Abstract
The effect of heat treatment on the microstructure and metallurgical properties of the interface between copper and austenitic stainless steel 304 formed by explosive bonding using different conditions of stand-off distance and material thickness was studied. Laboratory studies were carried out using optical microscopy, scanning electronic microscopy, and microhardness measurements to evaluate welded samples. Samples were subjected to heat treatment in a furnace at 300 °C for 22 or 30 h, then cooled in air. Microstructural studies showed that, as the stand-off distance and explosive load were increased, the interface between copper and stainless steel 304 became more wavy. The microhardness results showed that the hardness of the base metal was greater than in other areas near the interface because of extreme plastic deformation due to the explosive force. The results also showed that, as the temperature and heat treatment duration were increased, the hardness of the flyer plate at the interface decreased compared with before heat treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
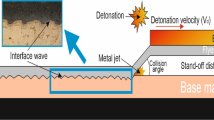
Joints between austenitic stainless steel and copper as dissimilar materials are now being applied, especially in expanding valves. In this materials system, the heat transfer is lower in the austenitic stainless steel heat than in the copper, motivating the use of explosive welding [1]. Indeed, one of the ways of welding austenitic stainless steel to copper as dissimilar materials is explosive welding. Explosive welding is one of the effective ways for joining dissimilar metals, resulting from a high-speed diagonal impact of a flyer plate with a baseplate [2]. Explosive welding, like other bonding methods, has some variables that can be controlled to achieve high-quality welds [3, 4]. Some studies and researches have revealed that the properties of the interface created between the flyer plate and baseplate play an essential role in its quality. The interface resulting from explosion welding is usually of three types: straight, wavy, or continuous frozen melt. However, such interfaces are usually wavy, and extreme deformations occur around them. As the explosive force is increased, there is the possibility of local melting and intermetallic compounds between the flyer plate and baseplate. If these solution combinations are solid, the welded zone becomes suitably flexible, whereas if intermetallic compounds form at the interface, the welded zone becomes brittle [3].
Many studies on explosive welding have focused on the morphological changes at the interface between static and flyer plates made of different materials [5,6,7,8].
Findic et al. studied the effect of the temperature and duration of heat treatment on explosive bonding of low-carbon steel to austenitic steel 304. They showed that the grain size, hardness, strength, and flexibility changed. Moreover, the hardness of austenitic steel is considerably greater than that of carbon steel, due to the changes that originate from the extreme plastic deformation and the density increase. Those authors found that high Ni, Cr, and Si content in the steel resulted in the formation of brittle intermetallic compounds at the interface [9] and suggested a shorter duration of heat treatment because, in this condition, the hardness and strength are suitable while the residual tension should be decreased. Also, Akbari et al. [10] investigated the effect of heat treatment on the behavior of the interface formed when explosively bonding steel 304 to Ti, revealing that, as the temperature was increased from 650 to 900 °C in 1 h, the nucleated Ti became coarser under the effect of recrystallization while the grain size in steel did not change notably. Studies have also shown that, as the temperature is increased, the width of the intermetallic zone at the interface grows because of increased diffusion of alloy elements. They also showed that the Ti diffusion length in steel 304 was shorter than that of alloying elements such as Cr, Ni, and Fe in Ti layers. The reason for this is claimed to be the compact cubic structure of austenitic steel in contrast to the open hexagonal structure of Ti.
The 304 steel has a compact structure, which alongside Fe, Cr, and Ni atoms, can increase the Ti lattice spacing, with an open hexagonal lattice structure. Consequently, Ti atoms becomes closer [10]. Bina et al. [11] studied the effect of heat treatment on improving the diffusion at the interface of joints between Cu and austenitic stainless steel 304 by varying the temperature and duration variables. They applied a temperature of 300 °C for 8 to 32 h in steps of 8 h, and their results revealed that the interface in joints between Cu and austenitic stainless steel 304 was wavy. They also conducted annealing heat treatment for 32 h, observing significantly increased diffusion. Microhardness measurements showed that the hardness of the samples near the interface was higher than elsewhere.
The mechanical and microstructural properties of various metals and their alloys subjected to explosive welding have been investigated by some researchers, some of whom have been cited above. The present investigation was prompted by recent applications of explosively welding of copper to stainless steel in a corrosive environment. Although there are some studies on explosive welding of copper to stainless steel produced in literature [12,13,14], there are no reports on the effect of post heat treatment on the bonding interface after explosively welding copper to stainless steel 304. The aim of this study is thus to investigate the effect of some parameters, viz. the medium layer thickness and explosive material thickness, on the microstructure and metallurgical properties and investigate the effect of post heat treatment on the properties of the bonding interface to determine the optimal conditions for explosive welding of copper to stainless steel 304.
Experimental Methods
Austenitic stainless steel 304 sheets with dimensions of 250 × 250 × 4 mm3 were used as the baseplate, and Cu with dimensions of 250 × 250 × 3 mm3 as the flyer plate (Table 1). Figure 1 shows the initial setup for explosive welding of the plates. The flyer plate and baseplate were Cu and austenitic stainless steel with thickness of 3 and 4 mm. Both samples were placed on a sand anvil for welding. A Cu spacer was used to control the standoff distance between the sheets, while high explosive was located on the surface of the flyer plate in a wooden box of suitable thickness [15]. To weld the sheets, Amatol 95/94 high explosive with a combination of 5% trinitrotoluene and 95% ammonium nitrate was used, reaching an explosion speed of 250 m/s [15].
After welding, heat treatment was performed at 300 °C. Due to the effect of the duration of the heat treatment on the intermetallic compounds, two samples were prepared and subject to heat treatment in a furnace for 22 or 30 h, then cooled at room temperature. In previous studies on explosive joints between copper and austenitic stainless steel, heat treatment was applied at 300 °C for durations in the range from 8 to 32 h. Since the duration and temperature were found to be critical, a temperature of 300 °C for 22–30 h was selected for these explosive joints between copper and stainless steel, according to the results of previous research [11]. For the microstructural investigation, samples with dimensions of 10 × 10 × 7 mm3 were cut by using a handsaw perpendicular to the direction of the explosion, to remove the effect of the temperature rise during the cutting process. Thereafter, the samples were mounted and sanded using no. 80 to 3000 papers, then polished using 3-µm alumina to remove lines and surface roughness.
After polishing, to prepare the samples for optical microscopy (OM), their surface was etched using 34 ml H2O + 33 ml HCL + 33 ml HNO3 solution, then washed and dried in alcohol. The microstructure of the interface and intermetallic compounds was studied at different magnifications before and after heat treatment by OM and scanning electron microscopy (SEM) equipped with energy-dispersive X-ray spectroscopy (EDS) to study the interface in the joint. Microhardness testing was used to study the hardness distribution around the interface. According to ASTM E384_11 standard, the (Vickers) microhardness was measured under a load of 50 g for 10 s at intervals of 50, 150, 250, and 350 µm from both sides of the interface into the base and flyer plate. This test was done twice on each sample to increase the accuracy and reliability of the microhardness rsults.
Results and Discussion
Microstructure of AS1
Figures 2 and 3 show metallographic and SEM images of the joint interface. In this case, the joint interface is wavy. A local frozen melt layer was created when bonding occurred. Also, the thickness of the diffusion layer increased because of the heat treatment. With increasing duration and temperature of the heat treatment, the thickness of the diffusion layer also increased. Before heat treatment, the thickness of the layers was measured to be 52.63 ± 9.04 µm, whereas after 22 h it reached 61.9 ± 8.94 µm, and after 30 h it reached 81.6 ± 6.09 µm. Studies have also shown that, as the temperature of the heat treatment is increased, the diffusion layer of the interface thickness also increases [12, 16, 17]. Therefore, performing heat treatment and increasing its duration at a constant temperature increases the thickness of the metallurgical layer formed by penetration. As seen in Fig. 2c, the development of this layer took 30 h, resulting in the formation of a continuous film.
Figure 3 shows SEM images of the interface of sample AS1 before and after heat treatment, revealing the formation of the local frozen melt layer and diffusion layer. The thickness of the local frozen melt layer is less than the diffusion layer after heat treatment at 300 °C for 30 h (point A). The reason for this (Fig. 3c) is the effect of the heat treatment and diffusion of alloying elements. Therefore, the diffusion layer thickness increased.
Figure 4 shows EDS analysis of the local frozen melt layers and diffusion layers of the interface. The results revealed that, before heat treatment, the frozen melt layer compound (Fig. 4a, point A) included 1.01 at.% Ni, 23.46 at.% Fe, and 46.13 at.% Cu. The area of the local frozen melt is created due to the combination of the flyer and base plates because of the effect of imprisoned jet rotation at the interface [9]. After heat treatment, the diffusion layer showed a higher Cu atomic content, since its diffusion coefficient and thermal conduction coefficient are higher than those of Fe. At the sample interface, the Cu and Fe content was measured to be 63.69 at.% and 21.28 at.% after heat treatment for 22 h (Fig. 4b, point A). After heat treatment for 30 h, the content of Ni, Cu, and Fe was measured to be 1.41 at.%, 59.68 at.%, and 32.52 at.%, respectively (Fig. 4c, point A). Meanwhile, the Cu and Fe content was 83.89 at.% and 13.48 at.%, respectively, at point B in Fig. 4d. By increasing the temperature, and because of the lower thermal conductivity of the austenitic stainless steel 304, the content of Ni and Fe elements decreased. Simultaneously, a large amount of Cu from the Cu part of the joint diffused to the interface. As the temperature was increased, the activation energy for diffusion increased, thus the width of the reactive area grew. The chemical composition of the intermetallic layers formed during diffusion depends on the amount of diffusion element, residual stresses and strains due to welding, changes in different crystal lattice parameters due to heat treatment and reduction of base metal strength near the weld zone due to structural changes [10, 18, 19].
The EDS analysis of the local frozen melt layers and diffusion layers of the interface is shown in Fig. 4. These results indicate that, before heat treatment, the frozen melt layer compound at point A in Fig. 4a had Ni, Fe, and Cu content of 1.01 at.%, 23.46 at.%, and 46.13 at.%, respectively.
After heat treatment, the diffusion layer showed that the Cu atomic content increased because its diffusion coefficient and thermal conduction coefficient are higher than those of Fe. The Cu and Fe content was measured to be 63.69 at.% and 21.28 at.% at the interface of the sample that was subject to heat treatment for 22 h (Fig. 4b, point A).
The content of Ni, Cu, and Fe was found to be 1.41 at.%, 59.68 at.%, and 32.52 at.%, respectively, in the sample subjected to heat treatment for 30 h (Fig. 4c, point A), whereas the Cu and Fe content at point B in Fig. 4d was 83.89 at.% and 13.48 at.%, respectively. As the temperature was increased, due to the lower thermal conductivity of the austenitic stainless steel 304, the content of Ni and Fe decreased and a large amount of Cu from the Cu part of the joint penetrated the interface. As the temperature was increased, the activation energy of diffusion also increased, so the width of the reactive area grew. Therefore, some factors like the amount of main elements diffusion remained tensions from welding, which ultimate creating yield in the diffusion area, creating yield caused by the physical-heat incompatibility of the material during heat treatment, and the base metal softening in the effect of the destruction of structural defects, affects the diffusion layers change [10, 18, 19].
Microstructure of AS2
The waves that were created perpendicular to the explosive direction are shown in Fig. 5. In this case, the joint interface is wavy. Because of the small stand-off distance (2 mm), the speed of the flyer plate was low, thus the joint interface was wavy before heat treatment. The sample subjected to heat treatment for 30 h exhibited a considerable amount of diffusion with increasing heat treatment duration, and the thickness of the diffusion layer reached 80.3 ± 4.32 µm. Studies have shown that, with increasing temperature and duration of the heat treatment, the width of the area of intermetallic compounds increases [13, 20].
Figure 6 shows SEM images of the interface and melted layer of sample AS2 welded with a standoff distance of 2 mm and thicker explosive material than was used for samples AS1. The thickness of the local frozen melt layer of this sample before heat treatment is shown in Fig. 6a. The diffusion layer after heat treatment (Fig. 6b, c) increased in comparison with the thickness of the local frozen melt layer of sample AS1 before heat treatment and the diffusion layer after heat treatment. The reason for this thickness reduction is the decrease in the standoff distance and kinetic energy of the collision. By increasing the heat treatment duration, the thickness of the diffusion layer got slightly thicker. Studies on the effect of heat treatment on the behavior of explosive joints between steel 304 and Ti have shown that, when strengthening the heat treatment, the intermetallic areas of the interface increase because of increased reaction and diffusion of alloy elements.
Microstructure of AS3
As shown in Fig. 7, the thickness of the local frozen melt layer and diffusion layer at the interface of sample AS2 obtained with a standoff distance of 3 mm increased compared with the sample obtained using a standoff distance of 2 mm, both before and after heat treatment.
This thickness was measured to be 228 ± 5.6 µm before heat treatment and 120.4 ± 13.9 µm after 30 h of heat treatment. Because of the increase in the standoff distance and the thickness of the explosive load compared with AS2, the velocity of the flyer plate was higher and more plastic deformation occurred at the interface of the joint. Also, as the kinetic energy of the collision increased, before heat treatment, the shape of the interface became more like a vortex compared with AS2 obtained with a standoff distance of 2 mm.
At a higher collision speed, the collision pressure and dynamic angle will be higher. Moreover, part of the kinetic energy turns into potential energy at the interface, causing the surface of the plates to deform.
Because of this increase, the materials tend to behave like a fluid; the velocity of the flyer plate increases with the standoff distance and, thus, the explosive load. Since the density and wave speed in the solids are different, the momentum pressure on either side of the interface changes when increasing the velocity of the flyer plate. Therefore, the collision point at the moment of joining will fluctuate. These vibrations will increase as the collision velocity is increased, and matter adjacent to the collision point will lose more strength and tend to behave like a fluid.
Heat treatment at 300 °C for 30 h led to the maximum diffusion layer thickness of 130 µm, which is 41 µm greater than that of the local frozen melt layer before heat treatment.
Figure 8 shows SEM images of the local frozen melt and diffusion layers of sample AS3 obtained a standoff distance of 3 mm. The thickness of the diffusion layer (Fig. 8) increased compared with AS2 due to the different standoff distance, explosive load, and kinetic energy at the interface.
Microstructure of AS4
Figure 9 shows the interface of sample AS4 obtained with a standoff distance of 2 mm. The interface morphology is wavy. Also, the thickness of the local melted frozen layer was 66.4 ± 8.33 µm before heat treatment. The greater the temperature, the thicker the diffusion layer.
After heat treatment for 22 and 30 h, the thickness of the diffusion layers was 80.7 ± 95.5 and 100.46 ± 8.29 µm, respectively. Because of the greater stand-off distance, explosive load, explosive velocity, and collision energy for sample AS2, the thickness increased, thus with the higher explosion load and flyer plate velocity, the kinetic energy of the collision increased. Thus, both before and after heat treatment, the thickness of the local frozen melt layer increased. Figure 10 shows images of the local frozen melt layer and diffusion layer of sample AS4. The thickness of the local frozen melt layer before heat treatment and the diffusion layer after heat treatment were lower than for sample AS3.
As mentioned above, as the duration of the heat treatment time was increased at constant temperature, the thickness of the intermetallic layer formed at the joint increased.
The reason is the reduction in the stand-off distance and kinetic energy of the collision at the interface. This sample had a stand-off distance of 2 mm and was subjected to heat treatment at 300 °C for 22 and 30 h. Before heat treatment, the maximum thickness of the frozen melted layer was 71 µm, but after 30 h, it reached 114 µm.
Figure 2 shows the changes in the thickness of the local frozen melted layer before and after heat treatment. With increasing the temperature and time of heat treatment, the thickness of intermetaic layer increased. This led to enhanced diffusion and a higher collision velocity and kinetic energy (Table 2). Such results have also been observed in previous studies.
Microhardness
Figure 11 shows the hardness results obtained at 50, 150, 250, and 350 µm from the interface of sample AS3, before and after heat treatment. Areas adjacent to the interface have higher hardness experience. Because of the explosive force in the joint area, the flyer metal collides strongly with the base metal, which results in plastic deformation of the flyer metal at the interface. This plastic deformation increases the hardness.
As the stoppage time increased, the hardness at the interface also increased (because of intense plastic deformation). The maximum microhardness of the AS3 sample was obtained at 50 µm away from the interface. Before heat treatment, the hardness of the stainless steel was 380 ± 15.5, 398 ± 8.5, and 40 ± 4.5 Vickers, while after heat treatment for 22 and 30 h it was 122 ± 4.8, 72.5 ± 15.5, and 67.5 ± 8.5 Vickers in the copper area. The hardness of copper was reduced due to the emission of energy produced by the collision stresses after heat treatment.
Table 3 presents the microhardness of the samples before and after heat treatment. Sample AS3 showed a greater hardness increase compared with AS1.
An increase in the thickness of the explosive material increases the energy, resulting in more kinetic energy during the collision. The outcome is intense plastic deformation at the interface. The microhardness in sample AS3, obtained using a standoff distance of 3 mm, was greater than that in AS4, which was obtained using a standoff distance of 2 mm.
This occurs because, by increasing the standoff distance, the velocity of the flyer plate and the dynamic angle are increased, and thereby the kinetic energy during the collision, resulting in higher hardness caused by explosive waves.
Conclusions
-
1.
As the stoppage time and the thickness of the explosive material are increased, the interface of the joint becomes like a vortex, and a local frozen melt layer is produced due to the increase in the collision pressure near the vortex waves. This effect is caused by the rotation of the flyer jet on the base metal alloy and flyer.
-
2.
Due to the presence of internal stresses, the hardness is reduced after heat treatment.
-
3.
Before heat treatment, the hardness in the copper section of the samples at a distance of 50 µm away from the interface was higher than in other areas. The reasons are the plastic deformation, and the hardness of the copper and austenitic stainless steel.
-
4.
The longer the heat treatment, the greater the penetration of alloy elements. The combinational diffusion layer includes metals present in the alloy and flyer material. Since the diffusion rate of copper is greater than that of steel and it has high thermal conductivity, more copper was found in the melt layer.
References
N. Kahraman, B. Gulenc, F. Findik, N. Kahraman, B. Gulenc, F. Findik, Joining of titanium/stainless steel by explosive welding and effect on interface. J. Mater. Process. Technol. 169, 127–133 (2005)
A.S.M. Handbook, Welding Brazing and Soldering (ASM International, Materials Park, 1993)
B. Crosland, Explosive Welding of Metals and Its Application (Clarendon, Oxford, 1982)
M.R.K.G. Shiran, H. Bakhtiari, S.A.A. Mousavi, G. Khalaj, S.M. Mirhashemi, M.R.K.G. Shiran, H. Bakhtiari, S.A.A. Mousavi, G. Khalaj, S.M. Mirhashemi, Effect of stand-off distance on the mechanical and metallurgical properties of explosively bonded 321 austenitic stainless steel-1230 aluminum alloy tubes. Mater. Res. 20, 291–302 (2017)
S.J. Kim, S.H. Paik, M.Y. Huh, S.J. Kim, S.H. Paik, M.Y. Huh, Applications of explosive welding. J. Korean Inst. Met. Mater. 32, 1558–1573 (1994)
D.G. Brasher, D.J. Butler, D.G. Brasher, D.J. Butler, Explosive welding: principles and potentials. J. Mater. Process. 3, 37–38 (1995)
M.H. Nishida, A. Chibia, Y. Honda, J. Hirazumi, K. Horikiri, M.H. Nishida, A. Chibia, Y. Honda, J. Hirazumi, K. Horikiri, Electron microscopy studies of bonding interface in explosively welded Ti/Steel clads. ISIJ Int. 35, 217–228 (1995)
S. Yano, H. Matsui, S. Morozumi, S. Yano, H. Matsui, S. Morozumi, Structural observations of the interface of explosion bonded Mo/Cu system. J. Mater. Sci. 33, 4857–4870 (1998)
F. Findik, R. Yilmaz, T. Somyurek, F. Findik, R. Yilmaz, T. Somyurek, The effects of heat treatment on the microstructure and microhardness of explosive welding. Sci. Res. Essays. 6, 4141–4151 (2011)
S.A.A. Akbari Mousavi, P. Farhadi Sartangi, S.A.A. Akbari Mousavi, P. Farhadi Sartangi, Effect of post-weld heat treatment on the interface microstructure of explosively welded titanium-stainless steel composite. J. Mater. Sci. Eng. A. 494, 329–336 (2008)
M. Bina, F. Dehghani, M. Salimi, M. Bina, F. Dehghani, M. Salimi, Effect of heat treatment on bonding interface in explosive welded copper/stainless steel. J. Mater. Des. 45, 504–509 (2013)
F. Findik, F. Findik, Recent developments in explosive welding. Mater. Des. 32, 1081–1093 (2011)
A. Durgutlu, H. Okuyucu, B. Gulenc, A. Durgutlu, H. Okuyucu, B. Gulenc, Investigation of effect of the stand-off distance on interface characteristics of explosively welded copper and stainless steel. Mater. Des. 29, 1480–1484 (2008)
A. Durgutlu, B. Gulenc, F. Findik, A. Durgutlu, B. Gulenc, F. Findik, Examination of copper/stainless steel joints formed by explosive welding. Mater. Des. 26, 497–507 (2005)
R. Kacar, M. Acarer, R. Kacar, M. Acarer, An investigation on the explosive cladding of 316L stainless steel-din-P355GH steel. J. Mater. Process. Technol. 153, 91–96 (2009)
L. Tricarico, R. Spina, D. Sorgente, M. Brandizzi, L. Tricarico, R. Spina, D. Sorgente, M. Brandizzi, Effects of heat treatments on mechanical properties of Fe/Al explosion-welded structural transition joints. J. Mater. Des. 30, 2693–2700 (2009)
I. Samardzic, B. Matesa, I. Kladaric, I. Samardzic, B. Matesa, I. Kladaric, The influence of heat treatment on properties of three-metal explosion joint: AlMg-Al-steel. Metabk. 50, 159–162 (2011)
Kengkla, N., Tareelap, N., Role of intermetallic compound on corrosion of aluminum/steel transition joint used in naval applications, in 1st Mae Fah Luang University International Conference (2012)
T.I. Khan, S.A. Rizvi, K. Matsuura, T.I. Khan, S.A. Rizvi, K. Matsuura, The effect on wear behavior of H13 tool steel surfaces modified using a tungsten arc heat source. J. Wear. 244, 154–164 (2000)
M. Acarer, B. Gulenc, F. Findik, M. Acarer, B. Gulenc, F. Findik, Investigation of explosive welding parameters and their effects on microhardness and shear strength. J. Mater. Des. 24, 659–664 (2003)
Acknowledgements
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Padash, E., Khanzadeh, M., Bakhtiari, H. et al. Effect of Heat Treatment Duration on Interface Characteristics of Explosively Bonded Cu/SS-304 Plates. Metallogr. Microstruct. Anal. 10, 74–85 (2021). https://doi.org/10.1007/s13632-021-00715-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13632-021-00715-z