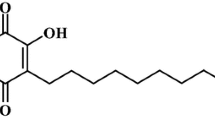
Abstract
Emergence of antiviral drug resistance in influenza virus remains a major public health concern worldwide. Nowadays, different herbs receive renewed attention because of their enormous antiviral potential. In this study, we investigated the antiviral activity of Camellia sinensis, Persicaria hydropiper, Persicaria orientale, Persicaria lapathifolia, Persicaria stagnina, Mucuna pruriens and Chenopodium album against different influenza strains using both in vitro and in silico approaches. Antiviral effect of plant extracts was evaluated by cytopathic effect (CPE) inhibition assay on influenza infected MDCK (Madin Darby Canine Kidney) cell line. Later, the herb demonstrating antiviral activity was virtually screened for their available bioactive compounds and multiple in silico tools were performed to prioritize and establish these compounds as potential inhibitor. The methanol, but not the n-hexane and ethyl acetate extracts of C. sinensis, P. hydropiper, M. pruriens and C. album exhibited anti-influenza effect with EC50 values within 32–46 µg/ml. Importantly, the extracts remained effective against both amantadine-resistant and -sensitive influenza isolates. The molecular docking analysis showed that flavonoids, steroid and derivatives had strong binding affinity to the target proteins which may remain responsible for the anti-influenza characteristics of plant extracts. Pharmacokinetic properties, bioavailability and drug-likeness score revealed that ferulic acid, sinapic acid, campesterol, cryptomeridiol, eupatin and genistein could be attractive leads as potential influenza inhibitors. Taken together, the botanical ingredients of these herbs could be used as valuable candidates for developing novel therapeutics to control influenza related illnesses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influenza (or ‘flu’) caused by influenza virus is a highly contagious respiratory illness that may cause mild to severe infections and may end to death occasionally, posing severe impact on public health and economy (Peteranderl et al. 2016; Moghadami 2017). Based on the antigenic specificity of the envelope proteins, influenza viruses are classified into influenza A, B and C, of which, the predominant type A infects both humans and other animals such as birds, horses and dogs. (Pulendran and Maddur 2014). The virus spreads rapidly from person to person by airborne droplets that attack the upper respiratory tract cells, causing typical flu symptoms like fatigue, fever, cough and body aches which may make immunocompromised and elderly people more vulnerable to potentially life-threatening secondary infections (McCullers et al. 1999; Dawood et al. 2012). Globally, more than 1 billion people become infected with influenza of which, 3 to 5 million suffer from severe illness and 300,000 to 500,000 people died each year (Naeem et al. 2020). In 2010, the estimated incidence rate of influenza associated respiratory illnesses was 6.5 and 1.3 per 1000 persons aged ˂ 5 and ≥ 5 years, respectively, resulted in an estimated economic loss of US$ 169 million in Bangladesh (Azziz-Baumgartner et al. 2012). Moreover, the estimated influenza associated mortality rate was 6 and 41 per 100 000 children < 5 years and persons > 60 years in 2010–2011, respectively which became 13 and 88 in 2011–2012 (Ahmed et al. 2018). Previously, we reported the prevalence of influenza 25.8% among children living in the slums of Dhaka city that was significantly associated with age, poor economic status, malnutrition and poor hygiene practices (Rahman et al. 2016).
Multiple approaches such as vaccines as well as antiviral drugs have been developed to minimize the burden of the influenza illnesses (Yamayoshi and Kawaoka 2019). Currently, three types of influenza vaccines: inactivated, live attenuated and recombinant are used in various countries that have several advantages as well as drawbacks (Rajão and Pérez 2018). Although World Health Organization (WHO) recommends to change the vaccine strain composition every year, antigenic mismatches between the vaccine viruses and the circulating strains often reduce the effectiveness of vaccination (Carrat and Flahault 2007; Chan et al. 2018). In addition, two classes of influenza antivirals: M2 channel inhibitor like amantadine and rimantadine, and neuraminidase inhibitor like zanamivir and oseltamivir are available at present to treat influenza infection (Betakova 2007). Among these, zanamivir and oseltamivir perform poorly against influenza in adults, while oseltamivir exhibit less effectiveness in reducing mortality among 2009A/H1N1 infected patients (Heneghan et al. 2016). Furthermore, emergence of antiviral- resistant influenza A subtypes H1N1, H1N2, H3N2, H4N2, H5N1, H5N2, H6N1, H6N6, H6N8, H9N2 and H11N3 from different regions of the world have been reported in many studies (Bright et al. 2005; Deyde et al. 2007; Cheng et al. 2009). A rapid increase of M2 inhibitors-resistant influenza A/H3N2 strain was reported between 2002 and 2007 (Bright et al. 2005; Deyde et al. 2007). Again, an elevated level of amantadine-resistant influenza was observed in South East Asia since 2007 (Barr et al. 2008). Recently, emergence of new influenza A variant have been reported that bore mutations associated with reduced susceptibility to Baloxavir marboxil, a new replication inhibiting drug against influenza (Imai et al. 2020). Thus, regular screening for new drugs against these drug-resistant influenza’s is essential.
Plant materials are the vital sources of lead compounds in modern drug development (Rajasekaran et al. 2013). For example, the extract of C. sinensis contains higher number of polyphenols that exhibit significant health protecting activity (Manzocco et al. 1998). Catechin derivatives are the major polyphenols in green tea that show antiviral effects against hepatitis C virus, human papilloma virus, rotavirus and enterovirus, influenza virus (Mukoyama et al. 1991; Song and Lee 2005; Gross et al. 2007; Chen et al. 2012). P. hydropiper is useful to mitigate inflammatory responses in treating viral infections (Ren et al. 2020). All parts of M. pruriens, widely known as “velvet bean”, possess antidiabetic, antineoplastic and antimicrobial properties (Sathiyanarayanan & Arulmozhi, 2007). C. album contains two antiviral proteins CAP-I and CAP-II that inhibited two plant viruses, Tobacco mosaic virus and Sunnhemp rosette virus (Dutt et al. 2003). However, the anti-influenza effect of these herbs yet remains vague.
In this study, we determined the antiviral activity of the different extracts of seven locally available herbs C. sinensis, P. hydropiper, P. orientale, P. lapathifolia, P. stagnina, M. pruriens and C. album against different influenza strains circulating in Bangladesh.
Materials and methods
The entire workflow is shown in Fig. 1.
Preparation of plant extracts
The plants were collected from their natural habitats from different parts of Bangladesh by an experienced plant taxonomist. The extracts of P. orientale, P. lapathifolia, P. stagnina, and P. hydropiper were prepared from whole plant, whereas leaves were used for C. sinensis, M. pruriens and C. album. The fresh plant materials were cut into small pieces, washed with tap water and allowed for sun drying in plastic boxes. The dried plant parts were crushed to prepare fine powder with mortar and pestle and kept in tightly closed containers at room temperature and away from light. For extraction, the dry powder (100 g) was soaked in 500 ml of solvent (hexane, ethyl acetate or methanol) for 2 days at room temperature. The extracts were then filtered through cotton and Whatman filter paper, transferred into a vacuum flask and dried at 45 °C under reduced pressure on a rotary evaporator. The resulting powder was stored in a tightly sealed falcon tube at room temperature. It was then weighed and dissolved in 100% DMSO to prepare stock solution and stored at 4 °C until use. To avoid the toxic effect of DMSO on cell line, the final concentration of DMSO was maintained at about 1% v/v during in vitro test.
Virus propagation
Eleven influenza strains (9 Influenza A and 2 Influenza B virus), isolated earlier from nasal and throat swab of different slums dwellers in Dhaka city (Rahman et al. 2016), were propagated on MDCK cell line as described previously (Rahman et al. 2017). Virus titration was performed by infecting MDCK cells. Viral suspension was diluted in serum free DMEM media and concentration that yielded 100% CPE by 48 h post-inoculation observed under microscope and confirmed by MTT assay was selected as optimum for antiviral assay.
Screening of anti-influenza effect
The anti-influenza effect of n-hexane, ethyl acetate and methanol extracts of herbs was determined by CPE inhibition assay against 6 influenza A/H1N1, 3 influenza A/H3N2 and 2 influenza B/Yamagata isolates. In brief, 125 μl of cell suspension (15 × 104 MDCK cells/ml in 10% FBS containing DMEM media) was seeded in each well of 96 well micro plate. When cell growth became confluent, media was removed, washed twice with PBS and 25 μl of virus inoculum was added into each well. After 1 h of incubation at 37 °C, the plate was washed twice with PBS to remove unbound viruses. Finally, 0, 10.0, 50.0, 90.0 and 120.0 μg/ml of extracts solution with DMEM containing 2% TPCK treated trypsin were added in each infected well. 25 μl of serum free DMEM media was used as control instead of virus inoculum in mock infected wells. Anti-influenza effect was determined based on survival of MDCK cells after 48 h of incubation.
MTT assay
MTT (3-(4,5- dimethyl thiazol-2yl)-2,5-diphenyl tetrazolium bromide) assay was performed to determine the EC50 value of extracts as described previously (Rahman et al. 2017). After observing CPE under light microscope, 20 μl of 5 mg/ml MTT solution (Sigma-Aldrich) was added for cell staining. After 2 h incubation at 37 °C, SDS (Promega, USA) solution was used to dissolve formazan crystals. Finally, absorbance was measured at 570 nm to determine the percentage of survivability of MDCK cells and plotted against extract concentration to determine EC50 values of extracts.
Ligand preparation
The phytochemical constituents of four extracts that were found to be effective in inhibition of influenza through in vitro experiments were retrieved from Indian Medicinal Plants, Phytochemistry and Therapeutics (IMPPAT) database (Mohanraj et al. 2018). The 3D structure of compounds was downloaded and converted into pdb (Protein Data Bank) format using OpenBabel tool integrated in PyRx (Dallakyan and Olson 2015). The Universal force field (UFF) was applied for energy minimization of ligands followed by conversion into pdbqt format for docking.
Protein preparation
The molecular structure of three target proteins was obtained from RCSB protein data bank. The PDB ID of the neuraminidase, transmembrane M2 protein and haemagglutinin protein of influenza virus were 2HU4, 6BKK and 6CF7, respectively. All the water molecules and heteroatoms including ions and bound molecules were removed from protein structure and polar hydrogen was added using Discovery Studio software v2020. The energy minimization of the cleaned structure was performed by GROMOS 43B1 forcefield in SWISS PDB viewer (Kaplan and Littlejohn 2001) and later saved in pdb format for further analysis. The active site of the target protein was predicted by Discovery Studio software v2020 and PDBsum (Laskowski 2001).
Molecular docking
The binding energy of the protein–ligand complex was calculated using PyRx Autodock Vina (Dallakyan and Olson 2015). The loaded protein structure was converted into pdbqt format and the grid box parameter was set manually to cover the active site of receptor with an exhaustiveness of 8. The maximum binding affinity was identified by observing the highest negative binding energy. Moreover, the docking procedure was validated by docking the active site of optimized protein with extracted ligand from retrieved protein structure following the same protocol (Shivanika et al. 2020). The protein ligand complex was visualized and their binding interactions were observed using PyMOL, Discovery Studio software v2020 and LigPlot + (Laskowski and Swindells 2011).
Exploration of drug-likeness properties
Compounds that had favorable docking score (< − 7.0 kcal/mol) were selected to study drug-likeness and ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) properties. The Lipinski rule is regarded as one of the most important factors for predicting a drug's oral drug-likeliness (Lipinski 2004; Benet et al. 2016). Pharmacokinetic properties were predicted by admetSAR (http://lmmd.ecust.edu.cn/admetsar2) and SwissADME (Daina et al. 2017). Drug-Likeness Tool (DruLiTo) was used for Quantitative estimation of Drug-likeness (QED) (Bickerton et al. 2012).
Results
Screening of in vitro anti-influenza activity of herbs
The methanol, n-hexane and ethylacetate extracts of seven available herbs: C. sinensis, P. hydropiper, P. orientale, P. lapathifolia, P. stagnina, M. pruriens and C. album were examined against a total of 11 influenza isolates. All the extracts were tested at concentrations ranging from 10.0, 50.0, 90.0, 120.0 μg/ml.
Among these, only methanol extracts of C. sinensis, P. hydropiper, M. pruriens and C. album exhibited antiviral effect with average EC50 of 41.36, 46.0, 41.68 and 32.22 µg/ml, respectively. All the extracts were also tested on uninfected cells (control). But no CPE was evident even in highest concentration. So, the extracts had no cytotoxicity in MDCK cell up to 120 µg/ml.
The average EC50 values of the methanol extract of C. sinensis against 6 influenza A/H1N1, 3 influenza A/H3N2 and 2 influenza B/Yamagata isolates were 40.91, 39 and 46.25 µg/ml, respectively. The average survivability of cells at 10.0, 50.0, 90.0 and 120.0 μg/ml of extracts were 29, 56, 77 and 90%, respectively for influenza A virus (Table 1).
The percentage of survivability at different concentrations of the methanol extract of P. hydropiper varied between isolates. The average EC50 values against influenza A and influenza B/Yamagata isolates were 45.1 and 50.25 µg/ml, respectively.
When six influenza A/H1N1, three influenza A/H3N2 and two influenza B/Yamagata viruses were challenged with methanolic extract of M. pruriens, the EC50 values ranged between 34 and 46.5 µg/ml (Table 1). The cell survivability pattern was similar to C. sinensis for all isolates.
Nearly 92% cell survivability was observed at 120 µg/ml concentration of the methanolic extract of C. album for influenza A/H1N1, influenza A/H3N2 and Influenza B. The EC50 values for all isolates were between 26.5 and 37 µg/ml.
The EC50 values of the methanolic extract of C. sinensis, P. hydropiper, M. pruriens and C. album against amantadine-resistant influenza strain A/ 105/ H1N1 were 42, 45, 43 and 33 µg/ml, respectively. The amount of these extracts needed to achieve the half of the maximum effect against Influenza B subtypes were quite higher than the same against Influenza A.
Though methanol, n-hexane and ethyl acetate extracts of P. orientale, P. lapathifolia, P. stagnina could not exhibit anti-influenza activity even at highest concentration, they were found to be non-toxic to MDCK cells.
Findings of the potential bioactive candidates
As the methanol extract of C. sinensis, P. hydropiper, M. pruriens and C. album were effective against the influenza virus, the phytochemicals of these antiviral herbs were virtually screened and retrieved their chemical structure with relevant information. Thereafter, docking protocols (Table 2) were utilized to observe the binding affinity as well as active interacting residues between the targeted viral receptors and phytochemicals of herbs. The 3D and 2D interactions between protein–ligand complex are shown in Figs. 2 and 3, respectively.
3D interaction between ligands (phytochemicals) and target protein. 3D images of target protein–ligand (phytochemicals) interactions. A1) 2HU4- CID 508,204 (C. album), A2) 6BKK- Cryptomeridiol (C. album), A3) 6CF7- CID 508,204(C. album), B1) 2HU4- Epicatechin gallate (C. sinensis), B2) 6BKK- Delphinidin (C. sinensis), B3) 6CF7- Theaflavin (C. sinensis), C1) 2HU4- Cardenolide B-3 (M. pruriens), C2) 6BKK- Hydroxygenistein (M. pruriens), C3) 6CF7- Cardenolide B-3 (M. pruriens), D1) 2HU4- Miquelianin (P. hydropiper), D2) 6BKK- Quercitin (P. hydropiper), D3) 6CF7- Daucosterol (P. hydropiper)
2D interaction between ligands (phytochemicals) and target protein. 2D images of target protein–ligand (phytochemicals) interactions. A1) 2HU4- CID 508,204 (C. album), A2) 6BKK- Cryptomeridiol (C. album), A3) 6CF7- CID 508,204(C. album), B1) 2HU4- Epicatechin gallate (C. sinensis), B2) 6BKK- Delphinidin (C. sinensis), B3) 6CF7- Theaflavin (C. sinensis), C1) 2HU4- Cardenolide B-3 (M. pruriens), C2) 6BKK- Hydroxygenistein (M. pruriens), C3) 6CF7- Cardenolide B-3 (M. pruriens), D1) 2HU4- Miquelianin (P. hydropiper), D2) 6BKK- Quercitin (P. hydropiper), D3) 6CF7- Daucosterol (P. hydropiper)
Among 98 phytochemicals of C. sinensis, half of the them showed favorable binding affinity to three different target receptors ranged from − 5.3 to − 10.4 kcal/mol. Epicatechin gallate, delphinidin and theaflavin were identified as the strongest binder to 2HU4 (− 9.4 kcal/mol), 6BKK (− 10.4 kcal/mol) and 6CF7 (− 8.9 kcal/mol), respectively. The binding energy of quercetin, fisetin and afzelechin to transmembrane domain of influenza virus, 6BKK were − 10.1, − 9.9 and − 9.1, respectively. ARG118, ASP151, ARG292, ASN294, ARG371, TYR347 of 2HU4; HIS37(A), HIS37(B), HIS37(C), VAL27(D) of 6BKK; and THR37(A), HIS38(A), THR49(B) of 6CF7 were found to be involved in forming hydrogen bond with epicatechin gallate, delphinidin and theaflavin, respectively (Fig. 3).
In case of 38 phytochemicals present in P. hydropiper, the highest binding score of miquelianin-2HU4, quercetin-6BKK, daucosterol-6CF7 complex were − 9.2, − 10.1, − 7.2, respectively. The amino acids that involved in hydrogen bond with ligands were ARG118, GLU119, ASN221, GLU227, GLY244, GLU277, ARG292, TYR347, TYR406 of 2HU4; HIS37(A), HIS37(B), HIS37(C), GLY34(B) of 6BKK and HIS38(A), GLY20(B) of 6CF7. Quercetin, kaempferol, confertiflorin were able to bind to all analyzed targets. Molecules that bound at least two targets were isoquercetin (− 8.7 kcal/mol to 2HU4, − 6.8 kcal/mol to 6CF7), astragalin (− 7.6 kcal/mol to 2HU4, − 6.8 kcal/mol to 6CF7), ellagic acid (− 8.4 kcal/mol to 2HU4, − 9.6 kcal/mol to 6BKK), 6-hydroxyluteolin (− 7.9 kcal/mol to 2HU4, − 6.8 kcal/mol to 6CF7) (Fig. 3). The average binding energy of all molecules to 2HU4, 6BKK and 6CF7 were − 6.5, − 6.14 and − 5.6 kcal/mol, respectively.
Cardenolide-B3 of M. pruriens showed highest binding activity to the active site of 2HU4 (− 8.6 kcal/mol) and 6CF7 (− 7.6 kcal/mol) that formed hydrogen bond with ARG118, GLU119, ASP151, ASN221, ARG224, GLU277, ASN294, TYR347, ARG371 of 2HU4 and HIS18(A), ALA19(A), ASN20(A), HIS38(A), THR15(B) of 6CF7 (Fig. 3). 2'-hydroxygenistein had highest binding affinity to 6BKK (− 8.8 kcal/mol) followed by genistein (− 8.7 kcal/mol) and glutathione (− 7.7 kcal/mol). Compounds that were observed to interact with all target proteins were 2'-hydroxygenistein, ambroxol hydrochloride and genistein. Eupatin, coumarin, tryptamine, levodopa bound to at least two targets. The average binding affinity of all molecules to 2HU4, 6BKK and 6CF7 were − 6.08, − 6.16 and − 5.5 kcal/mol, respectively.
CID 508,204 of C. album strongly bound to 2HU4 (− 8.9 kcal/mol) and 6CF7 (− 7.7 kcal/mol) whereas cryptomeridiol demonstrated the same to 6BKK. TYR347, TYR406 of 2HU4; HIS37(A), HIS37(D) of 6BKK and ASN50(B), SER113(B) of 6CF7 were involved in hydrogen bond with cid 508,204, cryptomeridiol and cid 508,204, respectively (Fig. 3). Metamitron, astragalin and cryptomeridiol were able to bind to all targets. The mean binding affinity of all molecules to 2HU4, 6BKK and 6CF7 were − 6.3, − 5.9 and − 5.6 kcal/mol, respectively.
The redocked structure of the extracted ligands oseltamivir, amantadine and jnj4796 bound to the target proteins 2HU4, 6BKK and 6CF7 with rmsd of 0.00, 0.042 and 0.035 Å, respectively. The lower rmsd value signified the accuracy and validation of docking protocol.
Analysis of pharmacokinetic properties
Most of the compounds belong to flavonoids class. Other classes include steroid and derivatives, purine nucleotides, carboxylic acid and derivatives, cinnamic acid and derivatives etc. Diverse patterns of absorption (human intestinal absorption, blood brain barrier, Caco-2 permeability), metabolism and toxicity (AMES toxicity, carcinogenicity) were observed. No molecules were found to be carcinogenic. All molecules except isoquercetin, astragalin, glutathione, ambroxol hydrochloride, levodopa were non-toxic in AMES test (Table 3). The bioavailability score of maximum molecules was 0.55. The drug-likeness of sinapic acid, lariciresinol, campesterol, cryptomeridiol, ferulic acid, genistein, eupatin and kaempherol were 0.797, 0.772, 0.769, 0.742, 0.748, 0.719, 0.71 and 0.618, respectively (Fig. 4).
Discussion
Medicinal plants have long been used for the treatment of influenza infections and now become increasingly popular as alternatives to synthetic drugs (Amić et al. 2003; Rajasekaran et al. 2013). Several studies reported the antiviral effect of different Asian medicinal plants against influenza virus through in vitro experiments (Enkhtaivan et al. 2015; Shoji et al. 2017; Liu et al. 2018). Here, we determined the anti-influenza activity of a number of traditional plant extracts that could serve as sources of new antivirals to combat drug-resistant viral infections. A significant criterion for an antiviral treatment is safety and it is critical to evaluate potential adverse effects when searching for novel medications. Herein, no cytotoxicity was observed (up to the highest concentration used) in the tested extracts. These non-toxic characteristics indicated the potential of extracts in the development of safer and less harmful medications. Generally, natural compounds with ethnomedical background are regarded as safer and more effective compared to substances missing this framework (Grienke et al. 2012; Rajasekaran et al. 2013).
The methanol extract of C. sinensis showed a potent antiviral effect that is in agreement with previous studies, describing the antiviral characteristics of tea against influenza A and B viruses (Nakayama et al. 1990; Zu et al. 2012). Aqueous extract of green tea contains different polyphenolic compounds such as catechins, theaflavins that are known to exert antioxidant, antiviral, antifungal and antibacterial effects (Friedman 2007). A study on antiviral potential of catechin in green tea reported the EC50 (the 50% effective inhibition concentration) of EGCG (epigallocatechin gallate), ECG (epicatechin gallate), and EGC (epigallocatechin) against influenza A virus were 22–28, 22–40 and 309–318 μM, respectively (Song and Lee 2005). The methanol extract of P. hydropiper was known to have anti-inflammatory properties (Yang et al. 2012). Here, the average EC50 value of the same extract was 46 µg/ml that was in line with another study that found the EC50 of P. chinese against influenza virus ranged between 38.4 to 55.5 µg/ml (Tran et al. 2017). Such anti-influenza effect might be attributed to their pharmacological and biological effects and the known phytochemicals of potential therapeutic importance that have been isolated so far (Fan et al. 2011). Phytochemical analysis of M. pruriens, an important medicinal plant, revealed the presence of a wide variety of chemicals such as alkaloids, β-sitosterol, glutathione, steroids, flavonoids, coumarins, cardenolides, tryptamine, alkylamines, oleic acid, linoleic acid, and palmitic acid having antiviral, antiparkinson, antioxidant, antimicrobial and antiprotozoal effects (Gupta et al. 1997; Adebowale et al. 2005; Rajeshwar et al. 2005; Sivaraman et al. 2010). We observed the moderate antiviral characteristics of M. pruriens, in which the average EC50 was 41.6 µg/ml. C. album is used in diet as a source of minerals, fiber, vitamins and essential fatty acids that has been traditionally used as a blood purifier, sedative, antiscorbutic laxative and anthelmintic (Poonia and Upadhayay 2015). In this study, the methanol extract of C. album was found to be most effective (EC50 = 32 µg/ml) in preventing influenza virus compared to other tested herbs. Such lower EC50 indicated the presence of higher amount of anti-influenza molecules that might be active at low concentration. Medicinal herbs have many secondary metabolites. Sequential method of extraction using n-hexane, ethyl acetate and methanol is mostly recommended if active metabolites of herbs are uncharacterized and thus it is possible to extract non polar, mid polar and polar compounds orderly (Poonia and Upadhayay 2015). As only methanolic extracts of different herbs showed anti-influenza activity, it can be interpreted that the active compounds of these herbs are polar in nature. Similarly, the polar extract of Jatropha multifida strongly inhibited influenza virus and thus promote the survivability of infected MDCK cells (Shoji et al. 2017). However, no anti-influenza activity was observed in n-hexane and ethyl acetate extracts of plants described herein which might be due to the chemical nature of solvents that did not facilitate the extraction of higher amount of polar compounds (da Costa Cordeiro et al. 2018).
Plant extracts contain many active components that can effectively prevent virus inhibition, playing a major role in exerting antiviral activity (Rajasekaran et al. 2013). The used extracts contain a lot of polyphenols such as catechins, isoquercetin, delphinidin, ellagic acid etc. and their antiviral activity had been demonstrated both experimentally and through molecular docking studies in numerous literatures (Kim et al. 2010; Liu et al. 2015; Sadati et al. 2019). In this study, catechin derivatives in tea exhibited noticeable interactions with target proteins. The binding affinity between C. sinensis phytochemicals and target protein revealed the catechin derivatives as potential inhibitors of influenza virus. Apart from the catechin derivatives, flavonoid compounds such as quercetin, delphinidin, cyanidin, fisetin and luteolin also showed strong binding affinity to neuraminidase, haemagglutinin and M2 protein. The derivatives namely miquelianin, isoquercetin, quercetin, confertiflorin, kaempferol, daucosterol from P. hydropiper exhibited differential binding affinity to receptor molecules. The binding of cardenolide from M. pruriens exerted strong affinity to neuraminidase and haemagglutinin of influenza which is similar to a study in which cardenolide was proven as an antiviral inhibitor of Influenza A virus (Boff et al. 2020). According to the in silico studies, the molecules abundant in C. album that were found to be responsible for exerting anti-influenza activities were cid508204, isoquercetin, metamitron, cryptomeridiol, astragalin, sinapic acid etc. The maximum nine hydrogen bond was observed in 2HU4-miquelianin interactions followed by eight in cardenolide-B3-2HU4 complex and six in 2HU4-epicatechin gallate complex. Importantly, based on higher docking score, better pharmacokinetic properties, higher drug-likeness and bioavailability score, it can be deduced that molecules such as sinapic acid, ferulic acid, lariciresinol, cryptomeridiol, genistein, eupatin, kaempherol, fisetin, scutellarein could have potential as drug candidate to control influenza.
Influenza virus has been a great threat to public health and health care system. Vaccines and a few anti-viral drugs are available as antiviral therapy for the treatment of patients. However, as evidenced by the appearance of the new 2009 H1N1 type pandemic virus of swine origin in april 2009, vaccinations are not always available in time (Pleschka et al. 2009). The acquisition of resistance to M2 channel inhibitors such as amantadine, is also identified as a potential health risk, globally. Therefore, alternative therapeutic approaches to overcome such resistance are urgently needed to minimize flu symptoms. To meet the growing need for new antiviral agents to overcome the increasing problem of antiviral drug resistance, plants can be suitable candidates due to their ability to produce a large number of phytochemical substances and have a long history of safe and successful use as traditional medications against infectious diseases (Guo et al. 2006). From our results, we speculated that these preparations can be promising choice in the prevention and treatment of influenza virus infections because of their antiviral characteristics, natural abundance and non-toxic properties.
Conclusions
The present study focused on the investigation of the efficacy of different herbs on isolated influenza viruses. The study reported the inhibitory effects of the methanol extract of C. sinensis, P. hydropiper, M. pruriens, C. album on different subtypes of influenza virus strains and later identified their compounds as the potential drug candidates by employing extensive in silico methods. However, further animal studies must be needed to confirm their effectiveness against the crucial targets of influenza virus.
References
Adebowale YA, Adeyemi A, Oshodi AA (2005) Variability in the physicochemical, nutritional and antinutritional attributes of six Mucuna species. Food Chem 89:37–48
Ahmed M, Aleem MA, Roguski K, Abedin J, Islam A, Alam KF, Gurley ES, Rahman M, Azziz-Baumgartner E, Homaira N (2018) Estimates of seasonal influenza-associated mortality in Bangladesh, 2010–2012. Influenza Other Respir Viruses 12:65–71
Amić D, Davidović-Amić D, Bešlo D, Trinajstić N (2003) Structure-radical scavenging activity relationships of flavonoids. Croat Chem Acta 76:55–61
Azziz-Baumgartner E, Alamgir ASM, Rahman M, Homaira N, Sohel BM, Sharker MA, Zaman RU, Dee J, Gurley ES, Al MA (2012) Incidence of influenza-like illness and severe acute respiratory infection during three influenza seasons in Bangladesh, 2008–2010. Bull World Health Organ 90:12–19
Barr IG, Deng YM, Iannello P, Hurt AC, Komadina N (2008) Adamantane resistance in influenza A (H1) viruses increased in 2007 in South East Asia but decreased in Australia and some other countries. Antivir Res 80:200–205
Benet LZ, Hosey CM, Ursu O, Oprea TI (2016) BDDCS, the rule of 5 and drugability. Adv Drug Deliv Rev 101:89–98
Betakova T (2007) M2 protein-a proton channel of influenza A virus. Cur Pharm Des 13:3231–3235
Bickerton GR, Paolini GV, Besnard J, Muresan S, Hopkins AL (2012) Quantifying the chemical beauty of drugs. Nat Chem 4:90–98
Boff L, Schreiber A, da Rocha MA, Del Sarto J, Brunotte L, Munkert J, Melo Ottoni F, Silva Ramos G, Kreis W, Castro Braga F (2020) Semisynthetic cardenolides acting as antiviral inhibitors of influenza A virus replication by preventing Polymerase complex formation. Molecules 25:4853
Bright RA, Medina M, Xu X, Perez-Oronoz G, Wallis TR, Davis XM, Povinelli L, Cox NJ, Klimov AI (2005) Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 366:1175–1181
Carrat F, Flahault A (2007) Influenza vaccine: the challenge of antigenic drift. Vaccine 25:6852–6862
Chan MCW, Wang MH, Chen Z, Hui DSC, Kwok AK, Yeung ACM, Liu KM, Yeoh YK, Lee N, Chan PKS (2018) Frequent genetic mismatch between vaccine strains and circulating seasonal influenza viruses, Hong Kong, China, 1996–2012. Emerg Infect Dis 24:1825
Chen C, Qiu H, Gong J, Liu Q, Xiao H, Chen X-W, Sun B-L, Yang R-G (2012) (−)-Epigallocatechin-3-gallate inhibits the replication cycle of hepatitis C virus. Arch Virol 157:1301–1312
Cheng PKC, Leung TWC, Ho ECM, Leung PCK, Ng AYY, Lai MYY, Lim WWL (2009) Oseltamivir-and amantadine-resistant influenza viruses A (H1N1). Emerg Infect Dis 15:966
da Costa Cordeiro BMP, de Lima Santos ND, Ferreira MRA, de Araújo LCC, Junior ARC, da Conceição Santos AD, de Oliveira AP, da Silva AG, da Silva Falcão EP, dos Santos Correia MT (2018) Hexane extract from Spondias tuberosa (Anacardiaceae) leaves has antioxidant activity and is an anti-Candida agent by causing mitochondrial and lysosomal damages. BMC Complem Altern Med 18:1–10
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:1–13
Dallakyan S, Olson AJ (2015) Small-molecule library screening by docking with PyRx. Springer, Berlin, pp 243–250
Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng P-Y, Bandaranayake D, Breiman RF, Brooks WA, Buchy P (2012) Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 12:687–695
Deyde VM, Xu X, Bright RA, Shaw M, Smith CB, Zhang Y, Shu Y, Gubareva LV, Cox NJ, Klimov AI (2007) Surveillance of resistance to adamantanes among influenza A (H3N2) and A (H1N1) viruses isolated worldwide. J Infect Dis 196:249–257
Dutt S, Narwal S, Kapoor HC, Lodha ML (2003) Isolation and characterization of two protein isoforms with antiviral activity from Chenopodium album L leaves. J Plant Biochem Biotechnol 12:117–122
Enkhtaivan G, John KMM, Ayyanar M, Sekar T, Jin K-J, Kim DH (2015) Anti-influenza (H1N1) potential of leaf and stem bark extracts of selected medicinal plants of South India. Saudi J Biol Sci 22:532–538
Fan D, Zhou X, Zhao C, Chen H, Zhao Y, Gong X (2011) Anti-inflammatory, antiviral and quantitative study of quercetin-3-O-β-D-glucuronide in Polygonum perfoliatum L. Fitoterapia 82:805–810
Friedman M (2007) Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res 51:116–134
Grienke U, Schmidtke M, von Grafenstein S, Kirchmair J, Liedl KR, Rollinger JM (2012) Influenza neuraminidase: a druggable target for natural products. Nat Prod Rep 29:11–36
Gross G, Meyer K, Pres H, Thielert C, Tawfik H, Mescheder A (2007) A randomized, double-blind, four-arm parallel-group, placebo-controlled Phase II/III study to investigate the clinical efficacy of two galenic formulations of Polyphenon® E in the treatment of external genital warts. J Eur Acad Dermatol Venereol 21:1404–1412
Guo J-P, Pang J, Wang X-W, Shen Z-Q, Jin M, Li J-W (2006) In vitro screening of traditionally used medicinal plants in China against enteroviruses. World J Gastroenterol 12:4078
Gupta M, Chakrabarti S, Bhattacharya S, Rath N (1997) Anti-epileptic and anti-cancer activity of some indigenous plants. Indian J Physiol Allied Sci 51:53–56
Heneghan C, Onakpoya I, Jones MA, Doshi P, Del Mar C, Hama R, Thompson MJ, Spencer EA, Mahtani KR, Nunan D (2016) Neuraminidase inhibitors for influenza: a systematic review and meta-analysis of regulatory and mortality data. Science 5:666
Imai M, Yamashita M, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Kiso M, Murakami J, Yasuhara A, Takada K, Ito M, Nakajima N (2020) Influenza A variants with reduced susceptibility to baloxavir isolated from Japanese patients are fit and transmit through respiratory droplets. Nat Microbiol 5:27–33
Kaplan W, Littlejohn TG (2001) Swiss-PDB viewer (deep view). Brief Bioinformatics 2:195–197
Kim Y, Narayanan S, Chang K-O (2010) Inhibition of influenza virus replication by plant-derived isoquercetin. Antivir Res 88:227–235
Laskowski RA (2001) PDBsum: summaries and analyses of PDB structures. Nucleic Acids Res 29:221–222
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. Science 2:556
Lipinski CA (2004) Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1:337–341
Liu Z, Zhao J, Li W, Wang X, Xu J, Xie J, Tao K, Shen L, Zhang R (2015) Molecular docking of potential inhibitors for influenza H7N9. Comput Math Methods Med 2:888
Liu J, Zu M, Chen K, Gao L, Min H, Zhuo W, Chen W, Liu A (2018) Screening of neuraminidase inhibitory activities of some medicinal plants traditionally used in Lingnan Chinese medicines. BMC Complem Altern Med 18:1–11
Manzocco L, Anese M, Nicoli MC (1998) Antioxidant properties of tea extracts as affected by processing. LWT Food Sci Technol 31:694–698
McCullers JA, Wang GC, He S, Webster RG (1999) Reassortment and insertion-deletion are strategies for the evolution of influenza B viruses in nature. J Virol 73:7343–7348
Moghadami M (2017) A narrative review of influenza: a seasonal and pandemic disease. Iran J Med Sci 42:2
Mohanraj K, Karthikeyan BS, Vivek-Ananth RP, Chand RPB, Aparna SR, Mangalapandi P, Samal A (2018) IMPPAT: a curated database of I ndian M edicinal P lants, P hytochemistry A nd T herapeutics. Sci Rep 8:1–17
Mukoyama A, Ushijima H, Nishimura S, Koike H, Toda M, Hara Y, Shimamura T (1991) Inhibition of rotavirus and enterovirus infections by tea extracts. Japan J Med Sci Biol 44:181–186
Naeem A, Elbakkouri K, Alfaiz A, Hamed ME, Alsaran H, AlOtaiby S, Enani M, Alosaimi B (2020) Antigenic drift of hemagglutinin and neuraminidase in seasonal H1N1 influenza viruses from Saudi Arabia in 2014 to 2015. J Med Virol 92:3016–3027
Nakayama M, Toda M, Okubo S, Shimamura T (1990) Inhibition of influenza virus infection by tea. Lett Appl Microbiol 11:38–40
Peteranderl C, Herold S, Schmoldt C (2016) Human influenza virus infections. In: Semin Respir Crit Care Med. Thieme Medical Publishers, pp 487–500
Pleschka S, Stein M, Schoop R, Hudson JB (2009) Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol J 6:1–9
Poonia AU (2015) Chenopodium album Linn: review of nutritive value and biological properties. J Food Sci Technol 52:3977–3985
Pulendran B, Maddur MS (2014) Innate immune sensing and response to influenza. Influenza Pathog Control II:23–71
Rahman SR, Ahmed MF, Islam MA, Rahman MM (2016) Effect of risk factors on the prevalence of influenza infections among children of slums of Dhaka city. Springerplus 5:1–6
Rahman M, Hoque SA, Islam MA, Rahman SR (2017) Molecular analysis of amantadine-resistant influenza A (H1N1 pdm09) virus isolated from slum dwellers of Dhaka, Bangladesh. Virus Genes 53:377–385
Rajão DS, Pérez DR (2018) Universal vaccines and vaccine platforms to protect against influenza viruses in humans and agriculture. Front Microbiol 9:123
Rajasekaran D, Palombo EA, Chia Yeo T, Lim Siok Ley D, Lee TuC, Malherbe F, Grollo L (2013) Identification of traditional medicinal plant extracts with novel anti-influenza activity. PLoS ONE 8:e79293
Rajeshwar Y, Gupta M, Mazumder UK (2005) In vitro lipid peroxidation and antimicrobial activity of Mucuna pruriens seeds. Science 5:7996
Ren C-Z, Hu W-Y, Li J-C, Xie Y-H, Jia N-N, Shi J, Wei Y-Y, Hu T-J (2020) Ethyl acetate fraction of flavonoids from Polygonum hydropiper L. modulates pseudorabies virus-induced inflammation in RAW264. 7 cells via the NF-κB and MAPK pathways. J Vet Med Sci 2:20–263
Sadati SM, Gheibi N, Ranjbar S, Hashemzadeh MS (2019) Docking study of flavonoid derivatives as potent inhibitors of influenza H1N1 virus neuraminidase. Biomed Rep 10:33–38
Sathiyanarayanan L, Arulmozhi S (2007) Mucuna pruriens Linn.-A comprehensive review. Pharmacogn Rev 1:6888
Shivanika C, Kumar D, Ragunathan V, Tiwari P, Sumitha A (2020) Molecular docking, validation, dynamics simulations, and pharmacokinetic prediction of natural compounds against the SARS-CoV-2 main-protease. J Biomol Struct 1:998
Shoji M, Woo S-Y, Masuda A, Win NN, Ngwe H, Takahashi E, Kido H, Morita H, Ito T, Kuzuhara T (2017) Anti-influenza virus activity of extracts from the stems of Jatropha multifida Linn. collected in Myanmar. BMC Complem Altern Med 17:1–7
Sivaraman D, Ratheeshkumar KS, Muralidaran P (2010) Effect of ethanolic seed extract of Mucuna pruriens (L.) DC var. utilis on haloperidol induced tardive dyskinesia in rats. Int J Pharm Sci Rev Res 3:6000
Song J-M, Lee K-H, Seong B-L (2005) Antiviral effect of catechins in green tea on influenza virus. Antivir Res 68:66–74
Tran TT, Kim M, Jang Y, Lee HW, Nguyen HT, Nguyen TN, Park HW, Le Dang Q, Kim J-C (2017) Characterization and mechanisms of anti-influenza virus metabolites isolated from the Vietnamese medicinal plant Polygonum chinense. BMC Complem Altern Med 17:1–11
Yamayoshi S, Kawaoka Y (2019) Current and future influenza vaccines. Nat Med 25:212–220
Yang Y, Yu T, Jang H-J, Byeon SE, Song S-Y, Lee B-H, Rhee MH, Kim TW, Lee J, Hong S (2012) In vitro and in vivo anti-inflammatory activities of Polygonum hydropiper methanol extract. J Ethnopharmacol 139:616–625
Zu M, Yang F, Zhou W, Liu A, Du G, Zheng L (2012) In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antivir Res 94:217–224
Acknowledgements
The study was funded by Ministry of Science and Technology, Government of the People’s Republic of Bangladesh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies involving animals performed by any of the authors. This article does not contain any studies involving human participants performed by any of the authors.
Conflict of interest
Md Abu Sayem Khan has no conflict of interest. Rifat Parveen has no conflict of interest. Sheikh Ariful Hoque has no conflict of interest. Md Firoz Ahmed has no conflict of interest. Abu Shara Shamsur Rouf has no conflict of interest. Sabita Rezwana Rahman has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, M.A.S., Parveen, R., Hoque, S.A. et al. Implementing in vitro and in silico approaches to evaluate anti-influenza virus activity of different Bangladeshi plant extracts. ADV TRADIT MED (ADTM) 23, 915–928 (2023). https://doi.org/10.1007/s13596-022-00669-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-022-00669-9