Abstract
Lung cancer is one of the deadliest cancers but also benefits from the greatest therapeutic advances. The advent of targeted therapies has revolutionized the management of non-small lung cancer (NSCLC) using molecular alterations but only concerns a limited number of patients. Antitumor immunotherapy, initially mainly an antigen-dependent approach with therapeutic vaccines, has been disappointing to treat lung cancer until recently. However, there are now positive results from clinical trials evaluating immune checkpoint inhibitors. This review summarizes the rational for exploiting each step of the antitumor immune cycle to treat lung cancer and summarizes the results from the main clinical trials. We report on the main strategies of enhancing antigen presentation, priming, and T cell activation, with a special focus on drugs that target the PD1/PD-L1 axis. This last option shows the best results and has undoubtedly become a new standard of care to treat lung cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the main cause of cancer-related mortality worldwide. Chemotherapies have reached a plateau of efficacy, and patient tolerance to them is often poor. Although targeted therapies have led to dramatic improvements in the management of selected patients, the tumors have inexorably developed mechanisms of resistance to these drugs. Thus, new strategies need to be developed to cover a larger population, to obtain better safety profiles, and to obtain more sustainable responses. Recently, although the antigen-dependent approach has seemed disappointing, antitumor immunotherapy has rapidly become integrated into the management of non-small lung cancer (NSCLC) after positive clinical trials evaluated the immune checkpoint inhibitors. Herein, we review the rational and results reported from different strategies for anticancer immunotherapy carried out at different steps during the anticancer immunity cycle in the context of lung cancer.
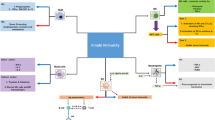
Antigen presentation: antitumor vaccines
Rational
The purpose of tumor-associated antigen (TAA) vaccines is to promote an antitumor immune response by enhancing the presentation of TAAs by dendritic cells to naïve T cells. Antigen-specific active immunotherapies have been tested in several clinical trials with, up to now, disappointing results. Most antigen-specific immunotherapies have incorporated a single antigen and have narrow epitope specificities, which may contribute to their lack of efficacy [1]. We briefly report on the main trials below.
Outcomes in thoracic oncology
Tecemotide, a therapeutic vaccine that targets cancer cells expressing MUC1, was tested in a phase 3 trial in unresectable stage III NSCLC, previously treated with chemoradiotherapy. Tecemotide failed to significantly prolong survival in the overall population. However, tecemotide improved survival in the preplanned group of patients who were treated with concurrent chemoradiotherapy. A further phase 3 trial is initiated in this population (START2). The TG4010 vaccine also targets MUC1 and IL2. In a phase 2 study on advanced stage NSCLC, a slight albeit non-significant difference in progression-free survival (PFS) at 6 months was observed, but there was no difference in terms of overall survival (OS). A phase 2–3 study where TG4010 was added to the chemotherapy for stage IV NSCLC (TIME trial) recently shows that TG4010 was efficacious with an acceptable safety profile in patients with stage IV NSCLC, particularly in the non-squamous population [2, 3]. The percentage of CD16 + CD56 + CD69+ cells, a phenotype of activated natural killer cells, was a potential predictor of outcome of patients who received TG4010 [4]. GSK1572932A is a vaccine that targets the melanoma-associated antigen-3 (MAGE-A3) peptide. A phase 2 study on patients with resected MAGE-A3-positive NSCLC initially suggested a trend towards improved outcomes [2, 3]. Unfortunately, these results were not confirmed in a recent large phase 3 study (MAGRIT trial), which showed no difference in terms of disease-free survival and no clear impact from the biomarkers [5].
GV1001, a peptide vaccine that corresponds to the active site of human telomerase reverse-transcriptase and GM-CSF, induced specific immune responses in 80 % of patients with unresectable stage III NSCLC [6]. A gain in median overall survival was reported among immune responders in a phase 1–2 study in which GV1001 was combined with a second telomerase peptide (I540). A phase 3 study is ongoing.
The CIMAvax EGF vaccine is designed to induce an antibody-mediated immune response [7]. However, a phase 2 study failed to demonstrate a gain in median OS although a subgroup of patients with a good antibody response appeared to derive some benefit from the drug.
The Belagenpumatucel-L vaccine (LucanixTM, NovaTx Corporation, San Diego, USA) consists of NSCLC cell lines transfected with a TGF-β2 antisense gene. Despite a promising phase 2 study [5], a recent phase 3 trial on advanced stage NSCLC was negative, although it showed a potential benefit for subgroups with adenocarcinoma and who had been previously treated with radiotherapy [8].
Tergenpumatucel-L (lung cancer cell lines transfected with a murine galactosyl-transferase gene) has been associated with an interesting median OS in a phase 2 study [9].
Altogether, the results are disappointing for the whole population to date, but some positive signals are reported in subgroups and new trials are currently being conducted in more closely selected patients.
Priming: CTLA-4 inhibition
Rational
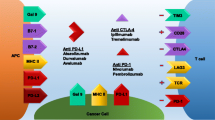
CTLA-4 regulates T cells, predominantly during their initial activation by dendritic cells and other antigen-presenting cells. CTLA-4 outcompetes the stimulatory receptor CD28 to bind to its ligands (CD80/CD86) due to its higher binding affinity and because it delivers a negative signal into the T cell, leading to inhibition of T cell activation and expansion [10]. Moreover, CTLA-4 is expressed by regulatory T cells (Treg) and can redirect T cells to the tolerogenic phenotype, CD25-Foxp3. The main function of these T cells is to maintain immune homeostasis and modulate immune responses, but they can also induce tumor proliferation [11]. CTLA4 is expressed on the surface of T lymphocytes but is also expressed in NSCLC tumors in 51–87 % of cases. CTLA4 expression was associated with non-squamous histology but is not significantly correlated with OS [12]. It is also associated with older age and poor tumor differentiation [13, 14].
Ipilimumab and tremelimumab are fully human monoclonal antibodies that target CTLA-4 and can lead to important, diffuse, and unspecific T cell activation.
Outcomes in thoracic oncology
Ipilimumab (Yervoy®) was first tested in metastatic melanoma before being evaluated for lung cancer. A phase 2 study for stage IIIB/IV NSCLC assessed the activity of ipilimumab plus paclitaxel and carboplatin. Patients were randomized to receive either paclitaxel with carboplatin and a placebo, or concurrent ipilimumab, or phased ipilimumab. Phased ipilimumab improved immune-related PFS (5.7 vs. 4.6 months with a placebo; HR 0.72, p < 0.05) and median OS (12.2 vs. 8.3 months with a placebo), but the difference in OS was not significant (p = 0.23). The PFS advantage was not observed in the concurrent ipilimumab arm [15]. Initial platinum-based chemotherapy is frequently efficient for small-cell lung cancer (SCLC), but usually fails to induce a durable response. A phase 1–2 study assessed nivolumab with or without ipilimumab to treat recurrent SCLC (CA209-032). Other objectives were safety, PFS, OS, and biomarker analysis. In 20 evaluable patients in the nivolumab-plus-ipilimumab arm, one patient had a complete response (CR), six (30 %) had a stable disease, and nine (45 %) had a partial response. Many other trials are ongoing, especially for SCLC. A phase 2 study is evaluating ipilimumab in addition to carboplatin and etoposide in a first-line setting to treat extensive stage SCLC (NCT01331525).
Two phase 2 studies (NCT01285609 and NCT01450761) have recently evaluated ipilimumab combined with paclitaxel–carboplatin or platinum–etoposide in patients with squamous NSCLC and SCLC. Results are awaited. Combinations with targeted therapies are also currently being tested in a phase 1b study (NCT01998126) where ipilimumab is associated with erlotinib or crizotinib for EGFR- or ALK-mutated stage IV NSCLC. A phase 2 trial is also assessing ipilimumab plus chemotherapy in a neo-adjuvant setting (NCT01820754).
Tremelimumab is another human IgG2 monoclonal antibody that targets CTLA-4. In a phase 2 open-label trial on advanced malignant mesothelioma that has relapsed after prior chemotherapy, the median PFS was 6.2 months and the median OS was 10.7 months [16]. This drug is being evaluated in combination with MEDI4736 vs. MEDI4736 alone vs. platinum-based chemotherapy as a first-line treatment for advanced or metastatic NSCLC (NCT02453282). We also await results from another phase 2b trial that is comparing tremelimumab as a monotherapy to a placebo for pretreated mesothelioma.
The PD-1/PD-L1 pathway: T cell expansion and activation
Rational
PD-1 is a transmembrane protein expressed on the surface of activated immune cells (T cells, B cells, dendritic cells, NK, NKT, macrophages [17]). PD-L1, its ligand, is also present on the surface of many hematopoietic (B cells, T cells, dendritic cells) and non-hematopoietic cells (epithelial cells, endothelial cells) [18]. The interaction between these two molecules constitutes a physiological checkpoint that prevents an excessive and uncontrolled immune response [19]. Ensuing inhibition of the T cell response (by limiting the secretion of cytotoxic mediators) can induce anergy or redirect T cells to a protumor T-reg FOXP3 phenotype [20, 21]. PD-L1 expression by tumor cells is a well-known mechanism of immune evasion that concerns 40–50 % of cases of NSCLC [22, 23]. This enables the tumor to be surrounded by a tolerogenic protumoral microenvironment. Blocking PD-1 or PD-L1 restores cytotoxic antitumor T cell activity and subsequently acts as an effective antitumor response [24•].
Outcomes in thoracic oncology
PD-1 inhibitors
Nivolumab (Opdivo®) was the first anti-PD1 approved for the treatment of locally advanced or metastatic squamous (EU, US) and non-squamous (US) NSCLC after prior chemotherapy [25]. This drug is a genetically engineered IgG4 monoclonal antibody that first demonstrated an interesting objective response rate (ORR) for squamous (33 %) and non-squamous (12 %) NSCLC (n = 76) in a phase 1 study. PD-L1 expression was strongly correlated with response in this study (33 vs. 0 % for PD-L1-positive or PD-L1-negative tumors, respectively) [26]. A single-arm phase 2 study, CheckMate 063, was then initiated, which tested nivolumab as a monotherapy for squamous NSCLC that had progressed after two or more previous lines of chemotherapy. An interesting objective response rate of 14.5 % was reported within this heavily pretreated population. Responses were durable, as a median response duration was not reached and 40.8 % of the population were still alive at 1 year [27].
CheckMate 017 was a phase 3 randomized trial comparing nivolumab to docetaxel in 272 pretreated squamous NSCLC patients. The ORR was significantly higher in the nivolumab arm (20 vs. 9 %). Moreover, responses appeared prolonged (9.2 months median OS vs. 6 months; median duration response was not reached in the nivolumab arm vs. 8.4 months in the docetaxel arm) and the toxicity profile was much more favorable (9 % grade 3/4 side effects vs. 71 % in docetaxel arm). PD-L1 expression (available for 83 % of patients on recent or archival tissues) was found to not be prognostic or predictive of a response, whatever the percentage of stained tumor cells in immunohistochemistry (1, 5 or 10 %). [28••]. The CheckMate 057, a study with a similar design, was then conducted on 582 cases of pretreated non-squamous NSCLC. The ORR was 19 vs. 12 % for the nivolumab and docetaxel arms, respectively, but, more interestingly, the median duration response was 17.2 vs. 5.6 months, and grade 3/4 toxicity was 10.5 vs. 53.7 %, respectively. In this study, PD-L1 expression was mostly assessed prospectively and strongly correlated with response, even when using a cutoff value of 1 % expression for tumor cells. The ORR was 9 vs. 31 % (cutoff 1 %), 10 vs. 36 % (cutoff 5 %), and 11 vs. 37 % (cutoff 10 %) for PD-L1-negative vs. PD-L1-positive tumors, respectively [29••]. CheckMate 026 (NCT02041533), a phase 3 study is recruiting patients to compare platinum-based chemotherapy and nivolumab as a first-line treatment for PD-L1-positive patients. Figure 1a, b shows an example of the response to nivolumab in a heavily pretreated patient.
Pembrolizumab (MK-3475, KEYTRUDA®) is another highly selective humanized IgG4 antibody that targets PD1 and has been approved by the FDA since October 2015 to treat PD-L1-positive metastatic NSCLC after failure of platinum-based chemotherapy. KEYNOTE-001 was a large phase 1 trial that enrolled 495 patients (394 pretreated, 101 not previously treated). The ORR was 19.5 % (18 % in the pretreated cohort, 24.8 % for untreated patients). Median duration of response was 23.3 months for previously untreated patients vs. 10.4 months for pretreated patients. Grade 3/4 adverse effects were reported in 9.5 % of patients (1.8 % pneumonitis). Strong PD-L1 expression, defined as expression of at least 50 % of tumor or tumor-infiltrating immune cells, strongly correlated with response to pembrolizumab, with a response rate of 45.2 % [30]. This justified the integration of PD-L1 expression for FDA approval. Clinical trials are ongoing to precisely identify the indications of pembrolizumab. KEYNOTE 010 is a phase 3 study comparing docetaxel versus pembrolizumab in previously treated patients. In total, 1034 patients have been enrolled: 345 received pembrolizumab 2 mg/kg, 346 pembrolizumab 10 mg/kg, and 343 docetaxel. The median OS was significantly longer for pembrolizumab 2 mg/kg (10.4 months) versus docetaxel (8.5 months) (HR 0.71, 95 % CI 0.58–0.88; p = 0.0008) and for pembrolizumab 10 mg/kg (12.7 months) versus docetaxel (HR 0.61, 0.49–0.75; p < 0.0001). Furthermore, for patients with strong PD-L1 expression (at least 50 % of tumor cells expressing PD-L1), OS was significantly longer with pembrolizumab 2 mg/kg than with docetaxel (median 14.9 vs. 8.2 months; HR 0.54, 95 % CI 0.38–0.77; p = 0.0002) and with pembrolizumab 10 mg/kg than with docetaxel (17.3 vs. 8.2 months; 0.50, 0.36–0.70; p < 0.0001). Treatment-related adverse events were less frequent with pembrolizumab than docetaxel [31]. KEYNOTE 024 (NCT02142738) is recruiting PD-L1 patients to receive either pembrolizumab or platinum-based chemotherapy as a first-line treatment for NSCLC. Finally, KEYNOTE 091 (NCT02504372) will compare pembrolizumab to a placebo in an adjuvant setting.
PD-L1 inhibitors
Atezolizumab (MPDL3280A), an antibody targeting PD-L1, showed encouraging results in a phase 1 study, leading to an ORR of 23 % and durable responses in pretreated patients. PD-L1 was predictive of a response in this study (39 % ORR and 88 % disease-control rate vs. 13 % ORR and 41 % disease-control rate in PD-L1-positive and PD-L1-negative patients, respectively) [32]. A phase 2 study (POPLAR) was then conducted, including 287 pretreated patients (interim analyses [33, 34]) randomized to receive either MPDL3280A or docetaxel. Patients were stratified by PD-L1 status (expression in immune cells and tumor cells), histology, and prior lines of treatment. Patients with the lowest PD-L1 expression (TC0 and IC0) did not derive any benefit from the treatment (ORR 8 %; median PFS 1.9 months). In contrast, strong immuno-histochemistry (IHC) staining (of TC3 or IC3) was strongly predictive of a response to atezolizumab, with an ORR of 38 vs. 13 % for docetaxel. Median PFS in this cohort was 9.7 months, and the median OS has not been reached. Atezolizumab was also better tolerated, with 11 % grade 3/4 side effects vs. 56 % in the docetaxel arm. A phase 3 study of a similar design has been recently completed (OAK, NCT02008227). Atezolizumab is also currently being evaluated in two single-arm phase 2 studies (FIR and BIRCH, NCT01846416 and NCT02031458) in PD-L1-positive patients. Preliminary results from the BIRCH trial were reported at the European Cancer Congress 2015 [35]. The ORR was 26, 24, and 27 % for tumors showing strong expression to PD-L1 (TC3 or IC3) in first-, second-, and third-line treatments, respectively (ORRs were 19, 17, and 17 % for TC2/3 or IC2/3 PD-L1 expression). The median response duration has not been reached, as most responses are still ongoing. Treatment-related overall adverse effect rate is 65 %, with only 11 % grade 3/4 adverse events.
MEDI4736 (durvalumab) is another PD-L1 inhibitor that achieved a 13 % ORR in a phase 1 study [36]. This drug is still undergoing ongoing phase 3 evaluation (ATLANTIC, NCT 02087423) as a monotherapy for heavily pretreated patients (at least two lines), with multiple arms, based on PD-L1 level of expression and mutational profile. The PACIFIC trial (NCT 02125461) is a phase 3 study that includes randomized patients receiving either durvalumab or a placebo after chemoradiation for stage III unresectable NSCLC. There are strong signals for a synergistic effect in this context, as radiation therapy may upregulate PD-L1 expression of tumor cells as a mechanism of resistance, which can be overcome by the PD-L1 blockade [37].
Table 1 summarizes the main results reported in clinical trials that have evaluated these anti-PD1 and anti-PD-L1 drugs.
Combinations of drugs
Even though immunotherapies have activities as single agents, more impressive activities can be seen when they are combined with other agents.
Chemotherapies and immunotherapies
Several conventional chemotherapies inducing immunogenic tumor-cell death can enhance a strong adaptive immune response. Indeed, tumor cells are converted into an anticancer vaccine (release of endogenous tumor antigens), and their effects may be enhanced by immune checkpoint inhibitors [38]. Numerous clinical trials are ongoing and are testing such combinations for SCLC and NSCLC.
Anti-PD-1/PDL-1 and anti-CTLA4 antibodies
Because of distinct levels of intervention, T cell activation, and expansion in the periphery of CTLA4 and CTL-effector functions in the tumor microenvironment of PD-1, it seems logical to combine these two drugs to enhance antitumor immune activity [39]. This has been demonstrated in melanoma. In lung cancer, the combination of ipilimumab plus nivolumab has shown response rates of 11–33 % depending on histology, but induces frequent and sometimes severe immune-related adverse effects [40].
Immunotherapy and targeted therapy
Activating the patient’s immune system during the time of tumor reduction and remission may be the best way to ensure that responses are converted into long-term and durable benefits. Unlike conventional chemotherapies, targeted therapies for EGFR-mutated or ALK-rearranged adenocarcinoma may achieve rapid and significant tumor shrinkage without the need for immunosuppression induced by chemotherapies. Moreover, oncogenic EGFR signaling remodels the tumor microenvironment to trigger an immune escape and mechanistically links the treatment response to PD-1 inhibition [41]. A first-line therapy combining nivolumab and erlotinib showed excellent response rates, even in EGFR–TKI pretreated patients, but with a grade 3–4 incidence of adverse events of 24 % [27]. Other trials that have combined, for example, gefitinib and tremelimumab in EGFR-mutated patients who progress under EGFR–TKI, or alectinib and atezolizumab in ALK-positive patients, are ongoing.
Biomarkers
Only a minority of patients currently benefit from active immunotherapy. Patient selection is therefore crucial. Strong arguments exist to consider PDL-1 expression in IHC analysis as a potential predictive biomarker for the response to drugs that target the PDL-1/PD-1 checkpoint. In the MPDL3280A phase 1 study, ORR was 46 % (6/13) in patients with a PDL-1 IHC score of 2 or 3, and 83 % (5/6) in those with a PDL-1 IHC score of 3 [42]. MEDI4736 response rate was also strongly correlated with PDL-1 expression (39 vs. 5 % ORR [36]). Response rates in the KEYNOTE-001 study (pembrolizumab) were 37, 17, and 10 % for strong-positive, weak-positive, or negative PDL-1 expression, respectively [43]. PD-L1 expression was found to be potent at discriminating responders from non-responders in the landmark phase 1 trial with nivolumab, and in the CheckMate 057 trial (for details, see above). The predictive value of PD-L1 expression was predefined as an additional endpoint in the CheckMate 017 and 057 studies, but the assay was not standardized and was conducted retrospectively on recent or archival tissue.
PD-L1 expression can vary with the microenvironment of the tumor. Thus, PD-L1 expression at a single time point may not represent a dynamic immune response. Overall, PD-L1 expression appears to be an imperfect marker, as the optimal test has not yet been clearly defined although constitutes an interesting basis for selecting patients. PD-L1 screening should be probably used in the future to select patients, especially in first-line settings, as chemotherapy (which can be associated with bevacizumab and maintenance therapy) has a response rate and a PFS that can reach 35 % [44] and 7.4 months [45], respectively. In contrast, in heavily pretreated patients who have less therapeutic options, biomarkers may be dispensable, as the response rates among PD-L1-negative patients remain higher than those observed in second- or multiple-line settings.
Other molecular biomarkers could also be used to help select the best candidates. Exome analysis of tumors from patients treated with another PD-1 inhibitor, pembrolizumab, showed that the best responses to PD-1 blockage were observed when there was a high mutation burden [46]. High molecular transversion associated with smoking is particularly associated with improved efficiency of pembrolizumab. These correlations with tobacco and the KRAS mutation have been confirmed among all stages of combined populations [47, 48]. This can be explained by the increase in tumor-specific antigens, such as ACE, KRAS, p53, and hTERT, from the frequently overexpressed or mutated genes observed in lung cancer, with these constituting targets for an efficient immune antitumor response.
Toxicity
Targeting immune checkpoints induced the emergence of a new form of toxicity. The side effects are less frequent and severe than the ones observed with chemotherapy and essentially concerned the lung (pneumonitis), the skin (rash), the gastrointestinal tractus (diarrhea and colitis), the liver (hepatitis), the kidneys (renal insufficiency), and the endocrine glands (hypophysitis and hypothyroidism) [49]. A rare but potentially fatal inflammatory pneumonitis has been observed in a few cases: pneumonitis was reported in 6 % of a group of patients (8/129) [50]. Figure 1c, d shows an example of lung toxicity. Pulmonary toxicity needs to be recognized and treated early. There is no validated recommendation for pneumonitis management, which is usually guided by clinical experience and observational reports [15, 51, 52]. Patients presenting with signs and symptoms of pneumonitis (dyspnea, cough, hypoxia, and interstitial radiological syndrome) must have CT scan. This scan can confirm pneumonitis (extensive, patchy, bilateral, peri-bronchial consolidation) or orient towards a differential diagnosis (infection, heart failure, tumor progression, pulmonary embolism). A bronchoscopy with an oriented broncho-alveolar lavage should be considered, including a cell count, analysis of T cell subsets, and microbial analyses. For grade 1 pneumonitis (according to CTCAE 4.0), it is possible to consider using steroids and to repeat the CT scan 3 weeks later. For grade 2 pneumonitis, it is recommended to delay immunotherapy and introduce prednisolone (1–2 mg/kg) [53]. For grade 3/4 pneumonitis, immunotherapy should be discontinued permanently and high doses of intravenous steroids (methylprednisolone 1 g/day) should be administered along with oxygen and ventilatory support. In cases of failure after 48 h or serious evolution, prophylactic antibiotics and additional immunosuppressive medication (infliximab, mycophenolate mofetil, or cyclophosphamide) can be discussed even if data are lacking to validate the use of such drugs [53].
Conclusion
After decades of frustration with negative results from antitumor vaccines for lung cancer, antitumor immunotherapy has finally entered the therapeutic arsenal to treat lung cancer after the positive results from clinical trials that have assessed immune checkpoint inhibitors. The PD1 and PD-L1 inhibitors are becoming a new standard of care for previously treated and advanced NSCLC. Nevertheless, if these drugs are well tolerated and offer durable responses, only a limited population can benefit. More fundamentally, translational and clinical research is needed to improve the selection of patients (biomarkers) and to find the best combinations (monotherapy, with chemotherapy, with a targeted therapy) to further improve their efficiency.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Slingluff CL. The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J. 2011;17(5):343–50.
Vansteenkiste J, Zielinski M, Linder A, Dahabreh J, Gonzalez EE, Malinowski W, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol. 2013;31(19):2396–403.
Quoix E, Losonczy G, Forget F, Chouaid C, Papai Z, Gervais R. 1055PD—TIME, a phase 2b/3 study evaluating TG4010 in combination with first line therapy in advanced non small cell lung cancer (NSCLC). Phase 2b results. ESMO2014;Abstr:5152.
Rotonda C, Anota A, Mercier M, Bastien B, Lacoste G, Limacher J-M, et al. Impact of TG4010 vaccine on health-related quality of life in advanced non-small-cell lung cancer: results of a phase IIB clinical trial. PloS One 2015;10(7):e0132568.
Vansteenkiste J, Cho B, Vanakesa T. MAGRIT, a double-blind, randomized, placebo-controlled phase III study to assess the efficacy of the recMAGE-A3 + AS15 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3 positive non-small cell lung cancer (NSCLC). Ann Oncol. 2014;25:iv409–16.
Brunsvig PF, Kyte JA, Kersten C, Sundstrøm S, Møller M, Nyakas M, et al. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res. 2011;17(21):6847–57.
Neninger Vinageras E, de la Torre A, Osorio Rodríguez M, Catalá Ferrer M, Bravo I, Mendoza del Pino M, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(9):1452–8.
Giaccone G. FDA backs continued study of belagenpumatucel-L for subgroups of patients based on data from phase III STOP trial. 2013. Available online: http://www.esmo.org/Conferences/Past-Conferences/European-Cancer-Congress-2013/News/Belagenpumatucel-L-Therapeutic-Tumour-Cell-Vaccine-for-Non-Small-Cell-Lung-Cancer.
Morris JC, Rossi GR, Harold N, Tennant L, Ramsey WJ, Vahanian N, et al. Potential chemo-sensitization effect of tergenpumatucel-L immunotherapy in treated ptients with advanced non-small cell lung cancer 8NSCLC). J Clin Oncol 2013;31:abstr 8094.
Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300–9.
Attridge K, Walker LSK. Homeostasis and function of regulatory T cells (Tregs) in vivo: lessons from TCR-transgenic Tregs. Immunol Rev. 2014;259(1):23–39.
Salvi S, Fontana V, Boccardo S, Merlo DF, Margallo E, Laurent S, et al. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2012;61(9):1463–72.
Sundar R, Soong R, Cho B-C, Brahmer JR, Soo RA. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer Amst Neth. 2014;85(2):101–9.
Zheng H, Li Y, Wang X, Zhang X, Wang X. Expression and significance of gp96 and immune-related gene CTLA-4, CD8 in lung cancer tissues. Zhongguo Fei Ai Za Zhi Chin J Lung Cancer. 2010;13(8):790–4.
Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–54.
Calabrò L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14(11):1104–11.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19(18):4917–24.
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–53.
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127–37.
McLaughlin JF, Schalper K, Carvajal-Hausdorf DE, et al. Domain-specific PD-L1 protein measurement in non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32:abstr 8064.
Yang C-Y, Lin M-W, Chang Y-L, Wu C-T, Yang P-C. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014;50(7):1361–9.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. A very elegant and exhaustive review of antitumor immunity.
Guibert N, Mazières J. Nivolumab for treating non-small cell lung cancer. Expert Opin Biol Ther. 2015;15(12):1789–97.
Gettinger S, Herbst RS. B7-H1/PD-1 blockade therapy in non-small cell lung cancer: current status and future direction. Cancer J. 2014;20(4):281–9.
Rizvi NA, Chow LQM, Borghaei H, Shen Y, Harbison C, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J Clin Oncol 32:5s, 2014;(suppl; abstr 8022).
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. A major phase III trial that led to approval for advanced squamous NSCLC.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. A pivotal phase III trial that will probably lead to approval for advanced non-squamous NSCLC.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet 19 December 2015[Internet]. Disponible sur: http://www.sciencedirect.com/science/article/pii/S0140673615012817
Herbst RS, Gordon MS, Fine GD, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J Clin Oncol. 2013;(suppl; abstr 3000).
Spira AI, Park K, Mazières J, Vansteenkiste JF, Rittmeyer A, Ballinger M, et al. Efficacy, safety and predictive biomarker results from a randomized phase II study comparing atezolizumab vs docetaxel in 2L/3L NSCLC (POPLAR). J Clin Oncol 2015;33:abstr 8010.
Vansteenkiste J, et al. Atezolizumab monotherapy vs docetaxel in 2L/3L non-small cell lung cancer: Primary analyses for efficacy, safety and predictive biomarkers from a randomized phase II study (POPLAR). ECC 2015; Vienna, Austria; 27 September 2015; 14LBA.
Besse B, Johnson M, Jänne PA, Garassino M, Eberhardt WEE, Peters S, et al. Phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L1-selected non-small cell lung cancer (NSCLC) Presented at: 2015 European Cancer Congress; September 25-29; Vienna, Austria. Abstract 16LBA. 2015.
Segal NH, Antonia SJ, Brahmer JR, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. Slides presented at the 50th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, USA, May 30‒June 03, 2014. J Clin Oncol (Meeting Abstracts). 2014;32(abstract 3002).
Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–68.
Vacchelli E, Aranda F, Eggermont A, Galon J, Sautès-Fridman C, Cremer I, et al. Trial Watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunolog. 2014;3(1):e27878.
Wolchok J, Kluger HM, Callahan MK, Postow MA, Gordon RA, Segal NH, et al. Safety and clinical activity of nivolumab (anti-PD-1, BMS-936558, ONO-4538) in combination with ipilimumab in patients (pts) with advanced melanoma (MEL). J Clin Oncol. 2013;31:(suppl; abstr 9012).
Antonia SJ, Gettinger SN, Chow LQM, Juergens RA, Borghaei H, Shen Y, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J Clin Oncol 32:5s, 2014:(suppl; abstr 8023).
Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–63.
Horn L. Longer follow-up confirms nivolumab OS benefit in nonsquamous non-small cell lung cancer. Presented at: 16th World Conference on Lung Cancer; Sep 6–9; Denver (CO). Abstract. 2015.
Garon EB, Gandhi L, Rizvi N, Hui R, Balmanoukian AS, Patnaik A, et al. Antitumor activity of pembrolizumab (Pembro; MK-3475) and correlation with programmed death ligand 1 (PD-L1) expression in a pooled analysis of patients with advanced non small cell lung carcinoma NSCLC. Ann Oncol. 2014;25(5):1–41. doi:10.1093/annonc/mdu438.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50.
Barlesi F, Scherpereel A, Rittmeyer A, Pazzola A, Ferrer Tur N, Kim J-H, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol. 2013;31(24):3004–11.
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8.
Ansen S, Schultheis AM, Hellmich M, Zander T, Brockmann M, Stoelben E, et al. PD-L1 expression and genotype in non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32:(abstr 7517).
Calles A, et al. Expression of PD-1 and its ligands, PD-L1 and PD-L2, in smokers and never smokers with KRAS-mutant lung cancer. J Thorac Oncol. 2015;10(12):1726--1735.
Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–7.
Brahmer JM, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients (pts) with advanced non-small-cell lung cancer (NSCLC): survival and clinical activity by subgroup analysis [abstract]. J Clin Oncol. 2014;32(5s):abstr 8112.
Inoue A, Kunitoh H, Sekine I, Sumi M, Tokuuye K, Saijo N. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys. 2001;49(3):649–55.
Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373(3):288–90.
Ramaligam S. et al. Phase 2 objective response rate and survival data for Opdivo (nivolumab) in heavily pre-treated advanced squamous cell non-small cell lung cancer. Chicago Multidisciplinary Symposium on Thoracic Oncology. 2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Myriam Delaunay, Julien Mazières, and Nicolas Guibert each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Lung Cancer
Rights and permissions
About this article
Cite this article
Delaunay, M., Mazières, J. & Guibert, N. Harnessing the antitumor immunity cycle to treat lung cancer. Curr Pulmonol Rep 5, 40–48 (2016). https://doi.org/10.1007/s13665-016-0136-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-016-0136-x