Abstract
Smoking cessation presents a daunting challenge for clinicians; tobacco use impacts every major organ system and is responsible for considerable morbidity and mortality. Despite significant reductions in the rate of smoking over the past 50 years, tobacco use continues to burden the healthcare system. First-line therapies for smoking cessation include nicotine replacement, novel partial nicotinic agonists as well as antidepressant therapy. Herein, we review recent updates to the literature regarding nicotine replacement, bupropion, and varenicline. Re-emerging therapies such as cytisine are reviewed, as well as the novel concept of vaccination. Finally, the controversy surrounding electronic cigarettes and their debatable role in cessation therapy will be addressed as their popularity continues to grow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite great strides in understanding the pathophysiology of addiction, tobacco use continues to burden society. The Surgeon General’s executive summary on the health consequences of smoking reports over 480,000 deaths attributed to smoking between 2005 and 2009 and over US$150 billion in yearly smoking-related productivity losses. Men and women are equally as likely to be affected by smoking-related diseases [1, 2]. Smoking cessation without the assistance of adjunctive therapy has an 80 % failure rate, and only 3 % of patients attempting to quit will find success at 1 year [3]. Despite these daunting statistics, pharmacologic management has provided powerful tools in assisting our patients to quit; smoking rates have been cut in half in the last 50 years [2]. In this article, we will update the clinician regarding advances to the mainstays of smoking cessation therapy including nicotine replacement, antidepressants, and partial nicotinic agonists. We will discuss recent controversy surrounding the explosive growth of electronic cigarettes and the complicated role they play as quit aids. Lastly, we will examine emerging therapies as well as new evidence for old pharmacologic agents as the future of smoking cessation interventions.
Nicotine replacement therapy
A variety of nicotine delivery systems have been devised to aid in smoking cessation including patches, gums, sprays, and lozenges. Nicotine replacement therapy (NRT) doubles quit rates when compared to placebo, regardless of the delivery route [4, 5]. The choice of delivery system does not appear to affect cessation rates, though the speed with which nicotine is delivered varies by agent [4]. Table 1 provides an overview of currently available therapies. Recent research on NRTs has focused on novel delivery routes, higher-dose delivery systems, and prolonged use of traditional therapy [6–9]. A 2014 meta-analysis by Brokowski et al. examined ten trials using transdermal delivery methods with nicotine doses of 42 mg or higher (twice what is currently approved by the FDA). They found that while smoking cessation rates improved during the initial study period (on average, 6 to 12 weeks), long-term cessation rates were no different than usual dosing at 6 to 12 months [6]. Moreover, 5 % of participants in the largest trial experienced dose-dependent adverse events including palpitations, GI upset, and local urticarial reactions resulting from the patch [10]. Cardiovascular events including MI and stroke were also noted in one study, though to such a small extent that their significance is unknown [6]. This preliminary data suggests that higher dosing may not provide benefit beyond what we have already achieved with the standard 21 mg dosing. The adverse effects of increased dosing may provide an impetus to abandon cessation therapy and return to smoking. Given the heterogeneity of results, more research will be needed before higher-dose nicotine patches can be prescribed in everyday practice.
In addition to higher dosing, studies of longer duration therapy are underway in an effort to improve the durability of smoking cessation. Transdermal nicotine is approved by the FDA for use beyond the standard 8-week treatment regimen based on the results of a large randomized control trial that showed improved cessation rates after 24 weeks of continuous therapy [11]. Schnoll and colleagues extended therapy to 52 consecutive weeks of transdermal nicotine. While they were able to reproduce the previous benefit noted at 24 weeks, an additional 28 weeks of therapy conferred no further benefit [9]. These results argue for a trial of extended therapy to 24 weeks rather than the traditional 8-week therapy to improve cessation rates [9, 11]. As noted by the authors of this study, the possibility of poor adherence to cessation therapy over time may account for the loss of benefit and future studies may focus on heavy smoking populations who could benefit from greater than 24 weeks of nicotine replacement.
On demand therapy to control cravings is the hallmark of nicotine gum and lozenges, though the time to maximal plasma concentration can be variable and may not provide the immediate relief smokers require. Nicotine mouth spray significantly reduces cravings within 3–4 min of use compared to lozenges [12]. Tonnesen and colleagues explored the efficacy of a 1-mg nicotine mouth spray versus placebo in a European population of 479 smokers (at least one cigarette or more per day). They found sustained abstinence in 13.8 % of the treatment arm versus 5.6 % of the placebo arm at week 52 [7]. These preparations are currently only available over the counter outside the USA. Their use should be considered when patients are unable to relieve cravings with gum or lozenges typically prescribed. Moreover, combination therapy with sustained release patches in addition to quick acting preparations such as the spray can improve early quit rates [13].
Bupropion
As the only FDA-approved antidepressant for smoking cessation, bupropion has been well validated [14–18]. Bupropion has dopaminergic, adrenergic, and nicotinic acetylcholinergic activity that all contribute to its cessation effects by blocking nicotine activity, relieving withdrawal symptoms and improving depressed mood [18]. Ebbert and colleagues attempted to take advantage of the antidepressant properties of bupropion in addition to the partial nicotine agonist effects of varenicline by combining the two therapies in their 2014 study of 506 smokers. They examined self-reported quit rates as well as biochemically confirmed cessation with exhaled CO levels in otherwise healthy individuals without a psychiatric history. The study found a statistically significant reduction in cessation at 12 and 24 weeks in the combination therapy group, but no significant improvement in cessation rates at 52 weeks with combination therapy compared to varenicline plus placebo [19]. Moreover, the combined therapy group reported more symptoms of anxiety and depression. In a similar study, Rose et al. found that, in 222 heavy smokers (≥10 cigarettes per day) who failed to respond significantly to first-line nicotine replacement therapy, patients randomized to combination bupropion and varenicline had a significant improvement in cessation rates at 12 weeks (confirmed by exhaled CO) compared to those on varenicline plus placebo [20]. While intriguing, these relatively small studies are subject to limited interpretation. It is unclear if improvement in cessation rates is population dependent given the difference in daily cigarette use between study groups. Likewise, Ebbert et al. had significant drop out (38 %), and it is unclear if this resulted in under- or overestimation of their results. More research with larger cohorts on the topic of combination therapy is needed to help clarify if this approach will prove to be an effective strategy for achieving prolonged cessation.
Varenicline
Varenicline is a novel compound that acts as a partial agonist of neuronal nicotinic acetylcholine receptors. It works by both inhibiting dopaminergic activation and thus reducing the rewarding effects of smoking, while at the same time relieving withdrawal symptoms [21]. Since 2008, varenicline has been considered a first-line agent for smoking cessation and current research has shown that it may assist current smokers unprepared to quit in reducing cigarette use prior to a quit attempt [22, 23]. Recent controversy has centered on potential cardiovascular and neuropsychiatric adverse events associated with its use. A 2010 multicenter randomized control trial of 714 patients found a nonsignificant trend towards more cardiovascular events including MI, angina, and nonfatal stroke in patients randomized to 12 weeks of varenicline therapy [24]. Based on these results, the FDA called on industry to further evaluate these findings through meta-analyses. A subsequent review by Singh and colleagues identified a small but significant increase in cardiovascular events, though ensuing meta-analyses did not share these findings [25–27]. The FDA continues to recommend weighing the benefits and risks of varenicline as an adjunct to smoking cessation, though the most recent 2014 meta-analysis echoes the findings that there are no statistically significant cardiovascular events associated with this therapy [28•]. Likely, the benefits of smoking cessation on overall cardiovascular health outweigh the aforementioned risks, though research is ongoing.
Continuing debate has also centered on reports of neuropsychiatric events related to varenicline use, and the drug currently carries a black box warning for neuropsychiatric adverse events including depression, suicide, and suicidal thoughts or behavior. First issued in 2009, the FDA raised concern of increased neuropsychiatric events seen in post-market surveillance [29]. Subsequent studies and meta-analyses have not shown a statistically significant increase in events, though the FDA is awaiting the results of a large clinical safety trial due in late 2015 [30–34]. Chief amongst the FDA’s concern is that many of the study end points, examined variables, and study population prevented generalizability of results [30]. Debate will likely continue, as the reported experiences in post-marketing surveillance are incongruent with current available data. Until such time, patients should be counseled and monitored for psychiatric events after initiating treatment.
Electronic cigarettes
Electronic cigarettes (e-cigarettes) have become a lucrative business in the USA [38]. Over 460 brands are now available to consumers with big tobacco aggressively investing in this growth market with projected sales of 10 billion dollars by 2017 [39]. Popularity amongst high school students alone increased from approximately 600,000 students in 2013 to close to 2 million in 1 year [40]. Greater than 7500 different “flavors” are now available to refill e-cigarette products sold as personal vaporizers [41]. At present, e-cigarettes are classified as a tobacco product rather than a drug with novel drug delivery system, falling outside the purview of FDA regulation [42]. However, the FDA has proposed extending its authority to reach these devices to ensure regulation similar to currently available tobacco products [43]. While marketed as a possible smoking cessation aid, there has been a paucity of data on their effectiveness. Concern has been raised that e-cigarette solution contains chemicals equally harmful as those found in traditional cigarettes, such as formaldehyde, diacetyl (recognized as a cause of “popcorn lung” in the flavor manufacturing industry), and metallic particles including but not limited to chromium, nickel, and tin [44–46].
To date, only two randomized control trials have examined the effectiveness of e-cigarettes compared to standard NRT prospectively. In 2013, Bullen et al. randomized 657 smokers to e-cigarettes, nicotine patches, or placebo e-cigarettes and prospectively followed cessation rates. At 6 months, 7.3 % had achieved abstinence in the e-cigarette group compared to 5.8 % with nicotine patches [47•]. Due to the low cessation rate throughout both groups, the study did not achieve adequate power to determine superiority of one modality over the next. Caponnetto et al. followed 300 patients not intending to quit smoking over 52 weeks, randomizing patients to a popular e-cigarette brand at two different nicotine doses versus placebo. Smoking cessation was documented in 8.7 % of the study population at 52 weeks compared to 4 % of the placebo group [48]. A meta-analysis of these trials concluded that while electronic cigarette use results in a statistically significant reduction in smoking, the small number of studies and short study duration limit confidence in the results [49•].
Research in this emerging market has lagged behind the explosive sales. The American Heart Association has advocated for further research regarding the safety and efficacy of electronic cigarettes as a smoking cessation aid [39]. In light of the current evidence, physicians should approach e-cigarette use as a smoking cessation aid with caution. While what limited evidence is available supports their use in smoking reduction, there is not yet sufficient understanding of safety and long-term cessation rates to suggest this over other well-established modalities.
Cytisine
New research into this relatively old alkaloid has renewed interest in its efficacy as a cessation aid [35, 36•]. Used as an alternative to nicotine as early as the nineteenth century, cytisine has been a mainstay in smoking cessation outside the USA since the 1960s [37]. Similar to varenicline, it is a partial agonist of nicotinic acetylcholine receptors. While less-rigorous studies are abundant, in 2011, West et al. published findings of their randomized placebo-controlled trial following 1542 adult smokers for 12 months after being randomized to cytisine versus placebo. They found that at 12 months, 8.4 % of patients remained abstinent compared to 2.4 % in the control arm. This absolute reduction in smoking rates is similar to NRT and antidepressant therapy [35]. An open label trial in 2014 comparing cytisine to conventional NRT found superior cessation rates at 1 week, 2 months, and 6 months, though cytisine had a higher adverse event rate including nausea, vomiting, and sleep disturbances [36•]. Though cytisine is not available in the USA, given its relatively low cost and similar efficacy to traditional therapies, it may represent a viable alternative to patients who have not had success with NRT or more costly antidepressants.
Vaccination
A conjugate vaccine to circulating nicotine has proved to be a novel approach to smoking cessation. In principle, antibodies bind to circulating nicotine, effectively preventing passage across the blood brain barrier. Phase II trials demonstrated proof of concept and safety [50]. Early phase III results, however, demonstrated no significant increase in smoking cessation [51]. In 2014, Hoogsteder et al. conducted a randomized placebo controlled trial of 558 smokers pairing vaccination with varenicline. At 52 weeks, the study failed to meet the primary endpoint of increased abstinence [52]. Although a vaccine offers the promise of a long-term solution without the addition of daily medications whether it manages to move beyond the bench and into meaningful clinical practice remains to be seen.
Our approach
At times, smoking cessation can feel like a daunting task to tackle in a single clinic visit, though straightforward counseling can prepare your patient for a successful quit attempt. In our clinical practice, we use the 5As of smoking cessation treatment: ask (about tobacco use), advise (smoking cessation), assess (readiness to quit), assist (with planning a quit attempt), and arrange (follow-up) [2].
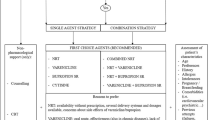
Asking about current smoking status and advising the patient to quit should occur during every visit. Assessment of readiness to quit should also include previous quit attempts, inquiries into past methods or medications used, and the barriers that patients faced to quitting. Once the patient has voiced interest in quitting, the various quit aids and their merits can be discussed; patient preference regarding the choice of a first-line therapy, i.e., nicotine replacement therapy versus a daily oral medication, can serve as a foundation for this discussion, as each method is associated with similar cessation rates. When choosing nicotine replacement, a combination of long-acting nicotine replacement in combination with on demand therapy can help to control nicotine cravings as nicotine doses up to 42 mg have been tolerated [6]. The duration of therapy can be extended to 24 weeks safely based on clinician discretion. When choosing between oral medications, the clinician should consider side effect profiles and comorbid conditions. The potential cardiovascular and neuropsychiatric risks attributed to varenicline should be discussed with patients suffering from heart disease or depression. Alternatively, bupropion may prove to be a beneficial in patients suffering from concomitant depression if deemed safe by their mental health provider. Once an agent has been chosen, a quit date should be set and, in the beginning, frequent follow-up and counseling is necessary. In patients who fail to quit with first-line therapy despite appropriate use, we attempt combination therapy with either varenicline and nicotine replacement, bupropion and nicotine replacement, or varenicline and bupropion, as each of these therapies have been found to slightly increase quit rates compared to monotherapy [19, 20, 53].
Conclusion
Smoking cessation continues to pose challenges for the clinician. Achieving long-term cessation remains a stumbling block despite mainstays of therapy including nicotine replacement, antidepressants, and partial nicotinic agonists. Recent evidence is growing to support longer duration of therapy and faster routes of nicotine delivery. Relatively old treatments such as cytisine may be added to our armamentarium in the future. Cessation counseling and treatment should be tailored to the individual patient given the availability of sustained release and on-demand agents now available. More research is needed to understand the impact and potential harms of electronic cigarettes and the role they will play, if any, in the future of smoking cessation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
The health consequences of smoking—50 years of progress: a report of the Surgeon General. 2014: U.S. Department of Health and Human Services.
Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. U.S. Department of Health and Human Services, Public Health Service 2008; Available from: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf.
Aubin HJ, Luquiens A, Berlin I. Pharmacotherapy for smoking cessation: pharmacological principles and clinical practice. Br J Clin Pharmacol. 2014;77(2):324–36.
Rennard SI, Daughton DM. Smoking cessation. Clin Chest Med. 2014;35(1):165–76.
Stead LF et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146.
Brokowski L, Chen J, Tanner S. High-dose transdermal nicotine replacement for tobacco cessation. Am J Health Syst Pharm. 2014;71(8):634–8.
Tonnesen P et al. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial. Eur Respir J. 2012;40(3):548–54.
Shahab L, Brose LS, West R. Novel delivery systems for nicotine replacement therapy as an aid to smoking cessation and for harm reduction: rationale, and evidence for advantages over existing systems. CNS Drugs. 2013;27(12):1007–19.
Schnoll RA et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175(4):504–11.
Hughes JR et al. Are higher doses of nicotine replacement more effective for smoking cessation? Nicotine Tob Res. 1999;1(2):169–74.
Schnoll RA et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med. 2010;152(3):144–51.
Hansson A, Hajek P, Perfekt R, et al. Effects of nicotine mouth spray on urges to smoke, a randomised clinical trial. BMJ Open 2012;2:e001618. doi:10.1136/bmjopen-2012-001618.
Caldwell BO, Adamson SJ, Crane J. Combination rapid-acting nicotine mouth spray and nicotine patch therapy in smoking cessation. Nicotine Tob Res. 2014;16(10):1356–64.
Gonzales D et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55.
Jorenby DE et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63.
Hurt RD et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337(17):1195–202.
Durcan MJ et al. The effect of bupropion sustained-release on cigarette craving after smoking cessation. Clin Ther. 2002;24(4):540–51.
Hughes JR et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;1:CD000031.
Ebbert JO et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311(2):155–63.
Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. Am J Psychiatry. 2014;171(11):1199–205.
Coe JW et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–7.
Hays JT, Ebbert JO. Varenicline for tobacco dependence. N Engl J Med. 2008;359(19):2018–24.
Ebbert JO et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687–94.
Rigotti NA et al. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121(2):221–9.
Singh S et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183(12):1359–66.
Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
Ware JH et al. Cardiovascular safety of varenicline: patient-level meta-analysis of randomized, blinded, placebo-controlled trials. Am J Ther. 2013;20(3):235–46.
Mills EJ et al. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28–41. Large meta-analysis examining the existing evidence regarding potential cardiovascular adverse effects of varenicline use for smoking cessation following FDA post-marketing reports of increased events.
Moore TJ et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6(11):e27016.
Gibbons RD, Mann JJ. Varenicline, smoking cessation, and neuropsychiatric adverse events. Am J Psychiatry. 2013;170(12):1460–7.
FDA Drug Safety Communication: FDA updates label for stop smoking drug Chantix (varenicline) to include potential alcohol interaction, rare risk of seizures, and studies of side effects on mood, behavior, or thinking. 2015 March 9, 2015 [cited 2015 June 1]; Available from: http://www.fda.gov/Drugs/DrugSafety/ucm436494.htm.
Thomas KH et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
Ahmed AI et al. Neuropsychiatric adverse events of varenicline: a systematic review of published reports. J Clin Psychopharmacol. 2013;33(1):55–62.
Meyer TE et al. Neuropsychiatric events in varenicline and nicotine replacement patch users in the Military Health System. Addiction. 2013;108(1):203–10.
West R et al. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med. 2011;365(13):1193–200.
Walker N et al. Cytisine versus nicotine for smoking cessation. N Engl J Med. 2014;371(25):2353–62. An open label trial of 1310 patients to try to establish non-inferiority of cytisine to traditional NRT. It examined self-reported quit rates at 1 month.
Prochaska JJ, Das S, Benowitz NL. Cytisine, the world’s oldest smoking cessation aid. BMJ. 2013;347:f5198.
Yamin CK, Bitton A, Bates DW. E-cigarettes: a rapidly growing Internet phenomenon. Ann Intern Med. 2010;153(9):607–9.
Bhatnagar A et al. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418–36.
McCarthy M. “Alarming” rise in popularity of e-cigarettes is seen among US teenagers as use triples in a year. BMJ. 2015;350:h2083.
Ebbert JO, Agunwamba AA, Rutten LJ. Counseling patients on the use of electronic cigarettes. Mayo Clin Proc. 2015;90(1):128–34.
Bartter T. Electronic cigarettes: aggregate harm. Ann Intern Med. 2015;163(1):59–60.
Deeming – Extending Authorities to Additional Tobacco Products. 2/26/2015; Available from: http://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm388395.htm.
Jensen RP et al. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–4.
Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493–4.
Orr MS. Electronic cigarettes in the USA: a summary of available toxicology data and suggestions for the future. Tob Control. 2014;23 Suppl 2:ii18–22.
Bullen C et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–37. Randomized controlled superiority trial comparing electronic cigarettes to placebo electronic cigarettes versus nicotine patches. Abstinence rates were examined 6 months following intervention.
Caponnetto P et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317.
McRobbie H et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;12:CD010216. An important examination of the two RCTs and cohort studies currently available reporting on the possible efficacy of electronic cigarettes as a smoking cessation tool.
Cornuz J et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008;3(6):e2547.
Raupach T, Hoogsteder PH, Onno van Schayck CP. Nicotine vaccines to assist with smoking cessation: current status of research. Drugs. 2012;72(4):e1–e16.
Hoogsteder PH et al. Efficacy of the nicotine vaccine 3′-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: a randomized placebo-controlled trial. Addiction. 2014;109(8):1252–9.
Koegelenberg CF et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014;312(2):155–61.
Compliance with Ethics Guidelines
Conflict of Interest
Drs Allam and Ochoa both state they have no conflicts to disclose.
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Smoking Cessation
Rights and permissions
About this article
Cite this article
Allam, J.S., Ochoa, C.D. Pharmacological therapies in smoking cessation: an evidence-based update. Curr Pulmonol Rep 4, 173–178 (2015). https://doi.org/10.1007/s13665-015-0125-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-015-0125-5