Abstract
This research work was focused on the performance of HVOF-sprayed 83WC–17CO and 86WC–10CO–4Cr coatings on boiler steel alloys ASME SA213 T22 and ASME SA213 T91 in a coal-fired boiler environment. Both coated and bare steel alloys were subjected to cyclic exposures, in the superheater zone of a coal-fired boiler for 10 cycles at 900 °C to compare the effect of the coatings in actual boiler environment. During the study, each cycle consisted of 100-h heating followed by 1-h cooling at ambient conditions and thereafter thermogravimetric method was used to establish the kinetics of corrosion. Corrosion products were examined by x-ray diffraction, scanning electron microscopy/energy-dispersive spectroscopy techniques. The hot corrosion resistance of both the coatings 83WC–17CO and 86WC–10CO–4Cr coating was found better on ASME SA213 T22, whereas 86WC–10CO–4Cr coating showed the higher corrosion resistance on ASME SA213 T22 as compared to 83WC–17CO coating.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In high-temperature applications of metals, the metals are heated up to the range of 700–900 °C in the presence of sodium chloride and sulfur compounds, the sulfate deposits are formed on the surface of metals, and this is regarded as hot corrosion [1, 2]. Hot corrosion of metals decreases the life span of components [3]. The corrosion leads to the failure of the components, which causes high monetary losses. In the USA, the losses due to hot corrosion are about $300 billion per year [4]. Once corrosion takes place, the metal component has to be replaced. To enhance the life of metal components in these high-temperature applications, thermal spray coatings have been used [3, 4]. Among various thermal spraying techniques, high-velocity oxy-fuel (HVOF) spray method has won reputation to formulate coatings over substrates of engineering components to guard them against hot corrosion, erosion, wear and excessive temperature oxidation. This approach employs a mixture of oxygen with another gas which includes hydrogen, propylene, propane, kerosene and hydrogen. High-velocity oxy-fuel spray technique develops coatings with high hardness, high bond energy, low porosity (much less than 1%), cost effectiveness, and low strain coatings. Many researchers have used HVOF coatings to increase the corrosion resistance of boiler steels. Singh et al. [5] evaluated the hot corrosion behavior of NiCrAlY, Ni–20Cr, stellite-6, and Ni3Al coatings at high temperature. The researches used plasma spray technique to deposit the coatings on iron-based superalloy. The results showed that NiCrAlY-coated specimens were having the highest corrosion resistance followed by stellite-6-coated specimens. Kawakita et al. [6] investigated the corrosion behavior of HVOF-sprayed stainless steel coatings. A nickel-based alloy was used as the substrate. The coatings were prepared by mixing molybdenum in stainless steel. It was found that 8% mass molybdenum coatings were most effective to reduce corrosion at high temperature. Sidhu et al. [7] did the comparative evaluation of hot corrosion performance of Cr2C3–NiCr and Ni–20Cr coatings. The coatings were deposited using HVOF technique on Ni-based superalloy. The experimentation was performed at high temperature in environment of molten salt Na2SO4–V2O5 at 900 °C under cyclic conditions. It was found that Ni–20Cr coating was more effective to reduce corrosion than other coating at high temperature. HVOF-sprayed different coatings were deposited successfully by Sidhu et al. [8]. The liquid petroleum gas (LPG) was used as fuel gas in HVOF process. It was concluded that the porosity of WC–Co coatings was less than that of other coatings. The Ni–20%Cr wire coatings were deposited using HVOF process by Sidhu et al. [9]. The Ni- and Fe-based superalloys were used as substrate materials. The coatings found were dense and were having low porosity. Sidhu and Prakash [10] investigated hot corrosion behavior of NiCrAlY, NiCr, stellite-6, and Ni3Al coatings on boiler steels in molten salt environment at 900 °C temperature. It was concluded that the corrosion resistance of stellite coating was more than that of other coatings, and Ni3Al coating was having the least corrosion resistance among all coatings. Sidhu et al. [11] showed that Ni–20Cr coating was having the highest hot corrosion resistance among NiCrBSi, Cr2C3–NiCr, Ni–20Cr, and stellite-6 coatings. The coatings were deposited on Fe-based superalloy with HVOF (high-velocity oxy-fuel) method. Sidhu et al. [12] investigated the hot corrosion behavior of HVOF-sprayed Cr2C3–NiCr and NiCrBSi coatings in molten salt environment. The Superni 718 was used as the substrate material. The coated specimens showed improved corrosion resistance than uncoated specimens. The ZrO2 coating was able to increase high-temperature performance in high-temperature application of Fe-, Co- and Ni-based superalloys [13]. The NiCrAl coating was successfully deposited on Ni- and Fe-based superalloys using HVOF process by Mahesh et al. [14]. The hot corrosion tests were performed in molten salt environment at 900 °C, and it was found that coated specimens showed the better corrosion resistance than uncoated specimens. The hot corrosion experiments on different types of coatings have been performed by various authors [15,16,17,18,19,20,21,22] which showed that thermal spray coatings have been able to enhance the corrosion resistance of boiler tube steels at high temperature.
It is clear from the literature review that thermal spray coatings have been used for enhancing the corrosion resistance of boiler steels at high temperature. Most authors have used Ni–Cr-based coatings, and there is very limited literature available on WC-, Co-, and Cr-based composite coatings. Therefore, in this experimental work, it was planned to investigate the high-temperature corrosion behavior and performance of 83WC–17CO and 86WC–10CO–4Cr coatings on ASME SA213 T22 and ASME SA213 T91 materials by assessing its surface and subsurface on heating it at 900 °C under cyclic conditions.
Experimental Procedure
Substrate Materials
ASME SA213 T22 and ASME SA213 T91 were two steel-based alloys that were selected as the substrate materials for the present research. These two steel alloys were procured from Guru Nanak Dev Thermal Power Plant, Bathinda, Punjab (India), in the form of cylindrical tubes. Then, these substrate materials were cut into the standard dimensions of 20 mm × 15 mm × 5 mm. After that, mirror polishing of cut specimens was done using emery papers of grit sizes 100, 150, 220, 320, 600, 800, and 1000. Alumina powder was used for grit blasting of samples. Tables 1 and 2 demonstrate (normal percentage and actual percentage) chemical composition of ASME SA213 T22 and ASME SA213 T91 boiler tube steels, respectively.
Coating Materials
83WC–17CO and 86WC–10CO–4Cr coating powders were used to deposit coatings. The composition and particle sizes of coating powders are given in the Table 3.
HVOF Spraying
High-velocity oxy-fuel thermal spray technique was opted for application of coatings on steel alloys specimens at Metallizing Equipment Co. Pvt. Ltd, Jodhpur, Rajasthan (India). Commercial HVOF (HIPOJET-2100) apparatus was used to deposit coatings. The various parameters used for spraying process are given in Table 4.
Experimentation in Coal-Fired Boilers
The experimental work was carried out in Guru Nanak Dev Thermal Plant, Bathinda, Punjab, where all the steel alloy specimens including bare as well as HVOF-coated were examined in an actual boiler environment in the central zone of platen superheater of the Stage-II boiler. The specimens were hung at 34.5-m height from the base of the boiler. The specimens were exposed for 10 cycles to the combustion environment, where each cycle consisted of 100-h heating followed by 1-h cooling at ambient conditions. In the boiler environment, the average temperature was about 900 ± 20 °C. The volumetric flow of flue gases was around 600 tonnes/h. Flue gases contained 12% CO2 and 5% O2 by volume. The SOx and NOx values of the flue gases were 315 and 1057 μg/m3, respectively. The gas stream contained the ash particles.
During experiment, the temperature was measured at normal intervals and the average temperature was measured to be about 900 °C with variation of ± 10 °C. At the end of each cycle, the weight of each specimen was measured. All the specimens were examined using x-ray diffraction (XRD), scanning electron microscopy/energy-dispersive spectroscopy. The hot corrosion behavior of all the specimens including bare and HVOF-coated ASME SA213 T22 and ASME SA213 T91 in the given environment was observed by computing the thermogravimetric data, metal thickness loss related to the corrosion scale formation and the depth of corrosion attack after 1000-h (10 cycles) exposure under cyclic conditions.
Results
Visual Examination of Bare and HVOF-Coated T22 and T91
The macrographs for all the specimens including uncoated, 83WC–17CO-coated and 86WC–10CO–4Cr-coated T22 and T91 after 1000-h exposure to the superheater zone of the coal-fired boiler are shown in Fig. 1.
Weight Change Measurements
Weight change measurements of all the specimens including bare and 83WC–17CO- and 86WC–10CO–4Cr-coated specimens subjected to cyclic oxidation for 10 cycles at 900 °C were measured. The minimum weight was gained by 86WC–10CO–4Cr-coated T22, whereas maximum weight was gained by bare T22 steel alloy specimen. Figure 2 shows the graph between weight change per unit area and number of cycles for each bare as well as HVOF-coated (T22, T91) specimens. The column chart of bare and coated samples for weight gained per unit area is shown in Fig. 3.
The uncoated specimen showed the highest weight gain per unit area both for T22 and T91 substrates. There was reduction in the weight gain substantially for all the coated specimens as shown in Fig. 3. The 86WC–10CO–4Cr-coated specimen showed less weight gain than 83WC–17CO-coated specimen for both substrate steels.
X-ray Diffraction
X-ray diffraction analysis patterns for bare and HVOF-coated specimens are shown in Fig. 4. XRD analysis of bare specimens indicated the presence of Fe2O3. The XRD analysis of 83WC–17Co-coated T22 and T91 steel alloys showed the presence of α-WC, γ-W, β-Co, and δ-W2C elements, whereas in the case of 86WC–10CO–4Cr-coated T22 and T91 specimens, the surface scale indicated the presence of α-WC and β-W2C. The presence of these compounds on the surface of coated specimens may be the reason for the increase in hot corrosion resistance of specimen at high temperature.
Scanning Electron Microscopy/Energy-Dispersive Spectroscopy
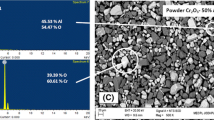
The scanning electron microscopy with energy-dispersive spectroscopy analysis of all the bare and coated specimens is shown in Fig. 5.
It is clear from the micrographs that scale of the specimens is having irregular flakes. The scanning electron microscopy and energy-dispersive spectroscopy analysis of 83WC–17CO-coated specimen shows the presence of W, C, and Co as the main as shown in Fig. 5a and b. The energy-dispersive spectroscopy analysis of 86WC–10CO–4Cr-coated specimen shows almost similar composition as that of coating powder as depicted in Fig. 5c and d.
Discussion
The bare T22 and T91 steel specimens indicated severe corrosion and high rate of weight gain during the exposure to the boiler environment. XRD analysis of these bare specimens showed the presence of ferrous oxide on the surface of exposed specimens. Kumar et al. [23] and Singh et al. [5] explained that the formation of Fe2O3 on the surface of bare steel specimens is the reason for high corrosion rate. The FE/scanning electron microscopy analysis further supplemented the XRD results, which indicated the presence of Fe and O as main elements in the case of bare steel specimens. The formation of Fe2O3 scale has also been discussed by Kumar et al. [23], Sidhu et al. [11], and Goyal et al. [16]. The macrographs of 83WC–17CO-coated and 86WC–10CO–4Cr-coated steel specimens reveal that the coating was intact even after experimentation. Both the coatings substantially reduced the weight gain rate during the experimentation as compared to bare steel specimens. FE/scanning electron microscopy micrographs of coated specimens show the formation of splats, and the formation of these splats may be the reason for the decrease in weight gain rate of coated specimens [12, 14, 24]. The XRD analysis of 83WC–17Co-coated T22 and T91 steel alloys indicated the presence of α-WC, γ-W, β-Co and δ-W2C elements, whereas in the case of 86WC–10CO–4Cr-coated T22 and T91 specimens, the surface scale indicated the presence of α-WC and β-W2C. The presence of these compounds on the surface of coated specimens may be the reason for the increase in hot corrosion resistance of specimen at high temperature [22, 25]. These elements might have restricted the penetration of corrosion species into the coated surface to decrease the overall weight change rate of coated boiler steel specimens. The scanning electron microscopy micrographs of all the exposed coated specimens indicate that the coating was intact with the substrate after the exposure of the specimens to the boiler environment. The energy-dispersive spectroscopy analysis indicates the presence of W, Co, C, and Cr as main elements on the exposed surfaces of coated specimens, which validated the formation of WC and W2C compounds on the coated surface. The presence of Al, Si, Fe, Mo, and Mn in energy-dispersive spectroscopy analysis may be due to the penetration of these elements from the substrate to the coating matrix [19, 21, 24].
Conclusions
The following conclusions have been drawn from this research work:
-
1.
HVOF spraying process has been effectively employed for depositing 83WC–17CO and 86WC–10CO–4Cr coatings on two steel alloy specimens, namely ASME SA213 T22 and ASME SA213 T91.
-
2.
Both the coatings on the steel alloy specimens were uniform, dense, and with the thickness between 200 and 250 µm
-
3.
The coatings used on steel alloys, i.e., ASME SA213 T22 and ASME SA213 T91, in the present research have proved to be corrosion resistant in coal-fired boiler environment in superheater zone when exposed for 10 cycles (each cycle consists of 100 h) at 900 °C.
-
4.
The coatings have shown the following order of corrosion resistance: 86WC–10CO–4Cr-coated T22 > 83WC–17CO-coated T22 > 86WC–10CO–4Cr-coated T91 > 83WC–17CO-coated T91.
-
5.
The bare samples have been observed to be the least corrosion resistant all bare and coated samples, among bare specimens ASME SA213 T22 has shown least resistance to corrosion.
References
H. Edris, D.G. McCartney, A.J. Sturgeon, Microstructural characterization of high velocity oxy-fuel sprayed coatings of Inconel 625. J. Mater. Sci. 32, 863–868 (1997)
P. Hancock, Vanadic and chloride attack of superalloys. Mater. Sci. Technol. 3, 536–544 (1987)
N. Priyantha, P. Jayaveera, A. Sanjurjo, K. Lau, F. Lu, K. Krist, Corrosion resistant metalic coatings for application in high aggressive environments. Surf. Coat. Technol 163–164, 31–36 (2003)
A. Vaidhya, T. Streibl, L. Li, S. Sampath, O. Kovarik, R. Greenlaw, An integrated study of thermal spray process–structure–property correlations: a case study for plasma sprayed molybdenum coatings. Mater. Sci. Eng. A 403, 191–204 (2005)
D.H. Singh, D. Puri, S. Prakash, Some studies on hot corrosion performance of plasma sprayed coatings on a Fe-based superalloy. Surf. Coat. Technol. 192, 27–38 (2005)
J. Kawakita, S. Kuroda, T. Fukushima, T. Kodama, Improvement of corrosion resistance of high velocity oxy-fuel sprayed stainless steel coatings by addition of Molybdenum. J. Therm. Spray Technol. 14, 224–230 (2005)
T.S. Sidhu, S. Prakash, R.D. Agrawal, Hot corrosion studies of HVOF sprayed Cr3C2–NiCr and Ni–20Cr coatings on nickel-based superalloy at 900 °C. Surf. Coat. Technol. 201, 792–800 (2006)
H.S. Sidhu, B.S. Sidhu, S. Prakash, Mechanical and microstructural properties of HVOF sprayed WC–Co and Cr3C2–NiCr coatings on the boiler tube steels using LPG as the fuel gas. J. Mater. Process. Technol. 171, 77–82 (2006)
T.S. Sidhu, S. Prakash, R.D. Agrawal, Characterisation of NiCr wire coating on Ni- and Fe-based superalloys by the HVOF process. Surf. Coat. Technol. 200, 5542–5549 (2006)
B.S. Sidhu, S. Prakash, Performance of NiCrAlY, Ni–Cr, Stellite-6 and Ni3Al coatings in Na2So4–60%V2O5 environment at 900 °C under cyclic conditions. Surf. Coat. Technol. 201, 1643–1654 (2006)
T.S. Sidhu, S. Prakash, R.D. Agrawal, Performance of high velocity oxyfuel-sprayed coatings on an Fe-based superalloy in Na2So4–60%V2O5 environment at 900 °C Part II: Hot corrosion behaviour of the coatings. J. Mater. Eng. Perform. 15, 130–138 (2006)
T.S. Sidhu, S. Prakash, R.D. Agrawal, Study of molten salt corrosion of high velocity oxy-fuel sprayed cermet and nickel-based coatings at 900 °C. Metall. Mater. Trans. A 38A, 77–85 (2007)
G. Goyal, H. Singh, S. Prakash, Effect of superficially applied ZrO2 inhibitor on the high temperature corrosion performance of some Fe-, Co- and Ni-base superalloys. Appl. Surf. Sci. 254, 6653–6661 (2008)
R.A. Mahesh, R. Jayaganthan, S. Prakash, Evaluation of hot corrosion behaviour of HVOF sprayed NiCrAl coating on superalloys at 900 °C. Mater. Chem. Phys. 111, 524–533 (2008)
G. Kaushal, H. Singh, S. Prakash, Surface engineering by detonation-gun spray coating of 347H boiler steel to enhance its high temperature corrosion resistance. Mater. High Temp. 28, 1–11 (2011)
R. Goyal, V. Chawla, B.S. Sidhu, State of art: thermal spraying and performance of hard coatings: a review. Int. J. Res. Mech. Eng. Technol 1, 22–26 (2011)
M. Kaur, Surface engineering analysis of detonation-gun sprayed Cr3C2–NiCr coating under high-temperature oxidation and oxidation–erosion environments. Surf. Coat. Technol. 206, 530–541 (2011)
S.S. Chatha, H.S. Sidhu, B.S. Sidhu, The effects of post-treatment on the hot corrosion behavior of the HVOF-sprayed Cr3C2–NiCr coating. Surf. Coat. Technol. 206, 4212–4224 (2012)
S.B. Mishra, K. Chandra, S. Prakash, Erosion–corrosion performance of NiCrAlY coating produced by plasma spray process in a coal-fired thermal power plant. Surf. Coat. Technol. 216, 23–34 (2013)
V.P.S. Sidhu, K. Goyal, R. Goyal, Corrosion behaviour of HVOF sprayed coatings on ASME SA213 T22 boiler steel in an actual boiler environment. Adv. Eng. Forum 20, 1–9 (2017)
S. Singh, K. Goyal, R. Goyal, Performance of Ni3Al And TIO2 coatings on T22 boiler tube steel in simulated boiler environment in laboratory. J. Mech. Eng. 46(1), 54–61 (2017)
S. Singh, K. Goyal, R. Goyal, Performance of Cr3C2-25 (Ni–20Cr) and Ni–20Cr coatings on T22 boiler tube steel in simulated boiler environment. J. Thin Films Coat. Sci. Technol. Appl. 3(2), 19–26 (2016)
M. Kumar, H. Singh, N. Singh, R.S. Joshi, Erosion–corrosion behavior of cold-spray nanostructured Ni–20Cr coatings in actual boiler environment. Wear 332, 1035–1043 (2015)
G. Singh, K. Goyal, R. Bhatia, Hot corrosion studies of plasma-sprayed chromium oxide coatings on boiler tube steel at 850 °C in simulated boiler environment. Iran. J. Sci. Technol. Trans. Mech. Eng. (2017). doi:10.1007/s40997-017-0090-4
X. Zhang, X. Zhang, X. Jie, X. Jie, L. Zhang, L. Zhang et al., Improving the high-temperature oxidation resistance of H13 steel by laser cladding with a WC/Co-Cr alloy coating. Anti-Corrosion Methods Mater. 63(3), 171–176 (2016)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sidhu, V.P.S., Goyal, K. & Goyal, R. Comparative Evaluation of Hot Corrosion Resistance of 83WC–17CO and 86WC–10CO–4Cr Coatings on Some Boiler Steels in Actual Boiler in Thermal Power Plant. Metallogr. Microstruct. Anal. 6, 512–518 (2017). https://doi.org/10.1007/s13632-017-0392-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13632-017-0392-3