Abstract
The notoriety of toxic metals has become a cause for concern across the world as their presence in the environment constitutes serious health hazard that affect not only the human populace but the ecosystem. This study aimed to explore the ameliorative potential of ethanol extract of Stachytarpheta cayennensis (SCE) leaf in Wistar rats exposed to arsenic, cadmium, and lead (ACL). Thirty Wistar rats were divided into five (5) groups of six (6) rats each. Group one served as the control group was given 300 ml of distilled water as their daily drinking water. Group two as ACL group was exposed to a combination of 30 mg/kg of lead, cadmium, and 1 mg/kg of arsenic, daily, via drinking water. Group three, four and five were the treatment groups administered a combination of 100 mg/kg of vitamins C and 30 mg/kg of vitamin E, 200, and 400 mg/kg of SCE with ACL in daily drinking water respectively. Experiment lasted for 28 days, the rats were sacrificed, blood and other organs were harvested for laboratory analysis. There were varying degrees of significant (P<0.05) amelioration of the heavy metals toxicity revealed by positive changes in the biochemical and hematological indices evaluated when the treated groups were compared to the ACL group. Therefore, the administration of SCE, vitamin C and E could potentially protect against the co-induced toxicity of arsenic, cadmium and lead in Wistar rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Environmental pollution remains a major challenge to our society. Heavy metal environmental pollution poses a serious threat and is a major source of public health concern (Ali et al. 2013; Ali and Khan 2017). The increased rates of urbanization and industrialization in the world at large have led to greater levels of environmental contamination by heavy metals and this had led to a gargantuan increase in the rate of transport and mobilization in the environment since the 1940s (Mohammad et al. 2021). Lead, cadmium and arsenic have been the most common heavy metals that induced human poisoning (Balali-Mood et al. 2021). Contamination of water and air by these toxic metals is an environmental concern and hundreds of millions of people are being affected around the world. Food contamination with lead, cadmium and arsenic is another concern for human and animal health (Luo et al. 2020). In developing countries, there is a paucity of information available to the general public on the dangers and consequences associated with exposure to these toxic metals (Afrin et al. 2015).
Lead is a toxic environmental contaminant with highly deleterious effects on numerous body organs. Lead is majorly absorbed from the respiratory and digestive system though it can be absorbed from the skin (Jacobs et al. 2009). Exposure to lead can induce neurological, respiratory, urinary and cardiovascular disorders due to immune modulation, oxidative and inflammatory mechanism. Lead can cause alteration in physiological functions of the body and can be associated with many diseases (Kianoush et al. 2012). Animal studies have shown that exposure of rats to lead acetate significantly reduced antioxidant indices such as glutathione peroxide, catalase and superoxide dismutase in hepatocyte and erythrocyte. In addition, lead significantly increased oxidative indices including malondialdehyde and H2O2 concentration (Omobowale et al. 2014).
Cadmium occurs naturally in soil and minerals such as sulfate, carbonate, chloride and hydroxide salt as well as in water. Anthropogenic activities contribute to high levels of cadmium in water, air and soil which could be substantial human exposure. A major exposure to cadmium is through ingestion of contaminated food and through smoking, which is capable of increasing cadmium level of blood and urine. The vital mechanism in the body could be disrupted by the presence of cadmium which may result in short or long term disorders (Richter et al. 2017; Cao et al. 2018). Rice, grains and seafood have been found to be contaminated by cadmium (Chunhabundit 2016), nonetheless, after ingestion, a little part of cadmium is absorbed. In Japan, there was an outbreak of itai-itai disease due to mass cadmium contamination of food and water. The patients suffered from painful bone degenerative disorder, kidney failure and lung disease (Nishijo et al. 2017). Study by Borowska et al’s (2017) revealed that rats exposed to 1 and 5 mg/kg cadmium were found to have a disorder in zinc (Zn) and copper (Cu) metabolism. Other studies suggest that cadmium decreases the absorption of Zn, Ca and Cu resulting in the low dietary intake of such essential elements (Balali-Mood et al. 2021).
Arsenic is one of the toxic heavy metals of public health concern. Sources of arsenic exposure are occupational or through the pollution of food and water, it has a long history of use as either a metalloid substance or as a medicinal product. Primarily arsenic absorption is from the intestine. Other routes of exposure are from skin contact and by inhalation (Gupta et al. 2017). Acute and chronic toxicity of arsenic is related to the dysfunctions of numerous vital enzymes. Arsenic can inhibit sulfhydryl group containing enzymes which leads to their dysfunction. Moreover, arsenic inhibits the pyruvate dehydrogenase by binding to the lipoic acid moiety of the enzyme. Pyruvate dehydrogenase inactivation can block the Krebs cycle and inhibits oxidative phosphorylation as a result ATP production decreases resulting in cell damage (Shen et al. 2013).
For years, nature has been seen as a source of ethnomedicinal agents and several drugs have been obtained from natural sources (Bradbury and Murray 2013). The application of plant parts for the management of diseases is as old as man and thus to a great extent has contributed to the dissemination of knowledge relating to the therapeutic virtues of these plants. It has been estimated that in Africa up to 80% of the population relies on plants for use as medications. Some of the plants that have proved to have high potential in treating heavy metal-induced toxicity are cilantro (Coriandrum sativum) (Bradbury and Murray 2013). Certain substances (for example, vitamin C, vitamin E, curcumin, and rutin), have the ability to either chelate these metals to prevent their absorption in tissues, or increase the body’s anti-oxidative capacity to reduce the likelihood of oxidative stress or damage to tissues like the kidney and liver (Al-Attar 2011; Mirani et al. 2012; Tarasub et al. 2012).
Stachytarpheta cayennensis is a purple colour flower and a seed producing weedy herbaceous plant, shrubby perennial and can grow up to 1.5 m high and belongs to the family verbenacceae. It is commonly called Brazilian tea, Oparara in south western Nigeria and Blue rat tail in English (Olayode et al. 2019). Stachytarpheta cayennensis is applied by many traditional healers to treat a host of diseases including diabetes, dysentery pain, wounds, eye infections, bacterial infection, chicken pox, measles, blood pressure and malaria. The phytochemical screening of the leaves of Stachytarpheta cayennensis revealed the presence of steroids, terpenoids, tannins, sapoinins, alkaloids and flavonoids (Otom and Okwute 2020). Pharmacological study of Stachytarpheta cayennensis in rat have provided evidence for the anti-inflammatory and gastroprotective properties supporting its use in folk medicine (Penido et al. 2006). Safety study carried out by Olayode et al. (2019) on the acute and repeated dose study of leaf extract of Stachytarpheta cayennensis showed that the LD50 of the extract was greater than 5000 mg/kg. Furthermore, the study concluded that the extract of Stachytarpheta cayennensis was relatively acutely non-toxic judging from the lack of serious alteration in functional and behavioural observations, lack of mortality following the administration of a single dose of 5000 mg/kg LD50 and repeated exposures to 250, 500 and 1000 mg/kg of the extract, daily, for 28 days. However, the haematological and biochemical parameters as well as, histological findings showed the potential of the extract to effect toxic action on the body at higher doses when given repeatedly for long duration (Olayode et al. 2019). The interest in natural antioxidants such as those of plant origin has increased greatly in recent years and thus has engineered this research to evaluate the ameliorative effect of Stachytarpheta cayennensis leaf extract on arsenic, cadmium and lead co-induced toxicity in Wistar rats.
Methods
Chemicals
Every chemical used was of analytical quality. 100 g of cadmium chloride (99.9% pure) and 25 g of sodium arsenate, manufactured by Cartivalue Chemical Limited, Mumbai, India were obtained from a chemical store in Benin City, alongside 100 g of lead acetate, manufactured by BDH Chemical Limited, Poole, England. Vitamin E (1000 I.U) capsules, manufactured by Korea United Pharmacy Inc., Chungam, Korea, and Vitamin C (1000 mg) tablets by Cornerstone Research and Development Inc., Utah, USA, were also obtained.
Preparation of plant materials and extraction
Fresh green plants of Stachytarpheta cayennensis (gervao-roxo) were collected from Okpara town Ethiope East Local Government Area of Delta State Nigeria (5.6380°N, 5.9658°E). Mr. P. Obaro, a Botanist in the Department of Plant Biology and Biotechnology, University of Benin, Edo State, identified the plant and a specimen was deposited at the herbarium with a voucher number UBHf0150. Leaves of the plant were detached from the stem before they were washed mildly in distilled water, air dried for a period of three weeks when they turned crisp, and then dried at 40–45 °C for about two hours in an incubator. The well-dried leaves were then transferred to a mechanical grinder, ground to a fine powder and stored in airtight bottles at room temperature for further use. Afterward, the dry weight was recorded. The maceration process was done by soaking the powdered material in ethanol inside a closed jar for a period of 48 h with shaking at 6-h intervals. The resultant mixture was filtered using Whatman filter paper number 1. The filtrate was collected and concentrated in a thermostatically regulated water bath at 55 °C. The yield was weighed and the percentage yield was calculated. The extract was introduced into a sterile bottle and kept at a temperature of 4 °C in the refrigerator.
Animals and experimental design
Male Wistar rats with an average weight between 160 and 250 g were bought from the animal house of the Pharmacology Department at the University of Benin. The animals were housed in standard plastic cages under controlled temperature (22–24 °C) and 12 h light/12 h dark cycle. Animals were fed standard rat pellets (Top feeds) and had unrestricted access to plain water for 14 days prior to the start of the experiment for acclimatization. The rats used for the study were maintained based on the guidelines of the National Institutes of Health guide for the care and use of laboratory animals and approval was obtained from the Department of Science Laboratory Technology, University of Benin with ethical number UBH/LS/SLT20114. Thirty male rats were randomly put into five groups of six rats each (control and four tested group). Group I animals which served as the control were given 1 ml of distilled water, each. Group II animals were given 30 mg/kg of lead, 1 mg/kg of arsenic and 30 mg/kg of cadmium dissolved in their drinking water (positive control). Group III animals were administered 100 mg/kg of vitamin C and 30 mg/kg of vitamin E orally, while 30 mg/kg of lead, 1 mg/kg of arsenic and 30 mg/kg of cadmium were dissolved in their drinking water. Animals in group IV were administered 200 mg/kg of Stachytarpheta cayennensis extract and 30 mg/kg of lead, 1 mg/kg of arsenic and 30 mg/kg of cadmium dissolved in their drinking water. Group V animals were administered 400 mg/kg of Stachytarpheta cayennensis extract and 30 mg/kg of lead, 1 mg/kg of arsenic and 30 mg/kg of cadmium dissolved in their drinking water. Administration of Stachytarpheta cayennensis extract and Vitamin C and E dissolved in distilled water, was by oral gavage, daily, for 28 days. Afterward the rats were fasted overnight and sacrificed on the 29th day under chloroform anesthesia, Blood was collected from the heart through the aorta in a heparinized bottle and taken to the laboratory for analysis.
Biochemical assays
Measurement of malondialdehyde
Malondialdehyde (MDA) which is produced when polyunsaturated fatty acids break down is a useful indicator of how much lipid peroxidation has occurred. Malondialdehyde and thiobarbituric acid (TBA) combine to generate an MDA-TBA2 adduct (red color complex), which absorbs light at 535 nm. This reaction is the basis for the assay (Ohkawa et al. 1979).
Measurement of antioxidant enzymes
The Cohen et al. (1970) method was used to measure catalase (CAT) activity. A common enzyme called catalase can be present in almost all living things that are in contact with oxygen, where it helps hydrogen peroxide break down into water and oxygen.
The Misra and Fridovich (1972) approach was used to measure the level of SOD activity. Superoxide dismutase is an enzyme class that catalyzes the deamination of superoxide into hydrogen peroxide and oxygen.
The Flohe and Gunzler (1984) method was used to measure glutathione peroxidase (GPx). The biological action of glutathione peroxidase is the conversion of free hydrogen peroxide to water and the reduction of lipid hydroperoxides to the corresponding alcohols.
Measurement of lipid profile
Serum triacylglycerol was estimated using the Tietz (1990) method. The triacylglycerol was determined after enzymatic hydrolysis by lipases. Enzymatically, the hydrolysis and oxidation of total cholesterol dictate its amount. In the presence of phenol and peroxidase, the indicator quinoneimine is created from hydrogen peroxide and 4-aminoantipyrine.
The addition of phosphotungstic acid in the presence of magnesium ions precipitated low-density lipoproteins (LDL and VLDL) and chylomicron fraction in a quantifiable manner. After centrifugation, the HDL (high-density lipoprotein) portion, which remained in the supernatant, had its cholesterol content measured.
Very low-density lipoproteins (VLDL), LDL, and HDL are the three main lipoprotein fractions that contain the majority of cholesterol. According to the connection, LDL cholesterol is calculated from observed values for total cholesterol, triglycerides, and HDL cholesterol.
Other biochemical tests (nephritic and hepatic tests)
After allowing the blood to coagulate and sediment, it was centrifuged at 704 g for 15 min. The concentration level of the hepatic and nephron biochemical parameters were obtained by pouring serum into auxiliary tubes and then placed in an analyzer (photometer 5010). The Reitman-Frenkel method was used to measure the activity of alanine and aspartate aminotransferases, the unified approach for color reaction with diacetylmonoxime was used to measure urea, and the Jaffe method with depolymerization was used to measure creatinine. TP, ALB, and ALP were assessed using a KonelabTM PRIME 60i automatic biochemical analyzer (Thermo Scientific, Vantaa, Finland) and commercialized assays from the same manufacturer at the University of Benin Teaching Hospital in Edo state, Nigeria.
Hematological parameters tests
Red blood cell count was carried out using a micropipette. Hayem’s fluid (3.99ml) as diluent was obtained to which was added 0.1ml of blood. The mixture was shaken properly before placing a drop, using a pipette, on a graticule slide over the counting area. The tip of the pipette was placed on the side of the slide to just touch the cover slip. The stopper was squeezed gently to allow a small quantity of the prepared blood to move under the coverslip by capillarity. This was viewed with a microscope.
White blood cell count was carried out using the automated differential counts method. The number of white blood cells and their indicators are crucial for immunological function. The platelets count was carried out using the QBC tube which was centrifuged and then read off on the QBC reader (QBC Autoread Plus Hematology System, USA).
Packed cell volume was determined using a centrifuge which was read out using a hematocrit reader.
Statistical analysis
Differences between the groups of rats were tested for significance using a one-way analysis of variance (ANOVA). All results are presented as means (± standard deviation). Statistical analysis and calculations were performed using the GraphPad PRISM 6.0 Ink software for Windows. Statistical significance was set at p < 0.05.
Results
The data in Table 1 shows that treatment with ACL mixture reduced the activities of SOD, GPx, and CAT when compared to negative control. There was a significant (p<0.05) increase of CAT, and GPx activities but a non significant increase in SOD activity in rats in administered vitamin C and E, 200 mg/kg and 400 mg/kg of Stachytarpheta cayennensis extract when compared to ACL group. MDA level was significantly (p<0.05) higher in the ACL group compared to the control group, vitamin C and E group and Stachytarpheta cayennensis extract at 200 mg/kg and 400 mg/ kg.
The effects of Stachytarpheta cayennensis, vitamin C, and E on TCHOL, TG, HDL, and LDL of Wistar rats exposed to heavy metal mixture (As, Cd, and Pb) are depicted in Table 2. Group II animal exposed to ACL mixture showed a significant (p<0.05) ) increase in TCHOL, TG, and LDL but marked decreased HDL relative to the control. However, group III, IV and V treated with vitamin C, and E, 200 mg/kg and 400 mg/kg Stachytarpheta cayennensis extract had significantly decreased TCHOL but significantly (p<0.05) increased HDL compared to the ACL mixture group.
The data in Fig. 1 show that ALP and ALT levels in the ACL mixture group were significantly (p < 0.05) increase when compared with the negative control group and the rats administered vitamins C and E, and 200 mg/kg and 400 mg/kg of Stachytarpheta cayennensis extract. The levels of AST, TP and ALB were reduced significantly (p<0.05) in the rats exposed to heavy metal (ACL) mixture compared to the negative control and the group administered vitamins C and E, and 200 mg/kg and 400 mg/kg of Stachytarpheta cayennensis extract.
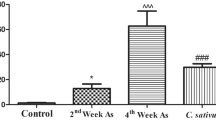
Rats in the group exposed to ACL mixture showed significantly (p < 0.05) increased levels of urea and creatinine in contrast to the control (distilled water) group. However, other groups treated with vitamins C and E, Stachytarpheta cayennensis extract at 200 and 400 mg/kg had significantly decreased urea and creatinine levels compared to the ACL mixture group as shown in Fig. 2.
Haematological parameters
The data in Fig. 3 show that haematocrit (HCT), white blood cells (WBC), platelets (PLT), and red blood cells (RBC) levels in the ACL mixture group were significantly decreased when compared to the negative control. Rat groups administered vitamins C and E, 200 mg/kg and 400 mg/kg of Stachytarpheta cayennensis extract, all showed a significant (p < 0.05) increase in HCT, WBC, PLT, and RBC levels in contrast to the ACL mixture group. There was no significant difference in the monocyte count between the control, heavy metal mixture and vitamins C and E groups. However, there was a marked elevation in monocytes count (p < 0.05) in the groups treated with 200 mg/kg and 400 mg/kg of Stachytarpheta cayennensis extract. For the number of lymphocytes, no significant difference was observed among the groups.
Discussion
The purpose of this study was to assess the effects of ethanol leaf extract of Stachytarpheta cayennensis on ACL co-induced toxicity in Wistar rats. The metals (arsenic, cadmium and lead) used in this study are known to be very notorious pollutants and toxicants to the bodily organs by accumulating inside the organ or tissue, causing harm, depending on the route of exposure and dose (Mitsiev 2015; Miller et al. 2002). The effects of these heavy metals become unpredictable and are often potentiated when exposure is to more than one metal (Jaishankar et al. 2014). This phenomenon necessitated the execution of this study to show how a mixture of the three heavy metals will affect certain important biochemical biomarkers such as alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), urea, creatinine, total cholesterol (TCHOL), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL).
The data obtained in this study showed that exposure to a mixture of ACL decreases the antioxidant status in the rat and caused the elevation of MDA. Research has demonstrated that a variety of antioxidant defence mechanisms shield cells against the toxicity caused by Cd (Ognjanovic et al. 2008). The reduction in the levels of antioxidants for the group of rats exposed to a mixture of ACL might be due to the oxidative stress caused by the ACL toxicity. Lead causes the production of ROS and blocks the activity of antioxidant enzymes in tissues (Jurczuk et al. 2007; Franco et al. 2009). Additionally, it has been demonstrated to raise lipid peroxidation levels (LPO) as seen in the elevation of MDA level in this study, which could be as a result of heavy metal enhanced ROS production, that induced oxidative stress and compromised the antioxidant defence system. In the vitamin C and E group, 200 and 400 mg/kg of Stachytarpheta cayennensis group, showed a significant elevation in the antioxidant levels, this could be as a result of the bioactive phytochemicals present in the extract. Most cells use their first line of defense, which includes enzymes that scavenge free radicals including SOD, CAT, and GPx, to combat oxidative stress (Sarkar et al. 2014). These are crucial for defending cells against oxidative stress. Previous studies with comparable findings have also been published (Ohkawa et al. 1979; Lakshmi et al. 2013).
Furthermore, the result of the lipid profile in this study showed a significant decrease in TCHOL, TG, and LDL of the treated group and an increase in HDL compared to the ACL mixture group. The increase in the TCHOL level observed in the ACL mixture group could exert a serious negative effect on the heart. According to studies, TCHOL plays a significant role in predicting the risk of cardiovascular disease (Conroy et al. 2003; Hippisley-Cox et al. 2010). Additionally, it has been demonstrated that ischemic heart disease and high cholesterol levels are related. (Kohli and Cannon 2012; Miller et al. 2011; Borena et al. 2011). In this study, it has been shown that heavy metals can increase the level of TG which could exert negative effects on the heart, and treatment with extract of Stachytarpheta cayennenis was shown to decrease the level of TG, suggesting a therapeutic effect. The high-density lipoproteins (HDLs) which is a good lipoprotein circulating in the human serum has proven to be a powerful marker to be used in the risk stratification of atherosclerotic cardiovascular disease (ASCVD). Increased high density lipoprotein, as seen in the Stachytarpheta cayennensis group (200 and 400 mg/kg) and the vitamin C and E group, can improve the normal functioning of the heart in a way that is consistent with earlier research on the association between HDL cholesterol and ASCVD (Di Angelantonio et al. 2009). HDL have the ability to prevent the onset of ASCVD by lowering atherosclerosis susceptibility. Low density lipoproteins (LDL), which are considered to be bad proteins, have been shown to increase the risk of cardiovascular disease. LDL concentration is regarded as a key indicator of atherosclerosis. LDL damages endothelial cells, which in turn promotes the development of fatty streaks and atherosclerosis. According to studies, lipid-related inflammation may have a significant role in the development of atherosclerosis (Weber and Noels 2011).
For the hepatic enzymes and proteins, a significant increase in ALT levels of the ACL mixture group is in accordance with results from Markiewicz-Górka et al. (2015). In 2015, Markiewicz-Górka et al. carried out a study to assess how heavy metals affected the levels of liver function indicators and if the effects were dose-related. Results from the experiment showed high AST and ALT activity and bilirubin levels, suggesting a case of liver dysfunction. An increase in serum aminotransferases can be linked directly to progressive liver injury and necrosis of liver tissue which causes the release of these enzymes. (Giannini et al. 2005). A significantly reduced AST level which was observed in the ACL mixture group as compared to the control group was not in agreement with the report from Markiewicz-Górka et al. (2015) and might be due to variance in experimental animal’s physiological state, differences in environmental factors, feed and water given to the animal. However, the decrease in AST can also be associated with severe liver diseases—necrosis or cirrhosis, caused by a significant decrease in the number of active cells that synthesize it (Tazitdinova et al. 2018). Furthermore, it is essential to note that the considerable increase in ALT levels observed obviously denotes cellular deterioration and hepatic membrane leakage. It is widely documented in the literature that heavy metal poisoning, particularly cadmium, causes hepatocellular damage (Baba et al. 2013; Lu et al. 2013). Both aminotransferases (AST and ALT) are highly concentrated in the liver; AST is found in both the cytosol and mitochondria of hepatocytes, but ALT is only found in the cytoplasm (Haidry and Malik 2014). Contrary to the group receiving Stachytarpheta cayennensis extract of 200 and 400 mg/kg, the up-regulation of ALT activity brought on by ACL exposure was notable. Overall, the administration of Stachytarpheta cayennensis extract showed a significant ameliorative effect similar to the effect observed in the group that received vitamin C and E, which is in agreement with the result of a study by Elgaml and Hashish (2014) that reported the amelioration of hepatic enzyme, total protein, albumin by Thymus vulgaris extract against cadmium induced toxicity.
The level of serum total protein (TP) is a marker for overall liver health. The serum total protein levels of the rats in the ACL mixture group significantly decreased, according to the current study. The research Awoke et al. (2020) supports these findings. It is well recognized that the liver is crucial to the production of serum protein. The reduction of serum protein in rats exposed to the mixture of ACL is supported by the finding of Andjelkovic et al. (2019). Administration of Stachytarpheta cayennensis extract showed a significant effect similar to the effect observed in the group that received vitamin C and E, which is in agreement with the result from similar studies by Awoke et al. (2020) on leaf extract of Ruspholia hypocrateriformis on successfully ameliorating the distorted redox imbalance and oxidative damage in the liver of the rats caused by exposure to the heavy metal.
For effect on the kidney, the parameters included the measurement of the creatinine levels and urea levels. It has been suggested that creatinine, which is produced when creatine phosphate is digested, is a measure of renal function. As a waste product of protein metabolism, urea can be used to measure the function of the kidneys (Adedapo et al. 2009). Creatinine is one of the metabolic waste products that the kidney excretes, and electrolytes are reabsorbed in the tubules to maintain the body's balance. Evaluation of serum urea and creatinine are crucial and sensitive biochemical markers that are typically used in the diagnosis of renal failure and damage because they are non-protein nitrogenous metabolites that are cleared by the body after glomerular filtration (Tietz 1990; Gowda et al. 2010). Results obtained from this study show that the urea and creatinine levels of the ACL mixture group increased significantly in contrast to the other groups. This is an indication that the mixture of ACL was responsible for the increased levels of creatinine and urea. This is in line with studies carried out by Salem and Salem (2012). This elevation indicates kidney dysfunction. When measuring renal function, urea and creatinine are employed, and their levels wouldn’t increase until at least 50 to 70 percent of the kidney nephrons were lost (Gaurav et al. 2010). The observation of a significant decrease in vitamin C and E group, the 200 and 400 mg/kg of S.C respectively were indicative of suppression of both creatinine and urea, indicating a therapeutic effect of Stachytarpheta cayennensis.
Evaluation of blood parameters is important for risk assessment because it can more accurately anticipate human toxicity from modifications in the haematological system when information from animal research is applied (Owolabi, et al. 2007). It has been established that the combination of lead, arsenic, and cadmium causes oxidative stress, which produces free radicals and changes the oxygen free radical scavenging enzyme system (Flora et al. 2004). This results in the damage of blood plasma membrane structure leading to abnormalities in haematological parameters. The results obtained from haematological tests and screening showed that the rat group exposed to the mixture of ACL had effects on each of the analysed haematological parameters. Haematological components are now understood to be pathological reflectors of healthy biological activities, pathological processes, or pharmacological reactions to therapeutic interventions, and as a result, they can change depending on a person's physiological condition (Olayode et al. 2019). In this study, there was a significant decreased in HCT, RBC, WBC, and PLT in the ACL mixture group, whereas monocytes and lymphocytes values were reduced but no statistical difference was seen when compared to other treated groups. A significant decrease in the number of red cells in the blood observed in ACL exposed rats maybe associated with the development of anaemia, due to the heavy metal generation of lipid peroxides that haemolysed the RBCs (Ali et al. 2013). For the haematological parameters, the study demonstrated that the heavy metal group increased the rate of abnormalities in the rats. The vitamin C and E, 200, and 400 mg/kg of Stachytarpheta cayennensis were able to abate the toxicity induced by the mixture of ACL.
Conclusions
This study for the first time has reported the ameliorative effect of ethanol leaf extract of Stachytarpheta cayennensis on co-induced toxicity to Wistar rats by exposure to mixture of arsenic, cadmium and lead in their drinking water. The study has shown the therapeutic ability of the extract to ameliorate the detrimental effects of arsenic, cadmium and lead intoxication on the biochemical enzymes of kidney, liver, and haematological system in Wistar rats. Furthermore, this study has demonstrated the efficacy of vitamin C and E against toxicity co-induced by arsenic, cadmium and lead metals. From the result of this present study, a recommendation is made for further mechanistic and molecular studies to be carried out on Stachytarpheta cayennensis in order to understand and extensively harness its therapeutic potential against arsenic, cadmium and lead toxicity.
Data availability
All the data generated or analysed during the study are included in this published article.
Abbreviations
- SCE:
-
Stachytarpheta cayennensis extract
- ACL:
-
Arsenic, cadmium and lead
- MDA:
-
Malondialdehyde
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxide.
- SOD:
-
Superoxide dismutase
- CVD:
-
Cardiovascular diseases
- TBA:
-
Thiobarbituric acid
- TBARC:
-
Thiobarbituric acid reactive compounds
- LDL:
-
Low density lipoprotein
- VLDL:
-
Very low density lipoprotein
- HDL:
-
High density lipoprotein
- TG:
-
Triglycerides
- TCHOL:
-
Total cholesterol
- AST:
-
Aspartate aminotransferase
- ALB:
-
Albumin
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- TP:
-
Total protein
- HCT:
-
Hematocrit
- WBC:
-
White blood cells
- PLT:
-
Platelets
- RBC:
-
Red blood cells
- MNT:
-
Monocytes
- LYM:
-
Lymphocytes
- ASCVD:
-
Atherosclerotic cardiovascular disease
References
Adedapo A, Mogbojuri O, Emikpe B (2009) Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. J Med Plants Res 3(8):586–591
Afrin R, Mia MY, Ahsan MA, Akbor A (2015) Concentration of heavy metals in available fish species (bain, Mastacembelus armatus; taki, Channa punctatus and bele, Glossogobius giuris) in the Turag river, Bangladesh. Pak J Sci Ind Res Ser B Biol Sci 58(2):104–110
Al-Attar AM (2011) Antioxidant effect of vitamin E treatment on some heavy metals-induced renal and testicular injuries in male mice. Saudi J Bio Sci 18(1):63–72. https://doi.org/10.1016/j.sjbs.2010.10.004
Ali H, Khan E (2017) Environmental chemistry in the twenty-first century. Environ Chem Lett 15(2):329–346
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals concepts and applications. Chemosphere 91(7):869–881
Andjelkovic M, Buha Djordjevic A, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z (2019) Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health 16(2):274. https://doi.org/10.3390/ijerph16020274
Awoke JN, Orji OU, Aja PM, Ezeani NN, Aloke C, Obasi OD (2020) Ethanol leaf extract of Ruspolia hypocrateriformis abrogated hepatic redox imbalance and oxidative damage induced by heavy metal toxicity in rats. Arab J Chem 13:8133–8145
Baba H, Tsuneyama K, Yazaki M, Nagata K, Minamisaka T, Tsuda T, Nomoto K, Hayashi S, Miwa S, Nakajima T, Nakanishi Y, Aoshima K, Imura J (2013) The liver in itai-itai disease (chronic cadmium poisoning): pathological features and metallothionein expression. Mod Pathol 26:1228–1234
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12:643972. https://doi.org/10.3389/fphar.2021.643972.PMID:33927623
Borena W, Stocks T, Jonsson H, Strohmaier S, Nagel G, Bjørge T, Manjer J, Hallmans G, Selmer R, Almquist M, Häggström C, Engeland A, Tretli S, Concin H, Strasak A, Stattin P, Ulmer H (2011) Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Causes Control 22:291–299
Borowska S, Brzóska MM, Gałażyn-Sidorczuk M, Rogalska J (2017) Effect of an extract from Aronia melanocarpa L. berries on the body status of zinc and copper under chronic exposure to cadmium: an in vivo experimental study. Nutrients 9(12):1374. https://doi.org/10.3390/nu9121374
Bradbury C, Murray J (2013) Investigating an incidental finding of thrombocytopenia. BMJ. https://doi.org/10.1136/bmj.f11
Cao ZR, Cui SM, Lu XX, Chen XM, Yang X, Cui JP, Zhang GH (2018) Effects of occupational cadmium exposure on workers’ cardiovascular system. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi = Zhonghua Laodong Weisheng Zhiyebing Zazhi = Chinese J Indust Hyg and Occupat Dis 36(6):474–477. https://doi.org/10.3760/cma.j.issn.1001-9391.2018.06.025
Chunhabundit R (2016) Cadmium exposure and potential health risk from foods in contaminated area, Thailand. Toxicol Res 32(1):65–72
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–38. https://doi.org/10.1016/0003-2697(70)90083-7
Conroy RM, Pyorala K, Fitzgerald AP (2003) Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 24:987–1003
Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J (2009) Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302:1993–2000
Elgaml SA, Hashish EA (2014) Clinicopathological studies of Thymus vulgaris extract against cadmium induced hepatotoxicity in Wistar Rats. Global J Pharmacol 8:501–509
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121. https://doi.org/10.1016/s0076-6879(84)05015-1
Flora SJS, Pande M, Kannan GM, Mehta A (2004) Lead induced oxidative stress and its recovery following co-administration of melatonin or N-Acetycysteine during chelation with succimer in male rats. Cell Mol Bio 50:543–551
Franco R, Sánchez-Olea R, Reyes-Reyes EM, Panayiotidis MI (2009) Environmental toxicity, oxidative stress and apoptosis: ménage à trois. Mut Res 674(1–2):3–22. https://doi.org/10.1016/j.mrgentox.2008.11.012
Gaurav D, Preet S, Dua KK (2010) Chronic cadmium toxicity in rats: treatment with combined administration of vitamins, amino acids, antioxidants and essential metals. J Food Drug Anal 18:464–470
Giannini EG, Testa R, Savarino V (2005) Liver enzyme alteration: a guide for clinicians. CMAJ Can Med Assoc J 172(3):367–379. https://doi.org/10.1503/cmaj.1040752
Gowda S, Desai PB, Kulkarni SS, Hull VV, Math AA, Vernekar SN (2010) Markers of renal function tests. N Am J Med Sci 2(4):170–173
Gupta DK, Tiwari S, Razafindrabe B, Chatterjee S (2017) Arsenic contamination from historical aspects to the present. Arsenic contamination in the environment. Springer, Berlin, pp 1–12
Haidry MT, Malik A (2014) Hepatoprotective and antioxidative effects of Terminalia Arjuna against cadmium provoked toxicity in Wistar Rats (Ratus Norvigicus). Biochem Pharmacol 3:130
Hippisley-Cox J, Coupland C, Robson J (2010) Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ 341:c6624
Jacobs DE, Wilson J, Dixon SL, Smith J, Evens A (2009) The relationship of housing and population health: a 30-year retrospective analysis. Environ Health Perspect 117(4):597–604. https://doi.org/10.1289/ehp.0800086
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Inter Toxicol 7(2):60–72. https://doi.org/10.2478/intox-2014-0009
Jurczuk M, Moniuszko-Jakoniuk J, Brzoska MM (2007) Hepatic and renal concentrations of vitamins E and C in lead- and ethanol-exposed rats: an assessment of their involvement in the mechanisms of peroxidative damage. Food Chem Toxicol 45:1478–1486
Kianoush S, Balali-Mood M, Mousavi SR, Moradi V, Sadeghi M, Dadpour B, Rajabi O, Shakeri MT (2012) Comparison of therapeutic effects of garlic and d-penicillamine in patients with chronic occupational lead poisoning. Basic Clin Pharmacol Toxicol 110(5):476–481
Kohli P, Cannon CP (2012) Triglycerides: how much credit do they deserve? Med Clin N Am 96:39–55
Lakshmi BVS, Sudhakar M, Aparna M (2013) Protective potential of black grapes against lead ilead-inducedtive stress in rats. Environ Toxicol Pharmacol 35:361–368
Lu Q, Lei YX, He CC, Lei ZN (2013) Blood translation elongation factor-1δ is a novel marker for cadmium exposure. Int J Mol Sci 14:5182–5197
Luo L, Wang B, Jiang J, Huang Q, Yu Z, Li H (2020) Heavy metal contaminations in herbal medicines: determination. Comprehensive risk assessments. Front Pharmacol 11:595335
Markiewicz-Górka I, Januszewska L, Michalak A, Prokopowicz A, Januszewska E, Pawlas N, Pawlas K (2015) Effects of chronic exposure to lead, cadmium, and manganese mixtures on oxidative stress in rat liver and heart. Arh Hig Rada Toksikol 66(1):51–62. https://doi.org/10.1515/aiht-2015-66-2515
Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S (2011) American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, & Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123(20):2292–2333. https://doi.org/10.1161/CIR.0b013e3182160726
Miller WH, Schipper HM, Lee JS, Singer J, Waxman S (2002) Mechanisms of action of arsenic trioxide. Cancer Res 62(14):3893–3903
Mirani N, Ashraf NJ, Siddique J, Rub A (2012) Protective effect of rutin against cadmium induced hepatotoxicity in Swiss Albino mice. J Pharmacol Toxicol 7:150–157
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Bio Chem 247(10):3170–3175
Mitsiev AK (2015) Change lipid peroxidation as a mechanism of renal disease under heavy metals. Patologicheskaia Fiziologiia i Eksperimental’naia Terapiia 59(2):65–69
Mohammad AM, Hossain D, Al-Imran SK, Begum M, Hasan OM (2021) Environmental pollution with heavy metals: a public health concern. Intech Open
Nishijo M, Nakagawa H, Suwazono Y, Nogawa K, Kido T (2017) Causes of death in patients with Itai-itai disease suffering from severe chronic cadmium poisoning: a nested case–control analysis of a follow-up study in Japan. BMJ Open 7(7):e015694
Ognjanovic BI, Markovic SD, Pavlovic SZ, Zikic RV, Stajn AS, Saicic ZS (2008) Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physio Res 57:403
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Olayode OA, Daniyan MO, Olayiwola G (2019) Biochemical, hematological and histopathological evaluation of the toxicity potential of the leaf extract of Stachytarpheta cayennensis in rats. J Tradit Complement Med 10(6):544–554. https://doi.org/10.1016/j.jtcme.2019.05.001
Omobowale TO, Oyagbemi AA, Akinrinde AS, Saba AB, Daramola OT, Ogunpolu BS (2014) Failure of recovery from lead induced hepatoxicity and disruption of erythrocyte antioxidant defence system in Wistar rats. Environ Toxicol Pharmacol 37(3):1202–1211
Otom OP, Okwute SK (2020) Chemical and biological screening of the leaves of Stachytarpheta cayennensis (l. vahl). Int J Res Sci Inn 7(12):2321–2705
Owolabi OJ, Omogbai EKI, Obasuyi O (2007) Antifungal and antibacterial activities of the ethanolic and aqueous extract of kigelia Africana (Bignoniaceae) stem bark. Afr J Biotech 6:882–885
Penido C, Costa KA, Futuro DO, Paiva SR, Kaplan MA, Figueiredo MR, Henriques MG (2006) Anti-inflammatory and anti-ulcerogenic properties of Stachytarpheta cayennensis (L. C. Rich) Vahl. J Ethnopharmacol 104(1–2):225–233. https://doi.org/10.1016/j.jep.2005.09.006
Richter P, Faroon O, Pappas RS (2017) Cadmium and cadmium/zinc ratios and tobacco-related morbidities. Int J Environ Res Public Health 14(10):1154
Salem EA, Salem NA, Maarouf AM, Serefoglu EC, Hellstrom WJ (2012) Selenium and lycopene attenuate cisplatin induced testicular toxicity associated with oxidative stress in Wistar rats. Urology 79:1184.e1–6
Sarkar S, Mukherjee S, Chattopadhyay A, Bhattacharya S (2014) A low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: expression of antioxidant genes. Ecotoxicol Environ Saf 107:1–8
Shen S, Li XF, Cullen WR, Weinfeld M, Le XC (2013) Arsenic binding to proteins. Chem Rev 113(10):7769–7792
Tarasub N, Junseecha T, Tarasub C, Ayutthaya WDN (2012) Protective effects of curcumin, vitamin C, or their combination on cadmium-induced hepatotoxicity. J Basic Clin Pharm 3:273–281
Tazitdinova R, Beisenova R, Saspugayeva G, Aubakirova B, Nurgalieva Z, Zandybai A, Fakhrudenova I, Kurmanbayeva A (2018) Changes in the biochemical parameters of rat blood under the combined effect of chronic intoxication with such heavy metals as copper, zinc, arsenic. Adv Anim Vet Sci 6(11):492–498
Tietz NW (1990) Clinical guide to laboratory tests, 2nd edn. W.B. Saunders Co., Philadelphia, p 566
Weber C, Noels H (2011) Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 17(11):1410–1422
Acknowledgements
The authors are thankful to Dr. and Dr (Mrs) P. Obaro for their support and contributions during the course of the work. The authors would like to acknowledge all the technicians and technologists in the Department of Science Laboratory Technology, University of Benin, Benin City, Edo State, Nigeria.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
OCE: Conceived, designed the research, wrote and revised the paper. EEI, MWE, SOH, BEO and JUE; were involved in material preparation, data collection, laboratory analysis and writing of the paper, SI was involved in interpretation of the data and revision of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Osazuwa Clinton Ekhator has no conflict of interest. Elijah Eshiokwemia Igbokah has no conflict of interest. Marvin Wisdom Eromosele has no conflict of interest. Sherifat Onosioriamhe Harun has no conflict of interest. Blessing Ejiro Oghenegweke has no conflict of interest. Jessica Uchechukwu Egbe has no conflict of interest. Success Isuman has no conflict of interest.
Ethical approval
The rats used for the study were maintained based on the guidelines of the National Institutes of Health guide for the care and use of laboratory animals and approval was obtained from the Department of Science Laboratory Technology, University of Benin with ethical number UBH/LS/SLT20114.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ekhator, O.C., Igbokah, E.E., Eromosele, M.W. et al. Ameliorative effect of Stachytarpheta cayennensis extract and vitamins C and E on arsenic, cadmium and lead co-induced toxicity in Wistar rats. ADV TRADIT MED (ADTM) 24, 823–833 (2024). https://doi.org/10.1007/s13596-023-00736-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-023-00736-9