(6):2651–2658.e1. https://doi.org/10.1016/j.jtcvs.2014.02.005.
Shah PL, Slebos D. Bronchoscopic interventions for severe emphysema: where are we now? Respirology. May 2020:resp.13835. https://doi.org/10.1111/resp.13835.
Evaluation of the Safety and Efficacy of TLD in Patients With COPD - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03639051?cond=NUVAIRA&draw=2&rank=1. Accessed 10 July 2020.
•• Slebos DJ, Klooster K, Koegelenberg CFN, Theron J, Styen D, Valipour A, et al. Targeted lung denervation for moderate to severe COPD: A pilot study. Thorax. 2015;70(5):411–9. https://doi.org/10.1136/thoraxjnl-2014-206146This is the first in human study that evaluated bronchoscopic targeted denervation of parasympathetic pulmonary nerves running around the airways in patients with COPD.
Koegelenberg CFN, Theron J, Slebos DJ, Klooster K, Mayse M, Gosens R. Antimuscarinic bronchodilator response retained after bronchoscopic vagal denervation in chronic obstructive pulmonary disease patients. Respiration. 2016;92(1):58–60. https://doi.org/10.1159/000447641.
Valipour A, Asadi S, Pison C, Jondot M, Kessler R, Bennedif K, et al. Long-term safety of bilateral targeted lung denervation in patients with COPD. Int J COPD. 2018;13:2163–72. https://doi.org/10.2147/COPD.S158748.
Johnson PJ, Mayse M, Rouw K. Targeted lung denervation; an evaluation of power dose effect. In: European Respiratory Journal. Vol 50. European Respiratory Society (ERS); 2017:PA821. https://doi.org/10.1183/1393003.congress-2017.pa821.
Mayse M, Hummel JP, Johnson PJ, Rouw K. Improved pulmonary resistance in healthy sheep following targeted lung denervation (TLD). In: European Respiratory Journal. Vol 50. European Respiratory Society (ERS); 2017:PA819. https://doi.org/10.1183/1393003.congress-2017.pa819.
Mayse M, Johnson P, Hummel J. Importance of surface cooling during targeted lung denervation for COPD. Eur Respir J. 2014;44(Suppl 58).
Mayse M, Johnson P, Rouw K. Demonstration of pulmonary denervation using the Hering-Breuer reflex following targeted lung denervation (TLD). In: European Respiratory Journal. Vol 50. European Respiratory Society (ERS); 2017:PA820. https://doi.org/10.1183/1393003.congress-2017.pa820.
Mayse M, Johnson P, Streeter J, Deem M, Hummel J. Targeted lung denervation in the healthy sheep model - a potential treatment for COPD. Eur Respir J. 2014;44(Suppl 58).
• Slebos DJ, Shah PL, Herth FJF, Pison C, Schumann C, Hübner RH, et al. Safety and adverse events after targeted lung denervation for symptomatic moderate to severe chronic obstructive pulmonary disease (AIRFLOW) a multicenter randomized controlled clinical trial. Am J Respir Crit Care Med. 2019;200(12):1477–86. https://doi.org/10.1164/rccm.201903-0624OCThis is a randomized controlled trial that demonstrate safety of TLD and its potential ability to reduce chronic obstructive pulmonary disease exacerbations that require hospitalization.
Valipour A, Shah PL, Pison C, Ninane V, Janssens W, Perez T, et al. Safety and dose study of targeted lung denervation in moderate/severe copd patients. Respiration. 2019;98(4):329–39. https://doi.org/10.1159/000500463.
Slebos DJ, Degano B, Valipour A, Shah PL, Deslée G, Sciurba FC. Design for a multicenter, randomized, sham-controlled study to evaluate safety and efficacy after treatment with the Nuvaira® lung denervation system in subjects with chronic obstructive pulmonary disease (AIRFLOW-3). BMC Pulm Med. 2020;20(1). https://doi.org/10.1186/s12890-020-1058-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Christian Ghattas is a site principal investigator of the AIRFLOW3 clinical trial.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Interventional Pulmonology
Rights and permissions
About this article
Cite this article
Ghattas, C. Lung Denervation for Advanced COPD: Basics and Beyond. Curr Pulmonol Rep 10, 98–102 (2021). https://doi.org/10.1007/s13665-021-00273-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-021-00273-3