Abstract
Purpose of Review
Bronchial thermoplasty (BT) is an endoscopic treatment for severe and uncontrolled asthma. This review provides an overview of the indications, selection criteria, treatment application, and scientific evidence regarding its mechanism of action and safety and efficacy of BT in current clinical practice.
Recent Findings
It seems that reduction in airway smooth muscle is one of the principal mechanisms of action, since it correlates with clinical benefits. Regarding safety and efficacy, recent case reports and small prospective studies appear to show benefit in more severe asthma patients.
Summary
Further research is needed to better understand the physiological benefits of BT and select the best candidates for treatment. In order to achieve these goals, experts recommend performing BT only in specialized centers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
International asthma guidelines place emphasis on treatment based on inhaled corticosteroids (ICS) and bronchodilators, since medical therapy achieves adequate disease control in most patients. However, it is estimated that the prevalence of severe and/or uncontrolled asthma may be as high as 5–10% [1]. This subgroup of patients with severe asthma accounts for most of the health care expenditures and hence is overrepresented in the economic burden of the disease.
A better understanding of the molecular and inflammatory pathways mediating asthma has led to the development of new molecules for the treatment of targeted phenotypes of severe asthma. Some of these drugs, including omalizumab or mepolizumab and other new incoming treatments, have demonstrated effectiveness in a highly selected group of patients. However, despite all the therapies available for this heterogeneous disease, some patients remain symptomatic.
Airway remodeling (AR) consists in structural changes in the airways characterized by increased thickness of the bronchial wall, involving hypertrophy and hyperplasia of airway smooth muscle (ASM), increase in mucous glands, thickening of reticular basement membrane, and angiogenesis [2].
Bronchial thermoplasty (BT) is the first non-pharmacological treatment approved by the US Food and Drug Administration (FDA) for uncontrolled severe asthma patients [3], which targets airway smooth muscle. However, little evidence is available regarding its putative mechanism of action.
This review provides an overview of the indications, selection criteria, treatment application, and scientific evidence regarding its mechanism of action and safety and efficacy of BT in current clinical practice.
Patient Selection
BT is indicated for patients with demonstrated severe and uncontrolled asthma despite adequate pharmacological treatment. The level of control may be defined by low scores in quality of life and standardized symptom-based questionnaires, frequent exacerbations, and/or hospitalizations.
The different asthma guidelines recommend BT for patients who do not respond to standard treatment, including monoclonal antibodies. Therapeutic compliance and comorbidities must be assessed prior to recommending BT. Recent studies have shown that patients treated in clinical practice are probably more severe than those included in pivotal trials [4,5,6] (Table 1).
In addition, asthma is a heterogeneous disease and clinical trials included a wide range of severity and disease phenotypes. It is not possible to define the profile of a “BT-responder patient.” There is no consistent information about cost-effectiveness studies of BT compared to other biological treatments in those patients with overlapping indications for different specific treatments for severe refractory asthma.
Regarding lung function, most patients included in clinical trials had a pre-bronchodilator forced expiratory volume in 1 s (FEV1) higher than 60% of the predicted value [4, 6], although limited evidence in more severely obstructed patients has shown that BT does not appear to increase adverse events in sicker patients [5, 8].
Possible candidates for BT treatment should be assessed by a multidisciplinary team with experience in the management of severe asthma and complex respiratory endoscopic techniques. Patients need to be optimally treated, ensuring treatment adherence via educational skill visits for example, and after excluding other respiratory conditions that could be responsible for uncontrolled disease. Little evidence exists regarding patients with an FEV1 < 60%. Vital risk crisis should be noticed to identify those patients with more labile disease, as BT sessions are prolonged and may lead to temporarily worsening of respiratory symptoms.
Contraindications to BT include the following: those for conventional bronchoscopy and placement of electrical devices such as pacemakers, pregnancy, and other airway diseases which do not respond to BT such as emphysema, bronchiectasis, etc.
Procedure Planning and Execution
Adequate asthma treatment should be maintained during BT sessions. Furthermore, oral prednisone (50 mg per day or 0.5 mg/kg/day) is recommended prior to each session and maintained 24 h after the procedure in order to prevent respiratory adverse events due to BT. However, there is no evidence supporting this recommendation. Some patients may need to increase their inhaled medication and/or systemic glucocorticosteroids during treatment.
Although the procedure is standardized [9, 10], variations regarding sedative drug use, intubation, and use of laryngeal masks or nasal vs oral intubation, abound.
BT is applied in three different sessions performed at 3–4-week intervals in order to ensure clinical recovery of the patient. The first session generally treats the right lower lobe. The second session is devoted to the left lower lobe, and finally, both upper lobes are treated during the third and final session. The middle lobe is excluded from treatment due to the risk of middle lobe syndrome. The number of activations is variable, depending on bronchial anatomy, but averages 40–50 activations for lower lobes and 60 activations for upper lobe treatment [11]. Recent data suggests that higher number of activations might result in greater benefit [12].
The Alair® bronchial thermoplasty system (Alair® Bronchial Thermoplasty System, Marlborough, MA, USA) includes an Alair® radio frequency controller, an Alair® catheter (one catheter per session) with an expandable basket with four electrodes, and a patient return electrode and requires a flexible bronchoscope with an external diameter of 4.9–5.3 mm and a working channel of at least 2 mm [9].
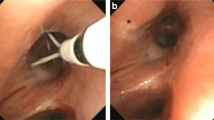
The BT catheter is inserted through the working channel of the bronchoscope with the electrode-containing basket closed and opened inside the bronchi to facilitate full contact with the bronchial wall. Radio frequency energy is automatically applied for 10 s to reach 65 °C by switching on the external pedal. All accessible bronchi more than 2 mm in diameter are treated at intervals of 5 mm by moving the basket in a distal to proximal direction guided by marks on the catheter (Fig. 1).
Detail from treated bronchi with bronchial thermoplasty. The electrode-containing basket (white arrow) is opened inside the bronchi to facilitate full contact with the bronchial wall. All accessible bronchi more than 2 mm in diameter are treated at intervals of 5 mm by moving the basket in a distal to proximal direction guided by black marks on the catheter (black arrow)
Mechanism of Action
Airway remodeling is considered as a process encompassing changes in the structural cells and tissues of the airways in obstructive diseases, particularly asthma. These include airway wall thickening, airway edema, subepithelial fibrosis, epithelial hyperplasia, airway smooth muscle hyperplasia and hypertrophy, and increasing number of myofibroblasts and inflammatory cells. Nevertheless, uncertainty persists regarding which mechanisms underlie AR, how AR alterations contribute to disease progression and severity and symptoms, and whether current therapies are effective at reversing AR. A standardized methodology for obtaining and analyzing biological samples is lacking and may be responsible for this uncertainty along with the absence of specific biomarkers for AR and technological limitations in the non-invasive assessing of AR. Recently, a research statement has been proposed by the ATS to address specific recommendations to achieve these goals [13].
Initial animal studies showed that BT reduces ASM. ASM hypertrophy and hyperplasia seem to be particularly common in individuals with more severe disease [14] and are correlated with permanent airflow obstruction [15].
BT applies controlled thermal energy through radiofrequency heating of the bronchial mucosa at 65 °C under direct visual guidance. The endoscopic view of the mucosa recently treated is nearly normal, although it is possible to observe immediate but transient white discoloration where electrodes have been placed. Preliminary studies in animal models showed reduction in ASM mass which led to a decrease in bronchial hyperreactivity as measured by methacholine challenge [16].
Petrolani et al. described a reduction of 12.93% in the ASM mass at 3 months after treatment in ten patients treated with BT, and this changed was not correlated with the number of radio frequency activations [17]. Interestingly, the study also found a significant reduction in ASM mass in the middle lobe, which was not treated, suggesting diffusion of the heat generated by radio frequency to non-treated areas. Similarly, other authors have reported short-term reductions in ASM, as well as changes in pro-inflammatory airway cytokine concentrations in the bronchoalveolar lavage of 11 BT-treated patients including lower transforming growth factor beta concentration and eosinophil counts, along with higher concentrations of tumor necrosis factor-related apoptosis-inducing ligand [18].
Chakir et al. analyzed changes in ASM mass and connective tissue from bronchial biopsies obtained in 17 patients at 8 weeks after BT treatment [19]. They found a significant average decrease of 8.3% in ASM mass and a significant reduction in type I collagen deposit. The same group extended the follow-up to those patients who consented to a follow-up diagnostic procedure at 24 months after treatment, including nine patients in which the reduction of ASM was maintained [20•].
In addition, Petrolani et al. also found significant reduction in ASM in 15 patients treated with BT at 3 months follow-up. This is the first study that found significant correlation between the reduction in ASM and clinical improvement (asthma control and quality of life, reduction in severe exacerbations, hospitalizations, and emergency department (ED) visits) [21•]. In contrast to the data reported by Chakir et al. [19], they found a significant increase in collagen deposition. The authors did not find changes in subepithelial mucous glands, eosinophils, and neutrophils. Neither changes in bronchial epithelium nor goblet cell counts were modified, suggesting that other cell/structural changes of AR are not targeted by BT.
Finally, the parasympathetic system is a constrictor neural pathway that controls airway tone, causing bronchoconstriction, mucous secretion, and bronchial vasodilation. Some authors identified neuroendocrine epithelial cells and submucosal nerves as targets whose reduction after BT correlated with clinical efficacy [21•].
Efficacy
Data from three randomized controlled clinical trials of BT in asthma patients are available. Results from the Asthma Intervention Research (AIR) study, enrolling 112 patients with moderate to severe asthma [4], revealed significant improvements in asthma control questionnaire scores (ACQ) and asthma quality of life questionnaire (AQLQ), reduction in mild exacerbations, asthma symptom-free days, and increase in morning peak flow rates, at 1-year follow-up. A second study, known as the Research in Severe Asthma (RISA) trial [5], reported significant improvements in ACQ and AQLQ scores as well as FEV1 after treatment, in patients with more severe disease.
The AIR2 trial was a multicenter, double-blind, sham-controlled study where 288 patients were assigned to BT or sham bronchoscopy, and the primary outcome was the AQLQ score [6]. The study failed to show significant differences. However, in a post-hoc analysis including only patients from both groups that scored the minimally important clinical difference in AQLQ (a score change of 0.5 or greater), the BT-group showed significant differences compared to the sham-group. Patients included in the BT-group showed significant improvements in secondary outcomes, including reduction in severe exacerbations, emergency department visits, and days missed from school or work. No differences regarding pulmonary function were observed. The AIR2 trial had some notable limitations including a significant placebo effect attributed to sham bronchoscopy and milder disease severity.
A recent meta-analysis reported significant differences in the reduction of severe exacerbations and moderate improvement in quality of life in patients treated with BT [22].
In a recent study, more severe asthma patients than those included in previous clinical trials were evaluated 12 months after BT treatment. The authors observed clinical benefits (reduction in severe exacerbations, hospitalizations, and emergency department (ED) visits) without significant changes in lung function [21•].
Safety
BT may cause temporary worsening of asthma symptoms during the treatment period, which comprises the first BT session and 1 month after the third session. In the AIR trial, hospitalizations for respiratory adverse events were more frequent among patients who underwent BT during the treatment period. No fatalities were registered during the study. In the extended follow-up period during 3 years post-treatment, no significant differences were observed between patients treated with BT and the control group [7]. A recent study showed transient peribronchial hyperdensities in computerized tomography (CT) scans after BT sessions, suggesting acute inflammatory changes induced by radio frequency [23]. These alterations were unrelated to clinical symptoms, and spontaneous resolution was observed without specific treatment after 1 month.
Worsening of pre-existing bronchiectasis or the appearance of new bronchiectasis following treatment was reported only in three patients (3%) in the AIR2 trial [6]. Higher hospitalization rates due to respiratory adverse events among BT-treated patients were also reported. However, BT-treated patients maintained low rates of exacerbations and ED visits during extended follow-up up to 5 years (reductions of 44 and 78%, respectively, compared to the 12 months pre-treatment) [24•]. They included annual follow-up with chest CT scans showing no structural changes attributable to BT.
Patients included in the RISA trial had a decrease in hospitalization rates and ED visits at 5-year follow-up [25].
Lung abscess has been reported in two patients treated with BT, including one occurring at 14 months post-treatment which was unlikely to be related to BT [7]. The other case was reported 3 days after the second session of BT (left lower lobe treatment) in a 43-year-old patient with severe uncontrolled asthma, on chronic oral corticosteroid therapy, who failed treatment with omalizumab, with no history of recurrent respiratory infections, or evidence of bronchiectasis in a chest CT scan prior to BT treatment [26]. In that case, the adverse event was attributed to treatment although the mechanism was unknown.
A 66-year-old woman with refractory asthma on anticoagulant therapy experienced a major hemorrhage from the right bronchial artery pseudoaneurysm, developing a mediastinal hematoma and haemothorax after the first session of BT (right lower lobe).
In a feasibility and safety study of BT, nine non-asthma patients scheduled to undergo lung resection for suspected lung cancer; BT was applied 3 weeks prior to prescheduled lung resection within the lobe scheduled to be removed. Three airway sections in two patients showed signs of thrombosis in the perichondral vessels [27].
A single case of hemoptysis requiring embolization occurred in the AIR2 trial [6]. Patients in need of anticoagulant therapy are excluded from BT.
A recent study evaluated short-term safety from routine UK clinical practice [28]. One hundred fifty-two procedures (59 patients) from two different and independent data sources were analyzed 30 days after BT treatment. Patients included in this registry were older and had worse pre-bronchodilator FEV1 values when compared to those in the AIR and AIR2 trials. A higher proportion of patients had adverse events in the registry when compared to clinical trials.
Conclusions
BT is an endoscopic treatment for severe and uncontrolled asthma patients who remain symptomatic despite optimal medical treatment. Although some evidence from small case series has shed light on BT’s mechanism of action, much remains to be known about its impact on AR and treatment-related improvements in quality of life and exacerbations reported by current trials. Although BT is associated with temporary worsening of respiratory symptoms during treatment, the procedure appears safe at a 5-year follow-up. Further research is needed to better select the best candidates for BT. It is highly recommended that treatment with BT be performed in specialized centers and if possible include patients in institutional registries and/or clinical trials in order to achieve these goals.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. https://doi.org/10.1183/09031936.00202013.
Bousquet J, Jeffery PK, Buse WW, Johnson M, Vignola AM. State of the Art: asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–45. https://doi.org/10.1164/ajrccm.161.5.9903102.
Dombret MC, Alagha K, Boulet LP, Brillet PY, Joos G, Laviolette M, et al. Bronchial thermoplasty: a new therapeutic option for the treatment of severe, uncontrolled asthma in adults. Eur Respir Rev. 2014;23:510–8. https://doi.org/10.1183/09059180.00005114.
Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327–37. https://doi.org/10.1056/NEJMoa064707.
Pavord ID, Cox G, Thomson NC, Rubin AS, Corris PA, Niven RM, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185–91. https://doi.org/10.1164/rccm.200704-571OC.
Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade LM, Shah PL, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116–24. https://doi.org/10.1164/rccm.200903-0354OC.
Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, Olivenstein R, et al. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011;11:8. https://doi.org/10.1186/1471-2466-11-8.
Doeing DC, Mahajan AK, White SR, Naureckas ET, Krishnan JA, Hogarth DK. Safety and feasibility of bronchial thermoplasty in asthma patients with very severe fixed airflow obstruction: a case series. J Asthma. 2013;50:215–8. https://doi.org/10.3109/02770903.2012.751997.
Bicknell S, Chaudhuri R, Thomson NC. How to: bronchial thermoplasty in asthma. Breathe. 2014;10:48–59. https://doi.org/10.1183/20734735.007813.
Mayse ML, Laviolette M, Rubin AS, Lampron N, Simoff M, Duhamel D, et al. Clinical pearls for bronchial thermoplasty. J Bronchol. 2007;14:115–23. https://doi.org/10.1097/LBR.0b013e318054dbed.
Cox G, Miller JD, McWilliams A, FitzGerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med. 2006;173:965–9. https://doi.org/10.1164/rccm.200507-1162OC.
Langton D, Sha J, Ing A, Fielding D, Thien F, Plummer V. Bronchial thermoplasty: activations predict response. Respir Res. 2017;18:134. https://doi.org/10.1186/s12931-017-0617-7.
Prakash YS, Halayko AJ, Gosens R, Panettieri RA Jr, Camoretti-Mercado B, Penn RB, et al. An official American thoracic society research statement: current challenges facing research and therapeutic advances in airway remodeling. Am J Respir Crit Care Med. 2017;195:e4–19. https://doi.org/10.1164/rccm.201611-2248ST.
Ramos-Barbón D, Fraga-Iriso R, Brienza NS, Montero-Martínez C, Verea-Hernando H, Olivenstein R, et al. T cells localize with proliferating smooth muscle α-actin+ cell compartments in asthma. Am J Respir Crit Care Med. 2010;182:317–24. https://doi.org/10.1164/rccm.200905-0745OC.
Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–8. https://doi.org/10.1164/rccm.200209-1030OC.
Danek CJ, Lombard CM, Dungworth DL, Cox PG, Miller JD, Biggs MJ, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol. 2004;97:1946–53. https://doi.org/10.1152/japplphysiol.01282.2003.
Petrolani M, Dombret MC, Thabut G, Knap D, Hamidi F, Debray MP, et al. Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med. 2014;190:1452–4. https://doi.org/10.1164/rccm.201407-1374LE.
Denner DR, Doeing DC, Hogarth DK, Dugan K, Naureckas ET, White SR. Airway inflammation after bronchial thermoplasty for severe asthma. Ann Am Thorac Soc. 2015;12:1302–9. https://doi.org/10.1513/AnnalsATS.201502-082OC.
Chakir J, Haj-Salem I, Gras D, Joubert P, Beaudoin ÈL, Biardel S, et al. Effects of bronchial thermoplasty on airway smooth muscle and collagen deposition in asthma. Ann Am Thorac Soc. 2015;12:1612–8. https://doi.org/10.1513/AnnalsATS.201504-208OC.
• Salem KH, Boulet LP, Biardel S, Lampron N, Martel S, Laviolette M, et al. Long term effects of bronchial thermoplasty on airway smooth muscle and reticular basement membrane thickness in severe asthma. Ann Am Thorac Soc. 2016;13:1426–8. https://doi.org/10.1513/AnnalsATS.201603-182LE. The reduction of airway smooth muscle in patients treated with bronchial thermoplasty persists after 2 years of treatment.
• Pretolani M, Bergqvist A, Thabut G, Dombret MC, Knapp D, Hamidi F, et al. Effectiveness of bronchial thermoplasty in patients with severe refractory asthma: clinical and histopathologic correlations. J Allergy Clin Immunol. 2017;139:1176–85. https://doi.org/10.1016/j.jaci.2016.08.009. This is the first study that explores the effects of bronchial thermoplasty on the parasympathetic system as a possible mechanism of action, and correlates histopathological changes of airway smooth muscle with clinical data.
Torrego A, Solà I, Munoz AM, Roqué i Figuls M, Yepes-Nuñez JJ, Alonso-Coello P, et al. Bronchial thermoplasty for moderate or severe persistent asthma in adults. Cochrane Database Syst Rev. 2014;3:CD009910. https://doi.org/10.1002/14651858.CD009910.pub2.
Debray MP, Dombret MC, Pretolani M, Thabut G, Alavoine L, Brillet PY, et al. Early computed tomography modifications following bronchial thermoplasty in patients with severe asthma. Eur Respir J. 2017;49:1601565. https://doi.org/10.1183/13993003.01565-2016.
• Wechsler ME, Laviolette M, Rubin AS, Fiterman J, Lapa e Silva JR, Shah PL, et al. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295–302. https://doi.org/10.1016/j.jaci.2013.08.009. This study shows durability of safety at 5 years follow-up of patients included in AIR2 trial who were treated with bronchial thermoplasty.
Pavord ID, Thomson NC, Niven RM, Corris PA, Chung KF, Cox G, et al. Safety of bronchial thermoplasty in patients with severe refractory asthma. Ann Allergy Asthma Immunol. 2013;111:402–7. https://doi.org/10.1016/j.anai.2013.05.002.
Balu A, Ryan D, Niven R. Lung abscess as a complication of bronchial thermoplasty. J Asthma. 2015;52:740–2. https://doi.org/10.3109/02770903.2015.1005844.
Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest. 2005;127:1999–2006. https://doi.org/10.1378/chest.127.6.1999.
Burn J, Sims AJ, Keltie K, Patrick H, Welham SA, Heaney LG, et al. Procedural and short-term safety of bronchial thermoplasty in clinical practice: evidence from a national registry and Hospital Episode Statistics. J Asthma. 2016;1:1–8. https://doi.org/10.1080/02770903.2016.1263652.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ana Maria Muñoz-Fernández and Alfons Torrego declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Interventional Pulmonology
Rights and permissions
About this article
Cite this article
Muñoz-Fernández, A.M., Torrego, A. Bronchial Thermoplasty in Severe Asthma. Curr Pulmonol Rep 6, 221–226 (2017). https://doi.org/10.1007/s13665-017-0191-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-017-0191-y