Abstract
In this work, the microstructural, mechanical, and physical properties of dissimilar welding between the 1.4742 ferritic and 310S austenitic stainless steels have been investigated. The welding was performed by the manual gas tungsten arc welding process utilizing ER310, ERNiCr-3, and ER446 filler metals. The weld metal microstructure of the ER310 and ERNiCr-3 filler metals was fully austenitic. The ER446 weld metal exhibited a ferritic-austenitic dual-phase microstructure. Carbide precipitates enriched with Cr, Fe, and or Nb were detected in all of the three weld metals. The unmixed zone was observed on both sides of the ERNiCr-3 weld metal, while for the ER310 and ER446 weld metals it was formed solely on one side. The ER446 weld metal displayed the maximum hardness values, 221.9 ± 5 HV, with relatively large fluctuations. The highest and lowest impact energy was achieved in the ERNiCr-3 weld metal, 40 ± 3 J, and ER446 weld metal, 25 ± 3 J, respectively. The ERNiCr-3 weld metal had a linear coefficient of thermal expansion (CTE) value about the mean value of the linear CTE of the two base metals. At last, for the dissimilar welding of these two base metals, it was concluded that the ERNiCr-3 filler metal is a better selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When it comes to using high-temperature-exposed materials, such as protection tubes, furnace parts, oil burner parts, and petroleum refining equipment, high-temperature properties are considered to be important [1, 2]. Stainless steels are well known for their corrosion resistance in the aqueous environments, and they can be found in high-temperature applications wherein corrosion resistance or strength is required [3]. The austenitic heat-resistant stainless steels with higher mechanical properties at elevated temperature are the most widely used steels in high-temperature applications. The ferritic heat-resistant stainless steels have a lower high-temperature mechanical property, as compared to those of austenitic grades, yet they offer a lower coefficient of thermal expansion (CTE) and better resistance to the cyclic oxidation and thermal fatigue [3, 4]. Moreover, due to the more costly nickel content of austenitic stainless steels, the low-to-medium ferritic grades are more cost-effective [3]. By taking into account the above distinct engineering requirements, i.e., the improvement of durability in the long-term operation or economic considerations by using low-cost materials, using dissimilar metal joints would be beneficial. Dissimilar metal welds (DMWs) are the joints that consist of different materials and have attracted considerable attention [5,6,7,8,9,10,11,12].
Hsieh et al. [7] evaluated the autogenous dissimilar welding of the 430 ferritic and 304 austenitic stainless steels using the gas tungsten arc welding (GTAW) process. They reported that the weld metal reveals higher hardness than the two base metals due to the precipitation of massive δ-ferrite in the austenite phase and ferrite-austenite interface boundaries. The dissimilar friction welding of the 430 ferritic and 304 austenitic stainless steels was performed by Satyanarayana et al. [8]. They stated that due to the greater high-temperature hardness and lower thermal conductivity of austenitic stainless steel in comparison with ferritic grades, the welding process-induced deformation is mostly confined to the 430 ferritic stainless steel. Shojaati et al. [9] evaluated the microstructural and mechanical properties of the 409 ferritic and 304 austenitic stainless steels dissimilar joints via the GTAW process. The ER310, ER316L, ER2209, and Nichrome 80/20 were used as filler metals. Eventually, they concluded that the ER316L weld metal offered the best mechanical properties from the economical viewpoint. Shanmugam et al. [10] studied the effect of the filler metals of ER308L, ER430, and ER2209 on the mechanical properties of GTAW-welded 409 ferritic stainless steel joints. From their investigation, it was found that the joint welded by ER2209 filler metals exhibits the best mechanical properties at room temperature because of its ferritic-austenitic dual-phase microstructure. The effect of the SMAW heat input on the chemical composition and microstructure of the weld overlay formed by the E309MoL covered electrode on the 410S ferritic stainless steel was investigated by Silva et al. [11]. They reported that by increasing the welding heat input, the amount of δ-ferrite is increased due to the compositional changes of the weld metals, made by dilution. In the investigation carried out by Kumar et al. [12], the 430 ferritic and 304 austenitic stainless steels were the dissimilar butt welded via the plasma arc welding process and the process parameters were optimized by the regression analysis based on the mechanical properties and microstructural aspects.
Despite the widespread application of DMWs in a wide variety of industries, there is a history of precocious failures associated with these welds [13, 14]. These accelerated failures can generally be attributed to operating at high-temperature services and thermal cycling conditions in combination with thermal expansion mismatch, differences in the chemical composition, hardness, and creep strength [14, 15]. In this regard, one of the main issues of using DMWs and developing techniques to increase their efficiency is choosing the proper filler metal [16].
Currently, flare combustion systems are made up of austenitic heat-resistant stainless steels, such as 310, 310S, 347, and 321 which can experience different types of failure mechanisms such as thermal fatigue, high-temperature corrosion, stress-assisted oxidation, and creep [17, 18]. Hence, 1.4742 ferritic heat-resistant stainless steel with better high-temperature corrosion, thermal fatigue resistance, and low costs can be a good candidate for the repairs of flare tips or new flare combustion systems fabrication [1,2,3,4]. According to the above mentioned, although several studies have attempted to evaluate the dissimilar welding of stainless steels, there has not been any report on the joint between the 1.4742 ferritic and 310S austenitic heat-resistant stainless steels. The aim of this study is to investigate the microstructural evolution, as well as the mechanical and physical properties of the dissimilar welding between the 1.4742 ferritic and 310S austenitic heat-resistant stainless steels via the GTAW process. It is worth mentioning that this type of dissimilar joint is especially applicable in the repair/fabrication of the flare combustion systems.
Materials and Methods
Materials and Welding
The as-received base metals used in this study were 310S austenitic (under hot-rolled conditions) and 1.4742 ferritic (under cold rolled conditions) stainless steel plates with dimensions 300 mm × 50 mm × 6 mm. Three types of filler metals, ER310 (austenitic stainless steel filler metal), ERNiCr-3 (Ni-base filler metal), and ER446 (ferritic stainless steel filler metal), with 2.4 mm in diameter were utilized. Table 1 shows the chemical compositions of the base (determined by optical emission spectroscopy) and filler (according to the manufacturer’s certificates) metals. Also, the chemical composition of the obtained weld metals corresponding to each filler metal (determined by optical emission spectroscopy) is given in Table 1.
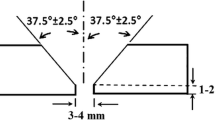
As shown in Fig. 1a and b, a single V butt joint geometry was created on the base metal plates by the machining process. Then the plates were wire brushed and degreased using acetone. The welding was performed by the manual gas tungsten arc welding (GTAW) process (machine model: DG-TIG PSQ400 AC/DC, Gaam Electric Co. Iran) in the flat position using commercially pure argon shielding/backing gas with the straight polarity. For all the weldments, no preheat and post-weld heat treatment was applied, and inter-pass temperatures were maintained at about 150 °C during the welding. The welding parameters and heat input for each welding pass are described in Table 2.
Microstructural Examination
For the purpose of metallographic examinations, the sections transverse to the welding direction (Fig. 1a) were ground and polished according to the standard procedures. Then, to ensure the proper etching and reveal the microstructures, different solutions were used depending on the material zone: (1) a solution consisting of 60 ml HNO3 + 40 ml H2O was used for the electro-chemical etching of the weld zones, (2) a marble solution (10 g CuSO4 + 50 ml HCl + 50 ml H2O) was utilized for the chemical etching of the 310S base metal and its heat-affected zone (HAZ), and (3) 45 g KOH + 60 ml H2O solution was used for the electro-chemical etching of the 1.4742 base metal and its HAZ. The microstructural examinations were performed by the MEIJI-IM7200 optical microscope (OM) and TESCAN-VEGA3 scanning electron microscopy (SEM) equipped with an energy-dispersive x-ray spectroscopy (EDS) analysis. Additionally, PHILIPS-PW1730 x-ray diffraction (XRD) measurements using Cu-Kα irradiation and EDS analysis were performed to identify the different phases in the weldments. The ferrite content of the as-received 310S base and ER446 weld metals was measured in terms of ferrite number (FN) and ferrite volume percentage (Fvol.%) by the Fischer-FMP30 Ferritescope.
Mechanical Testing
To determine the micro-hardness distribution throughout the middle of the weld cross sections (Fig. 1a), Vickers micro-hardness was measured at a load of 100 gf (machine model: EW-110/D2, Bowers Group Co. England). The sub-size transverse-longitudinal (T-L) Charpy V-notch impact test specimens were prepared according to the ASTM E23 standard and tested at room temperature (machine model: SIT-300AC, Santam Co. Iran). Figure 1a and c shows the position and schematic view of the impact test specimens, respectively. For the impact tests, at least the average values of three test specimens have been reported. The fracture surfaces of the impact test specimens were observed using SEM.
Physical Testing
In order to compare the linear thermal expansion behavior of the weld joints, longitudinally cylindrical specimens (Fig. 1a) with 5 mm in diameter and 20 mm in length were prepared from the center of the weld zones by the electric discharge machining process. The dilatometer instrument was the LINSEIS-L75H connecting rod dilatometer type, and the heating rate was 20 °C/min up to 1100 °C in the air atmosphere.
Results and Discussion
The Microstructure of the Base Metals
Figure 2a displays the OM microstructure of the 310S base metal in the as-received conditions. The microstructure comprised of austenite grains, annealing twins, and δ-ferrite stringers (3 FN ≡ 3 Fvol.%), elongated parallel to the rolling direction. This ferrite comes from the segregation of ferrite-stabilizing elements (mainly Cr) during the hot rolling or solidification processes [3]. Although the existence of the approximately 6–12 volume percentage of δ-ferrite in austenitic stainless steels improves the sensitivity to solidification cracking, it can reduce the toughness and corrosion resistance of austenitic stainless steels [3, 19]. The SEM micrograph (Fig. 2b) shows some precipitates which are mainly pulled out after etching. The EDS point analysis (Fig. 2c) revealed that these precipitates are Cr- and C-rich and can be identified as chromium carbide (Cr23C6), formed during solidification from the melt or during the hot rolling process. The presence of chromium carbide can result in a detrimental effect on the mechanical and corrosion performance of the alloy. The base metal of the 1.4742 represents a fully ferritic microstructure consisting of coarse and elongated grains (Fig. 3a) in the as-received conditions. These grains are elongated parallel to the rolling direction. Two types of intra-granular precipitates (in a low-amount distribution) were found in the ferritic matrix (Fig. 3b and c). As shown in Fig. 3b and d, one type is Al- and N-rich precipitates with cuboidal morphology and can be identified as aluminum nitride (AlN). Figure 3c and e shows that other types of precipitates with a racemose morphology, rich in C, Cr, and Fe, can be identified as two different carbides, (Cr,Fe)23C6 and (Cr,Fe)7C3 [3]. These precipitates cause losses in the ductility and toughness of the alloy and reduce the corrosion resistance of the alloy by removing Al and Cr elements from the ferritic matrix.
The microstructure of the 1.4742 base metal in the as-received conditions: (a) the OM micrograph, the SEM image of the: (b) cuboidal and (c) racemose precipitates (marked by arrows), the EDS point analysis of the: (d) cuboidal and (e) racemose precipitates that marked by arrows in (d) and (c), respectively
The Microstructure of the Weld Metals
In order to predict the weld metal microstructures, the Cr-equivalent (Creq) and Ni-equivalent (Nieq) values were determined (using their respective chemical composition in Table 1) based on the Schaeffler equivalency relationships [3]. As can be seen in Fig. 4, the Schaeffler constitution diagram predicts a fully austenitic microstructure for the ER310 and ERNiCr-3 weld metals, as well as a ferritic-austenitic dual-phase microstructure for the ER446 weld metal. The details about the microstructure of the weld metals can be explained as follows:
The Schaeffler constitution diagram for the prediction of the weld metal microstructures [3]
The cross-sectional optical photographs for all of the three weldments are shown in Fig. 5. The weld metal microstructure corresponding to the ER310 filler metal (the A regions in Fig. 5a) is fully austenitic with a cellular and dendritic morphology represented in Fig. 6a. Taking into account the chemical composition of the weld metal (Table 1), Fe, Cr, and Ni are the main alloying elements of this weld metal, which have a low tendency toward segregation in the inter-dendritic and inter-granular regions [20, 21]. Thus, there is a slight constitutional super-cooling driving force to transform the solidification morphology from cellular to dendritic morphology. However, the observation of dendritic morphology in the ER310 weld metal may be related to the use of high heat input during welding (Table 2) and the increment of constitutional super-cooling [22]. The solidification grain boundaries (SGBs), solidification sub-grain boundaries (SSGBs), and migrated grain boundaries (MGBs) are visible and have been indicated by arrows in Fig. 6a. The boundary that separates the neighboring sub-grains (cells or dendrites) is considered as SSGBs, whereas the SGBs arise from the intersection of sub-grain packets. The MGBs carry a high angle mis-orientation of the parent SGBs, and their migration driving force is related to reducing the energy of the grain boundary [3]. Cr- and C-rich precipitates were detected in the EDS point analysis of the microstructure of this weld metal (Fig. 6b and e). The most prominent active element present in the composition of the ER310 weld metal is the Cr element. As reported in the previous studies [23], Cr can be diffused into the SGBs and SSGBs during solidification and has led to the formation of chromium carbide precipitates (Cr23C6) along these boundaries. Figure 6c shows the microstructure of the interface (its path has been indicated by arrows) between the weld passes (the B regions in Fig. 6a) in the ER310 weld metal. As it is observed, each pass exhibits an epitaxial nucleation on the previous pass. In this type of nucleation/growth, the nucleation occurs by arranging atoms from the liquid metal upon the previous pass grains without altering their existing crystallographic orientations [22]. As shown in Fig. 6d, at the top and bottom of the weld zone and near the weld centerline (the C regions in Fig. 5a) the equiaxed dendrites are nucleated and grown. This morphology impedes the growth of columnar dendrites [22].
The micrograph of the ER310 weld metal microstructure (the A regions in Fig. 5a): (a) OM and (b) SEM (precipitates are marked by arrows), (c) the optical micrograph of the interface (its path is indicated by arrows) between the weld passes (the B regions in Fig. 5a), (d) the optical micrograph of the equiaxed dendrites at the top and bottom of the weld zone and near the weld centerline (the C regions in Fig. 5a), and (e) the EDS point analysis of the precipitates that marked by arrows in (b)
As can be seen in Fig. 7a, the microstructure of the ERNiCr-3 weld metal (the A regions in Fig. 5b) is fully austenitic and represents dendritic morphology. The presence of the Nb element in the weld metal chemical composition (Table 1) stabilizes the austenite phase at high temperature. Additionally, the solidification morphology is changed from the cellular state to the dendritic one due to the intense tendency of Nb to increase the degree of constitutional super-cooling driving force [24, 25]. As can be seen by the arrows in Fig. 7a, the SGBs, SSGBs, and MGBs are obviously visible. According to Fig. 7b and EDS point analysis in Fig. 7e, the ERNiCr-3 weld metal consists of inter-dendritic Nb- and C-rich precipitates. Based on the previous investigations [26, 27], as ERNiCr-3 filler metal is diluted with two Fe-base metals, i.e., 310S and 1.4742 base metals, due to an increment in the Fe content of the weld metal (Table 1), the Nb solubility in austenite phase decreases. Consequently, the segregation of Nb in the inter-dendritic region is increased and therefore the formation of niobium carbide (NbC) and laves (Fe2Nb) phases can take place. These two eutectic-type constituents significantly extend the solidification temperature range of the weld metal and can cause the solidification cracking [22, 26, 27]. Figure 7c and d portrays the epitaxial nucleation at the interface (its path has been indicated by arrows) of the weld passes (the B regions in Fig. 5b) and equiaxed dendrites (the C regions in Fig. 5b), respectively.
The micrograph of the ERNiCr-3 weld metal microstructure (the A regions in Fig. 5b): (a) OM and (b) SEM (precipitates are marked by arrows), (c) the optical micrograph of the interface (its path is indicated by arrows) between the weld passes (the B regions in Fig. 5b), (d) the optical micrograph of the equiaxed dendrites at the top and bottom of the weld zone and near the weld centerline (the C regions in Fig. 5b), and (e) the EDS point analysis of the precipitates that marked by arrows in (b)
The microstructure of the ER446 weld metal exhibited a dual-phase microstructure, as depicted in Fig. 8a. The XRD result (Fig. 9) taken from the top surface of this weld metal revealed that the matrix and second phases are ferrite and austenite, respectively. Elmer et al. [28] investigated the influence of the chemical composition and cooling rate on the microstructure of stainless steels using Fe–Ni–Cr alloys. They showed that if the primary solidification modes are ferritic or ferritic-austenitic (with a ferrite volume fraction which is nearly 100 percent) along with the cooling rate in the range of arc welding processes, the austenitic phase can be present in the microstructure via eutectic and solid-state transformations with blocky (allotriomorph) and Widmanstatten morphologies. As can be seen in Fig. 8a (the A region in Fig. 5c), the microstructure consists of large primary ferrite grains growing approximately parallel to the heat-flow direction accompanied by blocky and Widmanstatten austenite. Similar observations have been reported by other researchers [29, 30]. It is said that in these solidification modes, detecting the sub-grains is difficult and a cellular-dendritic morphology could generally be formed [28]. In this regard, it must be pointed out that Widmanstatten austenite is mainly formed on the grain boundary and blocky austenite has been arranged in chain shape inside the grains. This chain shape may be attributed to the existence of the sub-grains. As depicted in Fig. 8a–d (regions A-D in Fig. 5c), there is a gradual increase in the volume fraction of the austenitic phase moving from the top (the third pass) toward the bottom (the back weld pass) of the weld metal cross section. The obtained results from the ferritometry test revealed the ferrite content of 105 FN ≡ 74 Fvol.%, 95 FN ≡ 66 Fvol.%, and 89 FN ≡ 63 Fvol.% within the top, middle, and bottom of the weld metal, respectively. Considering the chemical composition of the base metals and ER446 filler metal (Table 1), within the bottom of the weld metal, a relatively large portion of the base metals, i.e., 310S austenitic with the highest content of gamma-genic Ni element and 1.4742 ferritic with the lowest content of alpha-genic Cr element, are melted and diluted into the weld metal. On the other hand, dilution is minimum within the third pass [31]. Hence, by moving from the top to the bottom of the weld metal cross section, dilution can lead to a gradual decrement in the Creq to Nieq ratio and increases the volume fraction of the austenitic phase [3, 31]. Although the authors acknowledge that EDS is a semi-quantitative analysis, we conducted the EDS point analysis on a polished cross section of the weld metal at a low magnification of 300X from the top, middle, and bottom of the weld. Table 3 summarizes the EDS point analysis measurements. It can be seen that the relative content of Ni (an indicator of 310S dilution) and Al (an indicator of 1.4742 dilution) elements increased the movement from the top toward the bottom of the weld metal cross section. The low-amount distribution of precipitates in the ER446 weld metal was observed using SEM in Fig. 10. The EDS point analysis (Fig. 10) revealed that these precipitates are Cr- and C-rich and can be identified as chromium carbide (Cr23C6).
The Microstructure of the Interface and HAZ
The interface between the ER310 weld metal and 310S base metal (the D region in Fig. 5a) is presented in Fig. 11a. The microstructure reveals an epitaxial growth in the fusion line formed due to the same crystallographic structure and chemical composition of the base and weld metal [22]. Additionally, the columnar growth and therefore Type-I boundaries that are approximately perpendicular to the fusion line can be seen in the weld metal near the fusion line. No clear grain growth in the HAZ of the 310S base metal has been detected (Fig. 11a). In the case of the solution-annealed and hot-rolled stainless steels, the grain growth in the HAZ tends to be restricted unless weld heat inputs are extremely high [3]. The HAZ microstructures of the 310S base metal were similar to the other two ERNiCr-3 and ER446 weld metals. As shown in Fig. 11b, the interface of the ER310 weld metal and 1.4742 base metal (the E region in Fig. 5a) reveals the presence of an unmixed zone (UZ) in which a part of the base metal has totally been melted and re-solidified without the proper mixing with the filler metal. The SEM micrograph together with an EDS line scan analysis across this zone is presented in Fig. 11c. The UZ existence is due to the difference in the chemical compositions and melting ranges of the filler metals and the base metals. This will be seen in the morphologies of the peninsula, island, and beach in the microstructure of the weld metal near the fusion line [32]. Figure 11b and c shows the UZ in the form of the peninsula.
The OM microstructure of the: (a) interface between the ER310 weld metal and 310S base metal (the D region in Fig. 5a) and HAZ of the 310S base metal and (b) interface of the ER310 weld metal and 1.4742 base metal (the E region in Fig. 5a) and high-temperature HAZ of the 1.4742 base metal, (c) the SEM micrograph along with the EDS line scan analysis of the interface between the ER310 weld metal and 1.4742 base metal, and (d) the OM microstructure of the low-temperature HAZ of the 1.4742 base metal
In comparison with austenitic stainless steels, generally, ferritic stainless steels experience an extreme grain growth in the HAZ. This behavior is mainly due to the lack of or negligible solid-state transformation during welding thermal cycles and higher diffusion rates in the body-centered cubic (BCC) structure of ferritic stainless steels. Moreover, the higher thermal conductivity of ferritic stainless steels can promote the heat flow and extend the width of HAZ [3]. As mentioned before, the microstructure of the 1.4742 base metal consisted of large elongated grains as a result of cold deformation (Fig. 3a). The HAZ of the 1.4742 base metal exhibited two distinct regions and could be divided into the high-temperature HAZ (Fig. 11b) and the low-temperature HAZ (Fig. 11d). The complete recrystallization and intense grain coarsening occurred in the high-temperature HAZ. Also, a great amount of inter- and intra-granular precipitates with different morphologies was observed by SEM (Fig. 12). The EDS point analysis (Fig. 12) specified that these precipitates can be complex carbides, such as (Cr,Fe)23C6 and (Cr,Fe)7C3, as reported by other researchers [33,34,35]. Intra-granular precipitates have linear, fine dispersed, and cuboidal (surrounded by fine dispersed precipitates) morphologies. Inter-granular precipitates have linear and cuboidal (surrounded by fine dispersed precipitates) morphologies. Linear intra-granular precipitates are formed on the sub-grains [34]. Cuboidal inter- and intra-granular precipitates may originate from the pre-existed racemose precipitates as depicted in Fig. 3c. It is only the recrystallization that is noteworthy next to the high-temperature HAZ and toward the 1.4742 base metal in the low-temperature HAZ. In the case of the other two ERNiCr-3 and ER446 weld metals, the similar HAZ microstructures of the 1.4742 base metal with different amounts of grain sizes and precipitates were obtained. The difference is due to the difference in the heat input [34]. As the heat input increases, the cooling rate is reduced and the duration time for the formation of precipitates and grain growth becomes longer. In this respect, the maximum and minimum of the heat input are attributed to the ERNiCr-3 and ER310 weld metals, respectively (Table 2). The UZ can be seen on both sides of the ERNiCr-3 weld metal (Fig. 13). The UZ at the interface of the ERNiCr-3 weld metal and 310S base metal (the D region in Fig. 5b) has a beach morphology (Fig. 13a) and its SEM micrograph with the EDS line scan analysis is demonstrated in Fig. 13c. The similarity of the crystal structure of the ERNiCr-3 weld metal and the 310S base metal (face-centered cubic-FCC) has led to the epitaxial growth from the UZ (Fig. 13a). Figure 13b and d shows the UZ in the form of a peninsula and an island at the interface of the ERNiCr-3 weld metal and 1.4742 base metal (the E region in Fig. 5b).
The SEM image of the high-temperature HAZ of the 1.4742 base metal along with the EDS point analysis of the precipitates (please note: linear inter- and intra-granular precipitates are shown by white and black arrows, respectively. cuboidal inter- and intra-granular precipitates are shown by dotted white and white circles, respectively)
The OM microstructure of the interface of the ERNiCr-3 weld metal and: (a) 310S base metal (the D region in Fig. 5b) and (b) 1.4742 base metal (the E region in Fig. 5b), the SEM micrograph along with the EDS line scan analysis of the interface between the ERNiCr-3 weld metal and: (c) 310S base metal, and (d) 1.4742 base metal
The interfaces of the ER446 weld metal with the 310S base metal (Fig. 14a and c: the E region in Fig. 5c), as well as with the 1.4742 base metal (Fig. 14b: the F region in Fig. 5c), indicate the presence of UZ with beach morphology and epitaxial growth, respectively. In the case of the interface between the ER446 weld metal and 1.4742 base metal, as mentioned before, the primary solidification mode of this weld metal was almost fully ferritic. Thus, this weld metal can nucleate/grow from the 1.4742 base metal side in epitaxial form.
Hardness
The micro-hardness profile results across the middle of the weldments are shown in Fig. 15. For the 310S base metal, the hardness value was 212.8 ± 2.6HV and did not reveal a meaningful variation in the HAZ near the fusion line. The hardness value of the 1.4742 base metal was 205.9 ± 1.9 HV, which is less than the 310S base metal. There is a gradual decrement corresponding to low-temperature HAZ, and then there is a considerable increment corresponding to high-temperature HAZ in hardness values from the 1.4742 base metal toward the fusion line. These changes within the HAZ of the 1.4742 base metal are related to the experienced thermal cycles and therefore the microstructural difference. The formation of precipitates and recrystallization/grain growth are the two main mechanisms that can explain these phenomena. In the low-temperature HAZ, the recrystallization/grain growth decreases hardness and the ERNiCr-3 weld metal with the highest heat input shows the maximum decrement. Yet in the high-temperature HAZ despite serious grain growth, the hardness increases and the maximum hardness increment belongs to the ERNiCr-3 weld metal. This hardness increment is attributed to the precipitation of complex carbides. Therefore, it seems that in the high-temperature HAZ, the effect of precipitation (a hardening factor) is a more dominant mechanism than the recrystallization/grain growth (a softening factor). For the ER310, ERNiCr-3, and ER446 weld metals, the hardness values were obtained as 207.7 ± 2.2 HV, 211.4 ± 3.2 HV, and 221.9 ± 5 HV, respectively. All the weld metals exhibited higher hardness than the 1.4742 base metal. Among the weld metals, the ER310 and ER446 weld metals showed the minimum and maximum hardness values, respectively. The higher hardness accompanied with large fluctuations (the high-standard deviation) of the ER446 weld metal could be attributed to the presence of the ferrite and different morphologies of the austenite.
Impact Toughness
The average impact energy for all of the three welds and base metals is listed in Fig. 16. Moreover, the SEM images of the fracture surfaces are depicted in Fig. 17. The 310S base metal with the FCC crystal structure and Ni content enjoys higher impact energy than the 1.4742 base metal. The impact energy for all the three weld metals is higher than the value relating to the 1.4742 base metal and indicates that all the three filler metals can provide the required toughness for this DMW. The impact energy of the ERNiCr-3 weld metal is the highest, even as compared to the 310S base metal. This can be due to the FCC crystal structure of the ERNiCr-3 weld metal coupled with the highest Ni content [23]. In contrast, the ER446 weld metal results in the minimum impact energy among the weld metals. This can be attributed to the presence of large and directional ferrite grains (Fig. 8). Large and directional ferrite grains signify a smaller number of grain boundaries and planar crack-growth paths, respectively. Hence, both of them promote the crack propagation and hence the lower impact energy [16].
The 310S base metal, ER310, and ERNiCr-3 weld metals did not undergo a complete rupture, and the notch deformation was occurred, whereas the 1.4742 base metal and ER446 weld metal experienced a complete rupture in all the trials. As depicted in Fig. 17a, c, and d, the fracture surfaces of the 310S base metal, as well as ER310, and ERNiCr-3 weld metals are ductile, including the morphology with fibrous patterns and dimples. For the ERNiCr-3 weld metal (Fig. 17d), some cracks, which are known as fish-mouth opening, can be observed [36]. Although we did not detect any solidification crack, these fish-mouth opening cracks may be propagated from the pre-existing solidification micro-cracks, facilitating the easy crack propagation and decrement in impact energy. As can be seen in Fig. 17b, a brittle fracture took place in the 1.4742 base metal, including the river patterns. The images of the fracture surface of the ER446 weld metal display the characteristics of a granular (brittle-cleavage) fracture along with some plastically deformed zones (as shown in Fig. 17e and f). These plastically deformed zones pertain to the austenite phase undergoing plastic deformation. The same fracture surface feature was reported by Kawaguchi et al. [37].
Dilatometry
As mentioned in the introduction, one of the main reasons for the precocious failure of dissimilar joints at high temperature, especially in thermal cycling conditions, is the induced thermal stresses resulting from the difference in the CTE. The induced thermal stresses due to the CTE mismatch in one of the metals associated with DMWs, close to the interface, can be estimated using the following equation [38]:
where σ is the induced thermal stresses (MPa), E is the elastic modulus of that metal (MPa), Δα is the linear CTE mismatch between the two metals (°C−1), and ΔT is the temperature changes (°C). According to Eq 1, the higher linear CTE mismatch produces higher induced thermal stresses. Therefore, the linear CTE for the base and weld metals as a function of temperature was measured and has been measured and is shown in Fig. 18. At temperatures above 600 °C, the maximum and minimum linear CTEs are related to the 310S and 1.4742 base metal, respectively. This large linear CTE mismatch, i.e., ~ 4 × 10−6/°C, at temperatures above 600 °C can cause a stress concentration along the joint interface, especially in thermal cycling conditions. Thus, a desirable candidate to be selected as a filler metal should have a linear CTE between the two base metals, leading to creating a weld joint with a graded and smooth transition in the interfacial properties [39, 40]. In order to meet CTE between the CTE of the 310S and 1.4742 base metals, the ERNiCr-3 and ER446 filler metals must be used. Additionally, the weld metal corresponding to the ERNiCr-3 filler metal with a linear CTE approximately equal to the mean value of the two base metals is more effective in reducing the induced thermal stresses resulting from the difference in the CTE.
Conclusions
Three types of filler metal, ER310, ERNiCr-3, and ER446, were used to obtain the dissimilar welding between the 1.4742 ferritic and 310S austenitic heat-resistant stainless steels using the GTAW process. The mechanical and physical properties of the weld joints, as well as the microstructural evolution, were investigated and the main outcomes are as follows:
-
(1)
The microstructure of the ER310 and ERNiCr-3 weld metals was fully austenitic. A cellular and dendritic morphology was seen for the ER310 weld metal, while a dendritic solidification structure occurred for the ERNiCr-3 weld metal. The microstructure of the ER446 weld metal exhibited a ferritic-austenitic dual-phase microstructure.
-
(2)
An unmixed zone with different morphologies was observed on both sides of the ERNiCr-3 weld metal, while for the ER310 and ER446 weld metals it was formed merely on one side.
-
(3)
The HAZ of the 1.4742 base metal side showed two distinct regions, including the high-temperature HAZ and low-temperature HAZ. The complete recrystallization and intense grain coarsening occurred in the high-temperature HAZ and in the low-temperature HAZ only recrystallization was noteworthy.
-
(4)
The presence of the ferrite and different morphologies of the austenite resulted in the highest hardness in the weld metal made by using the ER446 filler metal.
-
(5)
The ERNiCr-3 weld metal had the highest impact energy, due to the FCC crystal structure coupled with the highest Ni content. In contrast, the ER446 weld metal displayed the lowest value, as a result of the presence of large and directional ferrite grains.
-
(6)
The weld metal made by using the ERNiCr-3 filler metal with a linear CTE approximately equal to the mean value of the linear CTE of the two base metals is a better choice to reduce the induced thermal stresses across the joint interface.
-
(7)
Based on the mechanical and physical properties, as well as the microstructural evolution in this work, the ERNiCr-3 filler metal was proposed as a better filler metal for the dissimilar welding between the 1.4742 ferritic and 310S austenitic heat-resistant stainless steels.
References
P. Elliott, Materials for high-temperature environments. Chem. Eng. Prog. 97(2), 75–81 (2001)
G.Y. Lai, High-temperature corrosion and materials applications, 1st edn. (ASM International, Materials Park, 2007)
J.C. Lippold, D.J. Kotecki, Welding metallurgy and weldability of stainless steels, 3rd edn. (Wiley, Hoboken, 2005)
N. Nabiran, S. Weber, W. Theisen, Influence of intermetallic precipitates and heat treatment on the mechanical properties of high-temperature corrosion resistant ferritic steels. Procedia Eng. 10, 1651–1656 (2011)
M.H. Bina, M. Jamali, M. Shamanian, H. Sabet, Investigation on the resistance spot-welded austenitic/ferritic stainless steel. Int. J. Adv. Manuf. Technol. 75(9–12), 1371–1379 (2014)
M.K. Samal, M. Seidenfuss, E. Roos, K. Balani, Investigation of failure behavior of ferritic–austenitic type of dissimilar steel welded joints. Eng. Fail. Anal. 18(3), 999–1008 (2011)
C.C. Hsieh, D.Y. Lin, M.C. Chen, W. Wu, Precipitation and strengthening behavior of massive δ-ferrite in dissimilar stainless steels during massive phase transformation. Mater. Sci. Eng. A 477(1–2), 328–333 (2008)
V.V. Satyanarayana, G.M. Reddy, T. Mohandas, Dissimilar metal friction welding of austenitic-ferritic stainless steels. J. Mater. Process. Technol. 160(2), 128–137 (2005)
M. Shojaati, B. Beidokhti, Characterization of AISI 304/AISI 409 stainless steel joints using different filler materials. Constr. Build. Mater. 147, 608–615 (2017)
K. Shanmugam, A.K. Lakshminarayanan, V. Balasubramanian, Effect of weld metal properties on fatigue crack growth behaviour of gas tungsten arc welded AISI 409 M grade ferritic stainless steel joints. Int. J. Press. Vessels Pip. 8(8), 517–524 (2009)
C.C. Silva, H.C. Miranda, H.B. de Sant’Ana, J.P. Farias, Austenitic and ferritic stainless steel dissimilar weld metal evaluation for the applications as-coating in the petroleum processing equipment. Mater. Des. 47, 1–8 (2013)
S.R. Kumar, A.K. Singh, S. Sandeep, P. Aravind, Investigation on microstructural behavior and mechanical properties of plasma arc welded dissimilar butt joint of austenitic-ferritic stainless steels. Mater. Today Proc. 5(2), 8008–8015 (2018)
T.W. Nelson, J.C. Lippold, M.J. Mills, Nature and evolution of the fusion boundary in ferritic-austenitic dissimilar metal welds. Part 2: on-cooling transformations. Weld. J. 10, 267s–277s (2000)
J.N. DuPont, Microstructural evolution and high temperature failure of ferritic to austenitic dissimilar welds. Int. Mater. Rev. 57(4), 208–234 (2012)
A.K. Bhaduri, S. Venkadesan, P. Rodriguez, Transition metal joints for steam generators–an overview. Int. J. Press. Vessels Pip. 58(3), 251–265 (1994)
K. Ranjbar, R. Dehmolaei, M. Amra, I. Keivanrad, Microstructure and properties of a dissimilar weld between alloy 617 and A387 steel using different filler metals. Weld World 62, 1121–1136 (2018)
M. Yousefi, M.H. Farghadin, A. Farzadi, Investigate the causes of cracks in welded 310 stainless steel used in the flare tip. Eng. Fail. Anal. 53, 138–147 (2015)
S. Abolghasemi, J. Williamson, T.C. Lindley, R.M. Ward, P.D. Lee, Thermal imaging and stress analysis for predicting the behaviour and long-term performance of flare tips. J. Strain. Anal. Eng. 48(2), 103–111 (2013)
J.C. Lippold, W.F. Savage, Solidification of austenitic stainless steel weldments : part III–the effect of solidification behavior on hot cracking susceptibility. Weld. Res. Suppl. 1(12), 388–396 (1982)
H. Shah Hosseini, M. Shamanian, A. Kermanpur, Characterization of microstructures and mechanical properties of Inconel 617/310 stainless steel dissimilar welds. Mater. Charact. 62(4), 425–431 (2011)
H. Naffakh, M. Shamanian, F. Ashrafizadeh, Dissimilar welding of AISI 310 austenitic stainless steel to nickel-based alloy Inconel 657. J. Mater. Process. Technol. 209(7), 3628–3639 (2009)
S. Kou, Welding metallurgy, 2nd edn. (Wiley, Hoboken, 2003)
A. Mortezaie, M. Shamanian, An assessment of microstructure, mechanical properties and corrosion resistance of dissimilar welds between Inconel 718 and 310S austenitic stainless steel. Int. J. Press. Vessels Pip. 116, 37–46 (2014)
T.Y. Kuo, L.T. Lee, Effects of filler metal composition on joining properties of alloy 690 weldments. Mater. Sci. Eng. A 338(1–2), 202–212 (2002)
G. Sayiram, N. Arivazhagan, Microstructural characterization of dissimilar welds between Incoloy 800H and 321 austenitic stainless steel. Mater. Charact. 102, 180–188 (2015)
J.N. DuPont, S.W. Banovic, R. Marder, Microstructural evolution and weldability of dissimilar welds between a super austenitic stainless steel and nickel-based alloys. Weld. J. 82(6), 125s–135s (2003)
J.N. DuPont, Microstructural development and solidification cracking susceptibility of a stabilized stainless steel. Weld. J. 78, 253–263 (1999)
J.W. Elmer, S.M. Allen, T.W. Eagar, Microstructural development during solidification of stainless steel alloys. Metall. Trans. A 20(10), 2117–2131 (1989)
K.D. Ramkumar, P.S. Goutham, V.S. Radhakrishna, A. Tiwari, S. Anirudh, Studies on the structure–property relationships and corrosion behaviour of the activated flux TIG welding of UNS S32750. J. Manuf. Process. 23, 231–241 (2016)
V. Muthupandi, P.B. Srinivasan, S.K. Seshadri, S. Sundaresan, Effect of weld metal chemistry and heat input on the structure and properties of duplex stainless steel welds. Mater. Sci. Eng. A 358(1–2), 9–16 (2003)
J.N. DuPont, Welding fundamentals and processes—dilution in fusion welding, in ASM Handbook, vol. 6A, ed. by T. Lienert, T. Siewert, S. Babu, V. Acoff (ASM International, Materials Park, 2011), pp. 115–121
T. Soysal, S. Kou, D. Tat, T. Pasang, Macrosegregation in dissimilar-metal fusion welding. Acta Mater. 110, 149–160 (2016)
M. Pouranvari, S.P.H. Marashi, M. Alizadeh-Sh, Welding metallurgy of dissimilar AISI 430/DQSK steels resistance spot welds. Weld. J. 94(6), 203s–210s (2015)
E. Ranjbarnodeh, S. Hanke, S. Weiss, A. Fischer, Effect of welding parameters on the heat-affected zone of AISI409 ferritic stainless steel. Int. J. Miner. Metall. Mater. 19(10), 923–929 (2012)
C.C. Silva, J.P. Farias, H.C. Miranda, R.F. Guimarães, J.W.A. Menezes, M.A.M. Neto, Microstructural characterization of the HAZ in AISI 444 ferritic stainless steel welds. Mater. Charact. 59(5), 528–533 (2008)
K.D. Ramkumar, A. Singh, S. Raghuvanshi, A. Bajpai, T. Solanki, M. Arivarasu, N. Arivazhagan, S. Narayanan, Metallurgical and mechanical characterization of dissimilar welds of austenitic stainless steel and super-duplex stainless steel—a comparative study. J. Manuf. Process. 19, 212–232 (2015)
S. Kawaguchi, N. Sakamoto, G. Takano, F. Matsuda, Y. Kikuchi, L. Mra´z, Microstructural changes and fracture behavior of CF8M duplex stainless steels after long-term aging. Nucl. Eng. Des. 174(3), 273–285 (1997)
American Welding Society, Metals and their weldability—dissimilar metals, in Welding Handbook, vol. 4, 7th edn., ed. by W.H. Kearns (American Welding Society, Miami, 1997), pp. 519–552
J.F. King, M.D. Sullivan, G.M. Slaughter, Development of an improved stainless steel to ferritic steel transition joint. Weld. J. 56(11), 354s–358s (1977)
N. Sridharan, E. Cakmak, B. Jordan, D. Leonard, W.H. Peter, R.R. Dehoff, D. Gandy, S.S. Babu, Design, fabrication, and characterization of graded transition joints. Weld. J. 96(8), 295s–306s (2017)
Acknowledgements
Authors acknowledge the National Iranian South Oil Company (NIOC), Iran (Project No. 1397-DC-1271) and Shahid Chamran University of Ahvaz, Iran (Grant Nos. 97/3/02/26247 and 97/7/02/26247) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amra, M., Dehmolaei, R. & Alavi Zaree, S.R. On the Dissimilar Metal Welding of 1.4742 Ferritic to 310S Austenitic Stainless Steels Utilizing Different Filler Metals. Metallogr. Microstruct. Anal. 8, 623–641 (2019). https://doi.org/10.1007/s13632-019-00574-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13632-019-00574-9