Abstract
Nauclea pobeguinii (N. pobeguinii) is a plant used in African medicine to treat many gastroduodenal diseases. In this study, we determined the gastro-protective mechanisms and anti-Helicobacter pylori (H. pylori) properties of N. pobeguinii extracts. Wound healing activity (acetic acid test), anti-secretory properties (pyloric ligation, pyloric ligation/acetylcholine and pyloric ligation/histamine tests) and cytoprotective effects (ethanol test) were assessed in female rat, the anti-Helicobacter pylori (agar well diffusion method) was also evaluated. At doses of 100, 200 and 400 mg/kg, the extracts reduce (p < 0.001) the various ulceration parameters. In the acetic acid test, the extracts (200 mg/kg) reduced ulcerated areas by 99.23% (aqueous) and by 98.47% (methanol), levels of monocytes, lymphocytes, nitrogen, malondialdehyde and increased (p < 0.001) superoxide dismutase and catalase activities. Histological analysis showed repair of the mucosal epithelium at all doses of both extracts. Aqueous and methanol extracts inhibited ulceration indices by 99.68 and 99.33% (pyloric ligation), 83.81% and 61.07% (pyloric ligation/acetylcholine), 97.49% and 98.50% (pylorus ligation/histamine); they increased (p < 0.001) the mucus mass and uterine mass. In vitro, the different H. pylori isolates were sensitive to both extracts; the aqueous extract showed strong anti-urease activity, a large diameter of the inhibitory zone and a better minimum inhibitory concentration. Aqueous and methanolic extracts of N. pobeguinii healed ulcers through their estrogen-modulating anti-inflammatory, antioxidant, anti-secretory and cytoprotective properties. The aqueous extract of N. pobeguinii could be a good solution for the treatment of this infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastritis and gastric ulcers are major gastrointestinal disorders. The severity of inflammation of the gastric mucosa results in gastric ulcer formation. Complications of an undiagnosed or poorly treated gastric ulcer often include bleeding, gastric perforation, stricture, cancer, and death. About 8–10% of the world's population is affected by gastric ulcers, with an estimated prevalence of 80% in developing countries (Adinortey et al. 2013; Sidahmed et al. 2015). In Douala-Cameroon, the most affected age group is the group of people aged above 60 (Eloumou et al. 2016). Other epidemiological studies have reported that women have a lower prevalence of gastric ulcers than men (Wu et al. 2008; Kumral et al. 2014); pregnant women and those taking estrogen-containing pills have an even lower frequency of gastric ulcers (Ashokan et al. 2010). However, postmenopausal women are likely to have a high incidence of ulcers (Sangma et al. 2014). Many toxic agents can attack the stomach and cause ulcers in its mucous membrane. Hyperacidity, colonization of the gastric mucosa by Helicobacter pylori (H. pylori), alcohol consumption, overproduction of reactive oxygen species (ROS) and pro-inflammatory cytokines are the main causative agents of gastric ulcers (Ateufack et al. 2015).
Peptic ulcer, chronic gastritis, and gastric cancer result from the production of many virulent factors by these bacteria. These virulent factors mainly include vacuolating protein (Vac A) and urease (Ateufack et al. 2015). Colonization of the gastric epithelium by H. pylori generally results in elevated urease production, chronic inflammatory responses and tissue damage. The urease produced by all strains of H. pylori is responsible for neutralizing the surrounding acidity of the colonized area, but also, through its lipase and protease activities, urease destroys the mucosa resulting in the persistence of inflammation (Watari et al. 2014); while the secretion of mucus and bicarbonate, cell regeneration and maintenance of gastric blood flow contribute to the protection of the gastric mucosa. Recent advances for the knowledge of this disease and recent advances in the field have highlighted the contribution of female hormones, especially estrogens, in the cytoprotective mechanisms of the stomach (Kumral et al. 2014; Périco et al. 2018).
Conventional treatment of gastric ulcers regularly includes anti-secretory drugs such as type 3 muscarinic receptor antagonists (atropine sulfate), histamine type 2 receptor antagonists (cimetidine), proton pump inhibitors (omeprazole), and antibiotics (Bali 2016). The use of these drugs is very often associated with serious side effects. According to Kangwan et al. (2014), patients on anti-secretory treatment are likely to relapse. Although there are many antiulcer drugs available, the regimen for peptic ulcer treatment is considered to be one of the biggest clinical issues due to the lower efficacy and inconveniences associated with reciprocal antiulcer drugs on the market, that limit their use; this justifies why there is an increasingly growing tendency to discover new antiulcer agents from plants with high efficacy and little or no toxicity. Indeed, medicinal plants offer a wide chemical structural diversity (Sidahmed et al. 2016).
Nauclea pobeguinii (N. pobeguinii) (Pobeguin ex Pellegr.) or Sarcocephalus pobeguinii, of the Rubiaceae family, is a deciduous tree with an average height of 30 m (Mbiantcha et al. 2018), which is found in Central, Western and Eastern Africa. In Cameroon, this plant is called "koumkoma" in the "Tupuri" language and is found in swamp forests in the Southern, Eastern and Central Regions of the country. In Central Africa, root decoction of N. pobeguinii is used as anthelmintic and against malaria (Messia et al. 2012). In the South, East, and Central Regions of Cameroon, the powder from the bark of the stem helps to heal malaria, intestinal pain, jaundice, fever, ulcers, pain and inflammation (Seukep et al. 2016). Several scientific studies have shown that N. pobeguinii has antibacterial (Seukep et al. 2016), antioxidant (Kadiri et al. 2007; Mfotie et al. 2017), antimalarial (Kadiri et al. 2007), anti- inflammatory (Seukep et al. 2016), analgesic and antiarthritic properties (Tsafack et al. 2020). A toxicity study carried out by Tariq et al. (2011) showed that the LD50 of extracts from this plant was greater than 5 g/kg. In addition, aqueous and methanol extracts of N. pobeguinii contain flavonoids, tannins, saponins, steroids, alkaloids and terpenoids (Njimoh et al. 2015). Moreover, the bark of N. pobeguinii is capable of preventing the proliferation of Gram- negative bacteria and reverse the antibiotic resistance of bacteria (Njimoh et al. 2015; Seukep et al. 2016). Moreover, Qi et al. (2014) showed that N. pobeguinii contains compounds such as cadambine, 3α-dihydro cadambine, and 3α,5α-tetrahydrodesoxycordifoline. Other work carried out on this same plant has made it possible to isolate several other compounds, namely 3-acetoxy-11-oxo-urs-12-ene, p-coumaric acid, citric acid trimethyl ester, resveratrol, resveratrol β -D-glucopyranoside, strictosamide, strictosidine, naufoline, angustoline, naucleofficin D, 3,14-dihydroangustoline, magniflorine, naucleidinal, nauclefne, 3-O-β-fucosyl-quinovic acid, 19-O-methyl-3,14-dihydroangustoline, 3-ketoquinovic acid, O-acetyl-angustoline, naucleidinic acid, 3,14-dihydro-angustine and nauclequiniine (Zeches et al. 1985; Anam 1997; Kuete et al. 2015; Agnaniet et al. 2016). Our previous work has shown that extracts of N. pobeguinii have gastro-protective and healing effects, which could result from their anti-secretory, anti-acid activities or by stimulating proliferation and migration of epithelial cells which may have a cytoprotective effect by stimulating the prostaglandins production (unpublished work). However, there are no reports on the healing and anti-Helicobacter pylori activities of aqueous and methanol extracts of N. pobeguinii against established ulcers, as well as the mechanisms underlying its different activities. Thus, the present study was carried out to evaluate the gastro-protective mechanisms and the anti-Helicobacter pylori activities of aqueous and methanol extracts of N. pobeguinii.
Material and methods
Plant material and extraction
The barks, leaves, flowers and fruits of N. pobeguinii were harvested (August 2019) in the locality of Mbalmayo (department of Nyon and So'o, Center region, Cameroon) in order to carry out it identification. This identification was made in comparison with a sample referenced (n°11,367 (28,335/SRFcam)) at the National Herbarium of Cameroon (Yaoundé). Then, the harvested bark underwent in turn a cutting (thin piece) and a shade-drying, then were grind using a grinder into a fine powder. Part of the powder (500 g) was poured into a container with 5 litters of distilled water and boiled (20 min). After cooling, the mixture was filtered (filter papers No. 3 and No. 2), the filtrate obtained was dried in an oven (40 °C), 24 g of aqueous extract was obtained, i.e. 4.8% yield. Subsequently, the rest of the powder (500 g) was mixed with 5 litters of methanol in a hermetically sealed container, this mixture was macerated every day for 3 days, then filtered (filter papers n° 3 and n° 2) and evaporated under reduced pressure (170–180 mbar) and at a temperature of 70 °C; 41 g of methanol extract were obtained for a yield of 8.2%.
Quantitative phytochemical
Determination of phenolic constituents
The total amount of phenols present within the extracts was determined using Folin Ciocalteu’s reagent. In carrying out this study, gallic acid was used as our standard alongside total phenols which are expressed as mg/g of gallic acid equivalents (GAE). 0.01, 0.02, 0.03, 0.04 and 0.05 mg/mL of extracts were concentrated with gallic acid and prepared using methanol. After preparation, concentrations of 0.1 and 1 mg/mL of extract were also prepared using methanol. 0.5 mL from each of the samples was introduced in test tubes and mixed with 2.5 mL of a Folin Ciocalteu reagent which was diluted 10 times alongside 2 mL of a solution of 7.5 mg of gallic acid and 2 mL of 7.5% sodium carbonate. All the tubes were covered and placed on a stand for 30 min at room temperature, after which the absorbance was read using a spectrophotometer at 760 nm. As the Folin-Ciocalteu reagent is sensitive to reducing compounds, including polyphenols, it produced blue colour during the reaction (Savitree et al. 2004).
Determination of total flavonoids
For the measurement of total flavonoids in each extract, the method proposed by Zhishen et al. (1999) was used. For this purpose, 1 mL of each sample was mixed with 4 mL of distilled water and 0.3 mL of NaNO2 solution (10%). Five minutes later, 0.3 mL of AlCl3 solution (10%) was added into the mixture followed by 2 mL of NaOH solution (1%). Absorbance was read at 510 nm relative to blank.
In vivo tests
Reagents, chemicals, and equipment
Omeprazole®, Misoprostol®, Cimetidine®, Formaldehyde and estradiol valerate were obtained at a local pharmacy; while Sigma Chemicals Co. (St. Louis, MO, USA) made it possible to obtain acetic acid, ethanol, methanol, cysteine, histamine acid phosphate. NaOCl, glycerol, acetylcholine, Skirrow supplement, DMSO, NAOH and NaCl from Merck Chemicals, Darmstadt, Germany. All other used chemicals and reagents were of analytical grade.
Animals
Rats female Wistar rats (12 to 16 weeks, 170 to 200 g) from the animal facility of the Department of Animal Biology of the Faculty of Sciences of the University of Dschang were used in this study. They were fed with a standard rodent formula pellet, with free access to water. The use of these animals complied with the directives of the Ethics Committee of the Ministry of Scientific Research and Technology of Cameroon on the handling and preservation of Cameroonian flora and fauna according to the strict guidelines imposed by the European Union on Animal Care and Experimentation (Council CEC 86/609).
Ulcers induced by acetic acid
The method of induction of ulcers by acetic acid proposed by Shahrokhi et al. (2015) was used for this test. Briefly, 54 intact female rats starved for 24 h were anesthetized with Diazepam/Ketamine (2/1 v/v), then 48 rats underwent laparotomy, while 06 remaining animals were used as Sham-operated control. Then acetic acid (30%, 0.05 ml) was injected (sub serosa in the glandular part of the anterior wall of the stomach) in the animals having undergone laparotomy. Then, the outer surface of the stomach was washed with NaCl solution (0.9%) to prevent its adhesion with other neighbouring organs. Twenty-four (24) hours after administration of acetic acid, animals were treated once daily for 14 days.
Group 1 was used as Sham-operated and received distilled water with no ulcer; group 2 considered as negative control received distilled water; group 3 (positive control) treated with omeprazole (20 mg/kg); group 4 (100 mg/kg), group 5 (200 mg/kg) and group 6 (400 mg.kg) treated with the aqueous extract of N. pobeguinii; groups 7 (100 mg/kg), group 8 (200 mg/kg) and group 9 (400 mg/kg) treated with the methanolic extract of N. pobeguinii. On the fifteenth day, all the animals were sacrificed. The blood was collected by catheterization of the abdominal artery in tubes containing sodium heparin for a blood count; while the stomach was used to measure the production of mucus and to evaluate some ulceration parameters (ulceration area SU, ulceration percentages %SU, ulceration index, IU and percentage of healing % I). The glandular part of the stomach of each rat was divided into two: one part kept in 10% formaldehyde and used for the analysis of histological sections and the other part kept in a refrigerator at 20 °C and used for the determination of markers of oxidative stress (SOD, CAT, MDA) and inflammation (NO). In order to evaluate the antisecretory, cytoprotective and estrogenic properties of the extract, the intermediate effective dose for the previous test was used for further testing.
Pylorus-ligature ulcer model
After 24 h starvation, the animals received the different test substances following the protocol of Maheswari et al. (2020). Group 1 (negative control) received distilled water; group 2 (positive control) treated with omeprazole (20 mg/kg); group 3 (200 mg/kg) received aqueous extract of N. pobeguinii; group 4 (200 mg/kg) received methanol extract of N. pobeguinii. One hour after administration of the different test substances, a laparotomy was performed under anaesthesia (ethyl ether) and the pylorus of each rat was ligated with a cotton thread. Then, the external surface of the stomach was soaked by a NaCl solution (0.9%) to maintain the cells in a medium isotonic to that of the body. The incised abdomen was sutured with cotton thread and a needle. Six hours later, all rats were sacrificed by inhalation of ethyl ether in a tightly closed tank. The cardias were sectioned after opening the abdominal cavity of the animals and the stomach was isolated. The gastric juice was collected in the dry tubes and centrifuged at 3000 rpm for 10 min. The supernatant was collected for determination of gastric volume and gastric pH. Then, the stomach was opened following the great curvature for evaluation of gastric lesions and mucus collection.
Pylorus ligature/histamine ulcer model
For this test, the protocol of Amang et al. (2014) was used. The animals were divided as in the previous test. Here, cimetidine (50 mg/kg) was used as the reference substance. One hour after administration of the different test substances, the pylorus was ligated. One hour later, they received histamine intra-peritoneally at a dose of 2.5 mg/kg and two hours afterwards, all rats were sacrificed as previously described. The cardias were sectioned after opening the abdominal cavity of the animals and the stomach was isolated. The gastric juice and stomach of the animals underwent the same processing as previously described and the same ulceration parameters were evaluated.
Pylorus ligature/acetylcholine ulcer model
After starving the animals for 24 h, they were divided as previously described. In this test, Atropine sulphate (0.1 mg/kg) was used as the reference substance. One hour after administration of the different test substances, the pylorus was ligated as previously described and one hour afterwards, these animals received acetylcholine intraperitoneally at a dose of 1 mg/kg. Two hours later, all rats were sacrificed following the previously described method. The cardias were sectioned following the opening of the abdominal cavity of the animals and the stomach was isolated. The gastric juice and stomach of the animals underwent the same processing as previously described and the same ulceration parameters were evaluated.
Ethanol ulcer model on ovariectomized rats
The evaluation of the estrogenic actions of the aqueous and methanol extracts of N. pobeguinii was done on 30 rats. Twenty-four (24) of them underwent a bilateral ovariectomy while 6 were used as Sham operated under anaesthesia (Mvondo et al. 2012). Two weeks after this operation, the animals received the different test substances. Group 1 (non OVX/distilled water) was used as Sham-operated and received distilled water; group 2 (OVX/distilled water) was considered as negative control and received distilled water; group 3 (OVX/estradiol) positive control received E2V; group 4 (OVX/aqueous extract (200 mg/kg)) and group 5 (OVX/methanol extract (200 mg/kg)) received the extracts. All the treatments were administered once a day during 3 days. On the fourth day, all the groups received 60% ethanol and then one hour later they were sacrificed. The parameters of ulceration, the mass of mucus produced and the mass of the uteri were evaluated.
In vitro tests
Evaluation of the anti-urease activity of N. pobeguinii
For this test, a mixture of (25 μL of enzymatic solution (jack bean urease), 55 μL of buffers (100 mM urea), 5 μL of the different extracts (50 μg/ml)) was incubated (30 °C, 15 min) in 96-well plates. The indophenol method, which measures the production of ammonia, made it possible to determine the activity of urease (Messia et al. 2005). Into each well, 45 µL of phenol reagent containing phenol (1%, w/v) and sodium nitroprusside (0.005%, w/v) were added, then 70 µL of alkaline reagent containing NAOH (0.5%, w/v) and active NaOCl chloride (0.1%). After 50 min, a microplate reader (Molecular Device) set at 630 nm was used to read the absorbance at pH 6.8. The formula 100-(ODtest/ODcontrol) × 100 was used to calculate percent inhibition. The reference substance used in this test was thiourea.
Anti-H. pylori activity
Bacterial strains
In patients, with recurrent peptic ulcers, treated with metronidazole and amoxicillin and undergoing endoscopy at Dr. Panjwani’s Centre for Molecular Medicine and Drug at Research, International Centre for Chemical and Biological Sciences, 15 strains of H. pylori were been isolated from their gastric biopsies. The procedure was performed following Protocol No: ICCBS/IEC-015-BC-2019/Protocol/2.1 granted by Independent Ethics Committee, ICCBS, University of Karachi. The donors then gave a verbal agreement for the use of their biopsy for this study. The NCTC 11638 strain was used as a standard and the method of Tanih et al. (2010) was used to isolate and identify the samples. Biopsies were homogenized in a mixture of cysteine (0.2 g/L) and glycerol (20%) in BHI (Brain Heart Infusion Broth, Oxoid, England). A mixture of horse blood (6%) and a Skirrow supplement ((Oxoid, England) (2.5 mg of trimethoprim, 2.5 mg of cefulodin, 5 mg of vancomycin, 2.5 mg of amphotericin) was added to a freshly prepared Columbia agar base (Oxoid, England), and together allowed a small amount of the homogenate to be plated. After inoculation of plates, they were incubated (37 °C, 5 days) in microaerophilic conditions (5–6% O2, 10% CO2 and 80–85% N2) (Anaerocult Basingstoke, Hampshire, England); then the identification of the different isolates was made according to the morphology of the colonies, the urease, the amplification of the glmM gene, the positive tests for oxidase and catalase. The confirmed isolates were introduced in eppendorf tubes containing a mixture of BHI broth (1 mL) and glycerol (20%) and stored (-80 °C). Only biopsies from patients who did not take antibiotics and/or bismuth salts (for at least one week) and who gave their consent were used.
Anti-H. pylori test of N. pobeguinii
The agar well diffusion method described by Boyanova et al. (2005) was used for the realization of this test. On a BHI agar increased with horse blood (5%) and Skirrow supplement (Oxoid, England), the H. pylori inoculate prepared according to the McFarland Turbidity Standard 2 protocol were spread, then uniformly distributed on the plate which was left to dry (15 min). A sterile borer (stainless steel) was used to drill 6 mL (diameter) wells in the agar, then these wells were filled with extract (65 µL, 100 mg/kg), the positive and negative control wells received respectively clarithromycin (65 µL, 0.05 µg/mL) and DMSO (65 µL, 10%), then the plates were incubated (37 °C, 72 h) under microaerophilic conditions (Anaerocult, Oxoid, UK) and the diameters of the zones of inhibition (in mm) were determined. The breakpoint of sensitivity for extracts and clarithromycin, and then for the calculation of the percentage of sensitivity was a surface diameter ≥ 14 mm (Ndip et al. 2008).
Determination of the minimum inhibitory concentration (MIC90)
To further determine the MIC, the dilution (micro-broth) method of Bonacorsi et al (2009) was applied to strains sensitive to aqueous extracts and methanol using 96-well plates. The extract concentrations (5.0 mg/kg) were filtered using a filter (2.0 µm, Acrodisc Pall, MI, USA). A broth of BHI increased with horse serum (5%) and Skirrow supplement (Oxoid, England) introduced into the wells allowed a double dilution of the extracts to give a concentration varying from 0.001 to 5.0 mg/kg for each extract. Culture medium (100 µL) with each extract received 20 µL of H. pylori broth culture (18 h old, McFarland turbidity standard). Culture medium, broth and bacterial suspension were mixed for the negative control well; while amoxicillin and metronidazole were used at concentrations of 0.001–1.25 mg/mL and 0.005–5.0 mg/mL, respectively. The plates were incubated (37 °C, 72 h, microaerophilic) and their absorbance at 620 nm was read using an ELISA automatic microplate reader (Tokyo, Japan). The decrease or increase in bacterial growth was determined by comparing the initial absorbance and the post-inoculation absorbance. The MIC was recorded for the lowest concentrations of the extracts that caused 90% inhibition. (Ndip et al. 2008).
Statistical analyses
After analyzing the results using ANOVA one-way followed by Tukey's post test using R.3.5.0 software; the p < 0.05 threshold was used for statistical significance. The experimental results are given as the mean ± SEM.
Results and discussion
Quantitative analysis of extracts
Table 1 presents the results of the quantitative phytochemical content of each plant extract. It shows that in the aqueous extract, phenolic and flavonoids represent respectively 13.13 mg gallic acid and 3.45 mg quercetin equivalents/g; while in the methanol extract the phenol and flavonoid represent respectively 17.85 mg gallic acid and 3.36 mg quercetin equivalents/g.
Gastric ulcers arise due to imbalance between the aggressive (acid, pepsin) and defensive (blood flow, mucus, bicarbonate) factors of the gastric mucosa (Adediran et al. 2017). The study aimed to evaluate the in vivo healing, antisecretory, cytoprotective and estrogenic activities, and the in vitro anti-H. pylori effect of aqueous and methanolic extracts of N. pobeguinii on gastric ulcers using three different methods of experimental induction of gastric ulcers (acetic acid, pyloric ligation alone and ethanol).
In vivo tests
Effects of N. pobeguinii on acetic acid-induced gastric ulcers
The healing effects of extracts of N. pobeguinii were evaluated by measuring the gastric lesions (Fig. 1). The aqueous (400 mg/kg) and methanolic (200 mg/kg) extracts caused maximum healing percentages of 99.90% and 98.47%, while for omeprazole was 68.46%. Females with intact ovaries showed greater benefit in terms of ulceration index reduction after 14 days of treatment with N. pobeguinii extracts or with omeprazole (Fig. 2). In females with intact ovaries, omeprazole, aqueous extract (100, 200 and 400 mg/kg) and methanol extract (400 mg/kg) significantly (p < 0.001) reduced white blood cell counts. Lymphocyte levels were significantly reduced in all groups except animals treated with the methanol extract (400 mg/kg). The aqueous extract (200 and 400 mg/kg) and the methanol extract (100 mg/kg) were able to significantly reduce (p < 0.001) the levels of monocytes (Table 2). Acetic acid is a source of stress which leads to an increase in the amount of acid in the stomach as well as the release of inflammatory pro-12 cells (Tan et al. 2013). Clinically, an increase in the number of lymphocytes and monocytes in the blood is associated with chronic inflammatory states. The decrease in these markers of inflammation (monocytes and lymphocytes) would be due to the anti-inflammatory properties induced by the flavonoids contained in these different extracts (Maleki et al. 2019). The extracts significantly reduced NO levels, confirming their anti-inflammatory potential.
Administration of the aqueous and methanolic extracts resulted in a reduction in NO and MDA levels; furthermore, SOD and catalase levels increased after 14 days of treatment (Fig. 3). Rats that were not subjected to induction of gastric ulcer with acetic acid (Sham control) showed a healthy gastric wall consisting of the mucosa, submucosa, muscularis mucosa and serosa from top to bottom (Fig. 4); while those subjected to gastric ulcer induction by acetic acid gavage and did not received any treatment showed gastric wall with destruction of mucosal epithelium, tissue necrosis, craters and edema. After treatment of the animals with different doses of extracts, there was almost complete healing with significant reconstitution of the mucosal epithelium and no edema (Fig. 4). Both extracts demonstrated their antioxidant potential by decreasing the level of MDA and increasing the activities of CAT and SOD. These results are in agreement with the work of Kadiri et al. (2007) who showed the inhibitory activity of N. pobeguinii on lipid peroxidation. Classes of chemical compounds such as alkaloids, flavonoids, triterpenes, phenols and tannins are known for their antioxidant activity and are generally associated with the protection of the gastric mucosa (Mahmoud and Abd El-Ghffar 2019); their presence in our extracts would justify the healing and antioxidant capacity of N. pobeguinii. Moreover, N. pobeguinii in addition to reducing ulceration parameters also attenuates and repairs the loss of substance on the gastric mucosa. This indicates that its anti-inflammatory and antioxidant properties help maintain gastric integrity.
Effects of 14 days treatment of aqueous and methanolic extracts of N. pobeguinii stem bark on the levels of Malondialdehyde (MDA), superoxide dismutase (SOD), Catalase and nitric oxide (NO) in rats ulcerated by acetic acid. βp < 0.01, γp < 0.001 compare with Sham; bp < 0. 01, cp < 0.001 compare with distilled water (DW)
Histological study of acetic acid-induced gastric ulcer in rats (H&E: × 400) after treatment with aqueous and methanolic extracts of Nauclea pobeguinii: 1: mucosa, 2: submucosa, 3: muscularis externa, 4: serosa, D: glandular damage, C: crater, E: Edema, I: leucocyte inflammation, N: Necrotic lesion. SHAM: there is no disruption to the surface of epithelium with neither edema nor leucocytes infiltration of the submucosal layer; distilled water: There is severe disruption to the surface epithelium and necrotic lesions penetrate deeply into mucosa till muscularis externa with severe inflammation; omeprazole: There is ulceration till muscularis mucosa with glandular damage. They showed improved histological appearance compared to ulcer control rats with less glandular damage and healing of ulcerated portion
Effects of N. pobeguinii on the ligated pyloric ulcer model
After 6 h, ligation of the pylorus caused acid stasis in the stomach due to hydrochloric acid and pepsin which damages the stomach wall (Fig. 5). The extracts showed inhibition rates of ulcerated surfaces of 99.68 mg/kg (aqueous extract) and 99.33% (methanolic extract). Both extracts significantly reduced areas of ulceration, significantly (p < 0.001) increased mucus production, decreased gastric supernatant volume and gastric pH (Fig. 6).
Effects of aqueous and methanolic extracts of N. pobeguinii stem bark on ulceration areas, mucus weight, ulceration index, gastric pH and gastric content after induction of acute ulceration by pyloric ligation. Each bar represents the mean ± SEM, n = 6. ap < 0.05; cp < 0.001 compare with distilled water
Gastric mucosal damage following pyloric ligation results from gastric acid stasis and conversion of pepsinogen to pepsin with consequent accumulation of acid resulting in self-digestion via pepsin (Matah et al. 2020). On the other hand, this method activates the vagal reflex by activating the mechanoreceptors present in the antral mucosa and stimulates acid secretion through the nervous, endocrine and paracrine pathways (Prazeres et al. 2019). Extracts as well as omeprazole significantly relieve ulcers. This inhibition of ulcerated surfaces was associated with increased mucus weight with both extracts. This increase in mucus weight suggests that the extracts improve mucus production. Moreover, the fact that the aqueous extract significantly increases the amount of mucus produced suggests that this extract acts by stimulating the estrogen pathway, since this hormone is strongly involved in the production of gastric mucus (Milena et al. 2008). Extracts with activity similar to that of omeprazole suggest that the compounds present in this plant exert their cytoprotective activity by stimulating the production of mucus and estrogen; their antisecretory activity by inhibiting and neutralizing the diffusion of H+ ions from the proton pump, since tannins are known to have cytoprotective and anti-secretory activities, and are able to inhibit the H+/K+ pump (Demarque et al. 2018). Thus, the anti-secretory and cytoprotective activities of N. pobeguinii extracts could be linked to the presence of tannins.
Administration of acetylcholine one hour after pyloric ligation produced ulcerative lesions (Fig. 7). The treated rat showed a reduction in the ulcerated surface and a reduction in the volume of gastric supernatant (Fig. 8). Figure 9 shows lesions formed following histamine administration one hour after pyloric ligation. The aqueous and methanolic extracts (200 mg/kg) of N. pobeguinii protected the stomach from the attacks of the pyloric ligation/histamine association. The extracts decreased the ulceration index and significantly decreased the volume of gastric supernatant (Fig. 10).
Effects of aqueous and methanolic extracts of N. pobeguinii stem bark on ulceration areas, mucus weight, ulceration index, gastric pH and gastric content after induction of acute ulceration by pyloric ligation with acetylcholine. Each bar represents the mean ± SEM, n = 6. ap < 0.05; bp < 0.01; cp < 0.001 compare with distilled water
Effects of aqueous and methanolic extracts of N. pobeguinii stem bark on ulceration areas, mucus weight, ulceration index, gastric pH and gastric content after induction of acute ulceration by pyloric ligation with histamine. Each bar represents the mean ± SEM, n = 6. ap < 0.05, cp < 0.001 compare with distilled water
Basically, hydrochloric acid secretion is stimulated by three chemicals: acetylcholine (nerve pathway), gastrin (endocrine pathway), and histamine (paracrine pathway). Histamine binds to its G protein-coupled H2 receptors, causing increased intracellular calcium levels and activation of adenylyl cyclase for phosphorylation of ATP to cAMP. cAMP modulates the activity of protein kinases A which act on the H + /K+-ATPase pump responsible for acid secretion. Acetylcholine and gastrin stimulate acid and pepsinogen secretion by increasing intracellular calcium concentration (Amang et al. 2014). In the case of acetylcholine, both extracts significantly reduced various ulceration parameters, as did atropine sulphate. Quantitative analysis of N. pobeguinii extracts revealed that the plant was rich in polyphenols and that these extracts contain alkaloids (Tsafack et al. 2020), which are muscarinic M3 receptor antagonists (Silva et al. 2015; Fowler 2015); thus, the antisecretory activity of N. pobeguinii extracts in this model of pyloric ligation associated with acetylcholine could be due to the inhibition of type 3 muscarinic receptor activity. In the case of histamine, the cimetidine, an H2 receptor antagonist, used in this study as a standard, reduces the acidity and volume of gastric secretions by inhibiting the activation of adenylyl cyclase, thereby blocking the formation of cyclic AMP necessary for the production of HCI. Our results also showed that the extracts act like cimetidine in reducing secretion and volume of secretions in the stomach by inhibiting the histamine pathway. It is known that flavonoids are able to inhibit the activity of histidine decarboxylase, thus reducing the effect of histamine on the proton pump; thus, the anti-histaminic and anti-secretory activities of our extracts would thus be linked to the presence of flavonoids. In addition, the aqueous extract induced an increase in mucus mass which was significant, which could suggest that this extract may act by binding to ER-alpha estrogen receptors present in the stomach.
Effects of N. pobeguinii on ethanol-induced gastric ulcers in ovariectomized rats
Ethanol administration resulted in the formation of lesions with the appearance of elongated bands (Fig. 11). The effects of extracts and estrogens on ethanol-induced gastric lesions are shown in Table 3. The extracts produced percentage inhibition of ulcerations of 79.49% (aqueous extract) and 81.73% methanolic), while the Sham-operated and estrogen groups protected by 57.34% and 47.78%, respectively. The aqueous and methanolic extracts induced a significant decrease in the severity of ulcerations, a significant increase (p < 0.001) in the mass of mucus; only the aqueous extract caused an increase in uterine weight (Fig. 12), similar to that obtained with estradiol (p < 0.001).
Effects of aqueous and methanolic extracts from the bark of Nauclea pobeguinii and estradiol (E2V) on the relative weight of uterus in ovariectomized rats. Results are expressed mean ± SEM. Statistical significance was determined using one-way ANOVA followed by Tukey’s test. λp < 0.001 compare with Sham; bp < 0.01 compare with distilled water (DW)
Indeed, the gastric mucosa contains estradiol ER-alpha receptors present in the antrum and the gastric body, estradiol is known to be involved in the production of mucus (Milena et al. 2008), thus protecting the mucosa gastric. Ethanol exerts its direct toxic effect on the stomach wall through an ulcerogenic effect (Bassant et al. 2016), destroying the defense system of the gastric mucosa, which causes mucosal damage and can lead to necrosis (Kandhasamy and Nyuk 2014). It is known that 14 days after ovariectomy in laboratory animals, there is a decrease in endogenous estrogen levels which leads to atrophy of the uterus (Zemo et al. 2017). Our results showed that the uterine weight of ovariectomized and untreated control animals decreased compared to the Sham group of females with intact ovaries. After the 3-day treatment with the extracts and E2V, only the aqueous extract showed similar effects to E2V in causing an increase in uterine mass. These effects are known to be dependent on ER-alpha receptors and result from phenomena of water imbibition (Hewitt and Korach 2003) and/or cell proliferation (Mvondo et al. 2012; Zingue et al. 2016) in the uterus and vagina. The results also showed that E2V significantly reduced the ulcerated surfaces induced by ethanol. This result is almost similar to that of the Sham control of females with intact ovaries. Both N. pobeguinii extracts significantly reduced ulcerated surfaces demonstrating their cytoprotective activity. This result was accompanied by a significant increase in mucus weight in animals receiving the aqueous extract. Taken together, these results show that the aqueous extract of N. pobeguinii is endowed with estrogenic and cytoprotective properties, suggesting that this extract contains phenolic compounds that interact with estrogen ER-alpha receptors present both in the uterus and the gastric body. This interaction is responsible for the increase in uterine mass and mucus (Kamgaing et al. 2020).
In vitro tests
Anti-Helicobacter pylori effects of extracts
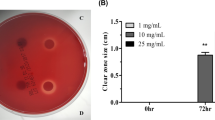
Anti-H. pylori of the two extracts on the 16 isolates of H. pylori lead to an increase in the diameters of the zone of inhibition ranging from 12 to 39 mm for the aqueous extract and from 8 to 36 mm for the methanolic extract (Table 4). The extracts recorded a larger mean zone diameter than clarithromycin (12.98 ± 4.87 mm). Figure 13 shows the percent sensitivity (> 50%) of H. pylori isolates to both extracts (Fig. 14). The aqueous extract of N. pbeguinii showed MICs between 1.11 and 2.5 mg/mL and the methanol extract gave MICs ranging from 2.2 to 6.65 mg/mL (Table 5). MIC values for amoxicillin and metronidazole ranged from 0.001 to 6 mg/mL. Aqueous and methanol extracts, such as thiourea, showed significant anti-urease activity. Indeed, the percentages of inhibition obtained for the aqueous extract, the methanol extract and the thiourea were respectively 90.00%, 73.33% and 94.33% (Fig. 13).
Generally, the antibacterial activity of a plant extract is considered significant when the MICs are below 100 g/mL, moderate (between 100 and 625 g/mL) and low (above 625 g/mL) (Kuete and Efferth 2010). The aqueous extract showed MICs below 1.24 mg/mL; this antibacterial activity would be due to the presence of classes of compounds such as flavonoids, tannins and terpenoids. However, it is known that flavonoids are synthesized by plants in response to a bacterial infection and are known for their anti-H pylori properties and their inhibitory activity on urease (Cushnie and Lamb 2011). However, the activities of our extracts, and particularly those of the aqueous extract, should not be neglected. Indeed, the antibiotics used in this experiment are in their purified form, while the extracts are a hodgepodge of pharmacological and non-pharmacological substances (Kouitcheu et al. 2017). In addition, extracts with little or no activity in vitro could have properties similar to those of pro-drugs whose active principles only act in vivo. The main virulence factors of this bacterium are Cag A, Vac A and urease. Through its lipase and protease activities, urease destroys the mucosa resulting in diaper inflammation or gastritis. This urease also converts urea into CO2 and ammonia which buffer the environment around the bacteria. Urease inhibition is one of the important factors for the eradication of H. pylori. Our extracts and in particular the aqueous extract inhibited urease activity. This anti-urease activity could be due to the presence of flavonoids in our extracts (Cushnie and Lamb 2011) and is an asset for the eradication of H. pylori infection.
Conclusion
The results of our work show that the extracts of N. pobeguinii have gastroprotective, estrogenic and cytoprotective properties, which would be due to the presence of many secondary metabolites present in plants. Moreover, these extracts and particularly the aqueous extract of N. pobeguinii possess metabolites with strong antimicrobial activity which would be useful against H. pylori infection. However, further in vivo evaluation of anti-H. pylori from this extract should be performed to confirm these results.
Data availability
All data supporting our findings are adequately contained within the manuscript.
References
Adinortey B, Charles A, Isaac G, Alexander N (2013) In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers 2013:1–12. https://doi.org/10.1155/2013/796405
Agnaniet H, Mbot EJ, Keita O, Fehrentz JA, Ankli A, Gallud A, Garcia M, Gary-Bobo M, Lebibi J, CresteilMenut TC (2016) Antidiabetic potential of two medicinal plants used in Gabonese folk medicine. BMC Complement Altern Med 16:71. https://doi.org/10.1186/s12906-016-1052-x
Amang PA, Tan PV, Patamaken SA, Mefe MN (2014) Cytoprotective and antioxidant effects of Eremomastax speciosa in rats. Afr J Tradit Complement Altern Med 11:165–171. https://doi.org/10.4314/ajtcam.v11i1.26
Anam EM (1997) Novel nauclequinine from the root extract of Nauclea pobeguinii (Pob. & Pellegr) Petit (Rubiaceae). Indian J Chem Sect B Org Chem Med Chem 36:54. https://doi.org/10.1002/chin.199740266
Ashokan K, Kurane M, Pillai M (2010) Effect of ovariectomy and of estrogen administration upon duodenal ulceration induced cysteamine. IUFS J Biol 69:7–16
Ateufack G, Mokam DEC, Mbiantcha M, Dongmo Feudjio RB, David N, Kamanyi A (2015) Gastroprotective and ulcer healing effects of Piptadeniastrum africanum on experimentally induced gastric ulcers in rats. BMC Complement Altern Med 15:1–10. https://doi.org/10.1186/s12906-015-0713-5
Bali A (2016) Drug treatment of peptic ulcer disease. Peptic ulcer disease 1–19.
Bassant MMI, Abeer AAS, Heba MIA, Sally AEA, Nermeen MS (2016) Study of the protective effects of flaxseed oil on ethanol induced gastric mucosal lesions in non-ovariectomized and ovariectomized rats. Int J Pharmacol 12:329–339. https://doi.org/10.3923/ijp.2016.329.339
Bonacorsi C, Maria Stella G, Raddi IZ, Carlos MS, Vilegas W (2009) Anti-Helicobacter pylori pylori activity and immunostimulatory effect of extracts from Byrsonima crassa Nied. (Malpighiaceae). BMC Complement Altern Med. https://doi.org/10.1186/1472-6882-9-2
Boyanova L, Gergova G, Nikolov R, Derejian S, Lazarova E, Katsarov N, Mitov I, Krastev Z (2005) Activity of Bulgarian propolis against 94 Helicobacter pylori strains in vitro by agar-well diffusion, agar dilution and disc diffusion methods. J Med Microbiol 54:481–483. https://doi.org/10.1099/jmm.0.45880-0
Cushnie T, Lamb J (2011) Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Agents 38:99–197. https://doi.org/10.1016/j.ijantimicag.2011.02.014
Demarque DP, Callejon DR, de Oliveira GG, Silva DB, Carollo CA, Lopes NP (2018) The role of tannins as antiulcer agents: a fluorescence-imaging based study. Rev Bras Farmacogn 28:425–432. https://doi.org/10.1016/j.bjp.2018.03.011
Eloumou Bagnaka SAF, Luma Namme H, Noah Noah D, Essomba NE, Malongue A, Manga A, Tzeuton C, Biwole Sida M (2016) Facteurs de risques associés aux lésions gastroduodénales dans un Hôpital de référence de Douala (Cameroun). Med Sante Trop 26(1):12–15. https://doi.org/10.1684/mst.2015.0521
Fowler C (2015) Plant sources of antimuscarinics. BJU Int 115:4–7. https://doi.org/10.1111/bju.13074
Hewitt S, Korach K (2003) Estrogen receptor knockout mice: roles for estrogen receptors alpha and beta in reproductive tissues. Reproduction 125:143–149. https://doi.org/10.1530/rep.0.1250143
Kadiri H, Adegor E, Asagba S (2007) Effects of aqueous Nauclea pobeguinii leaf extracts on rat induced with hepatic injury. Res J Med Plant 1:139–143. https://doi.org/10.3923/rjmp.2007.139.143
Kamgaing WMT, Mvondo MA, Poualeu KSL, Minko ES, Wansi NSL (2020) The aqueous extract of Dacryodes edulis (Burseraceae) leaves inhibits cell proliferation induced by estradiol on the uterus and vagina of ovariectomized female wistar rats. Adv Pharmacol Sci 2020:1–11. https://doi.org/10.1155/2020/8869281
Kandhasamy S, Nyuk L (2014) Antioxidant and anti-ulcer effects of ethyl acetate fractions of Merremia tridentata (L.) Hallier F. root. Agric Agric Sci Procedia 2:406–414. https://doi.org/10.1016/j.aaspro.2014.11.057
Kangwan N, Park J, Kim E et al (2014) Quality of healing of gastric ulcers: natural products beyond acid suppression. World J Gastroenterol 5:40–47. https://doi.org/10.4291/wjgp.v5.i1.40
Kouitcheu MLB, Nanfack NB, Eyoum BB, Tchuenteu TR, Nguepi E (2017) Anti- Helicobacter pylori and antiulcerogenic activity of Aframomum pruinosum seeds on indomethacin-induced gastric ulcer in rats. J Pharma Biol. https://doi.org/10.1080/13880209.2017.1285326
Kuete V, Efferth T (2010) Cameroonian medicinal plants: pharmacology and derived natural products. Front Pharmacol 1(123):1–19. https://doi.org/10.3389/fphar.2010.00123
Kuete V, Sandjo LP, Mbaveng AT, Seukep JA, Ngadjui BT, Efferth T (2015) Cytotoxicity of selected Cameroonian medicinal plants and Nauclea pobeguinii towards multi-factorial drug-resistant cancer cells. BMC Complement Altern Med 15:309. https://doi.org/10.1186/s12906-015-0841-y
Kumral ZNO, Memi G, Ercan F, Yeğen BC (2014) Estrogen alleviates acetic-acid-induced gastric or colonic damage via both ERα- and ERβ- mediated and direct antioxidant mechanisms in rats. Inflammation 37:694–706. https://doi.org/10.1007/s10753-013-9786-9
Maheswari B, Rajyalakshmi Devi P, Ajith K, VedPrakash P, SeshaSai Gayatri K (2020) Evaluation of antiulcer activity of ethanol extract leaves of Lactuca Sativa. J Drug Deliv Ther 10:196–199. https://doi.org/10.22270/jddt.v10i4.4190
Mahmoud I, Abd El-Ghffar A (2019) Spirulina ameliorates aspirin-induced gastric ulcers in albimo mice by alleviating oxidative stress and inflammation. Biomed Pharmacother 109:314–321. https://doi.org/10.1016/j.biopha.2018.10.118
Maleki S, Crespo J, Cabanillas B (2019) Anti-inflammatory effects of flavonoids. Food Chem 299:124–125. https://doi.org/10.1016/j.foodchem.2019.125124
Matah Marthe VM, Ateufack G, Mbiantcha M, Yousseu NW, Atsamo AD, Adjouzem FC, Djuichou Nguemnang SF, Tsafack EG, Tadjoua TH, Emakoua J (2020) Cytoprotective and antisecretory properties of Distemonanthus Benthamianus (Caesalpiniaceae) stem bark on acute gastric ulcer in rats. J Altern Complement Med 2020:1–14. https://doi.org/10.1515/jcim-2019-0216
Mbiantcha M, Tsafack EG, Ateufack G, Nana Yousseu W, Bomba TFD, Djuichou Nguemnang SF, Mbankou NS, Wego KMT (2018) Analgesic, anti-inflammatory and anti-arthritic properties of aqueous and methanolic stem bark extracts from Nauclea pobeguinii (Rubiaceae) in rats. J Altern Complement Med 15:1–7. https://doi.org/10.1515/jcim-2017-0140
Mesia GK, Tona GL, Penge O, Lusakibanza M, Nanga TM, Cimanga RK, Apers S, Van Miert S, Totte J, Pieters L, Vlietinck AJ (2005) Anti-malaria activities and toxicities of three plants used as traditional remedies for malaria in the Democratic Republic of Congo: Croton mubango, Nauclea pobeguinii and Pyrenacantha standii. Ann Trop Med Parasitol 99:345–357. https://doi.org/10.1179/136485905X36325
Mesia K, Tona L, Mampunza MM, Ntamabyaliro N, Muanda T, Muyembe T, Musuamba T, Mets T, Cimanga K, Totté J, Pieters L, Vlietinck AJ (2012) Antimalarial efficacy of a quantified extract of Nauclea pobeguinii sterm bark on human volunteer. Planta Med 7:234–248. https://doi.org/10.1055/s-0031-1298488
Mfotie N, Munvera A, Mkounga P (2017) Phytochemical analysis with free radical scavenging nitric oxide, inhibition and antiproliferative activity of Sarcocephalus pobeguinii extract. Complement Altern Med 17:190–199. https://doi.org/10.1186/s12906-017-1712-5
Milena SS, Teresa NG, Armando GD, Guillermo RP, Ignacio CA (2008) Estrogen and progesterone isoforms expression in the stomach of Mongolian gerbils. World J Gastroenterol 14:5701–5706. https://doi.org/10.3748/wjg.14.5701
Mvondo MA, Njamen D, Tanee Fomum S, Wandji J (2012) Effects of Alpinumisoflavone and Abyssinone V-4’- methyl ether derived from Erythrina lysistemon (Fabaceae) on the genital tract of ovariectomized female wistar rat. Phytother Res 26:1029–1036. https://doi.org/10.1002/ptr.3685
Ndip RN, Malange Takang AE, Ojongokpoko JEA, Luma HN, Malongue A, Akoachere JFTK, Ndip LM, MacMillan M, Weaver LT (2008) Helicobacter pylori isolates recovered from gastric biopsies of patients with gastro-duodenal pathologies in Cameroon: current status of antibiogram. Trop Med Int Health 13:848–854. https://doi.org/10.1111/j.1365-3156.2008.02062.x
Njimoh DL, Assob JCN, Mokake SE, Nyhalah DJ, Yinda CK, Sandjon B (2015) Antimicrobial activities of a plethora of medicinal plant extracts and hydrolates against human pathogens and their potential to reverse antibiotic resistance. Int J Microbiol 2015:1–15. https://doi.org/10.1155/2015/547156
Périco LL, Rodrigues VP, Ohara R, Bueno G, Nunes VVA, Dos Santos RC, Camargo ACL, Júnior LAJ, De Andrade SF, Steimbach VMB, Da Silva LM, Da Rocha LRM, Vilegas W, Dos Santos C, Hiruma-Lima CA (2018) Sex-specific effects of Eugenia punicifolia extract on gastric ulcer healing in rats. World J Gastroenterol 24:4369–4383. https://doi.org/10.3748/wjg.v24.i38.4369
Prazeres LDKT, Aragão TP, Brito SA, Almeida CLF, Silva AD, de Paula MMF, Farias JS, Vieira LD, Damasceno BPGL, Rolim LA, Veras BO, Rocha IG, Silva Neto JC, Bittencourt MLF, Gonçalves RCR, Kitagawa RR, Wanderley AG (2019) Antioxidant and antiulcerogenic activity of the dry extract of pods of Libidibia ferrea Mart. ex. Tul. (Fabaceae). Oxid Med Cell Longev 2019:1–23. https://doi.org/10.1155/2019/1983137
Qi W, Yue SJ, Sun JH, Simpkins JW, Zhang L, Yuan D (2014) Alkaloids from the hook-bearing branch of Uncariarhynchophyllaand their neuroprotective effects against glutamate-induced HT22 cell death. J Asian Nat Prod Res 16(8):876–883. https://doi.org/10.1080/10286020.2014.918109
Sangma T, Jain S, Mediratta P (2014) Effect of ovarian sex hormones on non-steroidal anti-inflammatory drug-induced gastric lesions in female rats. Indian J Pharmacol 46:113–116. https://doi.org/10.4103/0253-7613.125191
Savitree M, Isara P, Nittaya SL, Worapan S (2004) Radical scavenging activity and total phenolic content of medicinal plants used in primary health care. J Pharm Sci 9(1):32–35
Seukep JA, Sandjo LP, Ngadjui BT, Kuete V (2016) Antibacterial activity of the extract and compounds from Nauclea pobeguinii against gram negative multi drug. BMC Complement Altern Med 16:181–193. https://doi.org/10.1186/s12906-016-1173-2
Shahrokhi N, Keshavarzi Z, Khaksari M (2015) Ulcer healing activity of Mumijo aqueous extract against acetic acid induced gastric ulcer in rats. J Pharm Bioallied Sci 7(1):56–59. https://doi.org/10.4103/0975-7406.148739
Sidahmed H, Mohd Hashim N, Mohan S, Abdelwahab S, Mohamed Elhassan Taha M, Dehghan F, Yahayu M, Mohd Hashim N, Loke MF, Vadivelu J (2015) Evidence of the gastroprotective and anti- Helicobacter pylori activities of β-mangostin isolated from Cratoxylum arborescens (vahl) blume. Dove Press Journal 10:297–313. https://doi.org/10.2147/DDDT.S80625
Sidahmed HMA, Mohan NMAS, Abdelwahab SI, Taha MME, Dehghan F, Yahayu M, Lian Ee GC, Loke MF, Vadivelu J (2016) Evidence of the gastroprotective and anti- Helicobacter pylori activities of β-mangostin isolated from Cratoxylum arborescens (vahl) blume. Drug Des Dev Ther 10:297–313. https://doi.org/10.2147/DDDT.S80625
Silva B, Molina-Fernández C, Ugalde MB, Tognarelli EI, Angel C, Campusano JM (2015) Muscarinic ACh receptors contribute to aversive olfactory learning in Drosophila. Neural Plast 2015:658918. https://doi.org/10.1155/2015/658918
Tan PV, Mezui C, Enow-Orock GE, Agbor G (2013) Antioxidant capacity, cytoprotection and healing actions of the leaf aqueous extract of Ocimum suave in rats subjected to chronic and cold-restrain stress ulcers. Ulcers 2013:1–9. https://doi.org/10.1155/2013/150780
Tanih NF, Okeleye BI, Naidoo N, Clarke AM, Mkwetshana N, Green E, Ndip LM, Ndip RN (2010) Marked susceptibility of South African Helicobacter pylori strains to ciprofloxacin and amoxicillin: clinical implication. S Afr Med J 100:49–52
Tariq SA, Ahmad MN, Obaidullah KA, Choudhary MI, Ahmad W, Ahmad M (2011) Urease inhibitors from Indigofera gerardiana Wall. J Enzyme Inhib Med Chem 26:480–484. https://doi.org/10.3109/14756366.2010.528415
Tsafack EG, Djuichou Nguemnang SF, Atsamo AD, Nana Yousseu W, Tadjoua TH, Matah MMV, Mbiantcha M, Ateufack G (2020) In vitro anti-inflammatory, anti-oxidant and in vivo anti-arthritic properties of stem bark of Nauclea pobeguinii (Rubiaceae) in rats. Asian Pac J Trop Biomed 10:65–77. https://doi.org/10.4103/2221-1691.275421
Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, Miwa H, Lim KJ, Das KM (2014) Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions and pathogenesis of gastric cancer development. World J Gastroenterol 20(18):5461–5473. https://doi.org/10.3748/wjg.v20.i18.5461
Wu HC, Tuo BG, Wu WM, Gao Y, Xu QQ, Zhao K (2008) Prevalence of peptic ulcer in dyspeptic patients and the influence of age, sex, and Helicbacter pylori infection. Dig Dis Sci 53:2650–2656. https://doi.org/10.1007/s10620-007-0177-7
Zeches M, Richard B, Gueye-M’Bahia L, Men-Olivier L, Delaude C (1985) Constituants des ecorces de racine de Nauclea pobeguinii. J Nat Prod 48:42–46. https://doi.org/10.1021/np50037a007
Zemo GF, Djiogue S, Ketcha WGJM, Seke EPF, Yonkeu TFG, Djikem TRN, Awounfack CF, Njamen D (2017) Fourteen days post-ovariectomy estrogens declines is associated with anxiogenic effects on Wistar rats. J Pharm Pharmacol 5:869–876. https://doi.org/10.17265/2328-2150/2017.12.004
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4):555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Zingue S, Tchoumtchoua J, Ntsa DM, Sandjo LP, Cisilotto J, Nde CBM, Winter E, Awounfack CF, Ndinteh DT, Clyne C, Njamen D, Halabalaki M, Creczynski-Pasa TB (2016) Estrogenic and cytotoxic potentials of compounds isolated from Millettia macrophylla Benth (Fabaceae): towards a better understanding of its underling mechanisms. BMC Complement Altern Med 16:1–17. https://doi.org/10.1186/s12906-016-1385-5
Author information
Authors and Affiliations
Contributions
MKYK, AG and MM designed the work. MKYK, AG, MM, FZNL, NAE, TEG, DNSF, ACF and MMVM conducted the work and collected and analysed the data. MKYK, AG and MM drafted the manuscript and revised it critically. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical approval
The experimental procedures have been approved by the local ethics committee and are in accordance with the guidelines for the study of pain in awake animals, published by the NIH (publication no. 85-23), “Principles of Animal Protection,” Laboratory, Study of Pain, Ministry of Scientific Research and Technology, which adopted the European Union Guidelines on Animal Care and Experimentation (EWC 86/609).
Conflict of interest
MKYK is PhD students in the Department of Animal Biology, Faculty of Science, University of Dschang, Cameroon. MM (PhD) is a senior lecturer in the Department of Animal Biology, Faculty of Science, University of Dschang, Cameroon. AG is an associate professor in the Department of Animal Biology, Faculty of Science, University of Dschang, Cameroon. FZNL, NAE, TEG, DNSF, ACF and MMVM are PhD students in the Department of Animal Biology, Faculty of Science, University of Dschang, Cameroon. The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yacine Karelle, M.K., Marius, M., Zenab Linda, F.N. et al. Gastro-protective effects and anti-Helicobacter pylori activities of the aqueous and methanol extracts of the stem-back of Nauclea pobeguinii (Rubiaceae). ADV TRADIT MED (ADTM) 24, 223–242 (2024). https://doi.org/10.1007/s13596-023-00686-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-023-00686-2