Abstract
Mesua ferrea Linn. flowers have been used in Ayurveda as a brain tonic and as an ingredient in memory-enhancing formulations such as Brahma Rasayan and Chyawanprash. However, this ethnomedicinal use has not been investigated scientifically. This study evaluated the effect of the ethanolic extract of Mesua ferrea flowers (MFE) on memory in scopolamine-induced models of cognitive dysfunction. MFE was administered to rats (100, 200 and 400 mg/kg, p.o) for a period of 14 days, after which amnesia was induced by giving scopolamine (1 mg/kg, s.c) on the 14th day. Piracetam (200 mg/kg, p.o) was given as a positive control. The models employed to assess memory in the rats were the T-maze continuous alternation task (T-CAT) and novel object recognition test (NORT). Pretreatment with MFE ameliorated the memory deficit caused by scopolamine; which was evidenced by a significantly greater relative proportion of spontaneous alternation percentage in the T-CAT, and a significant increase of discrimination index in the NORT. Further, MFE significantly inhibited anticholinesterase activity in the brain, elevated the levels of reduced glutathione and catalase, and decreased malondialdehyde and nitrite levels in the brain. The results of this study show that MFE exhibited significant anticholinesterase and antioxidant activities in scopolamine treated rats, which could be the possible underlying mechanism of its memory-enhancing activity and of its ethnomedicinal use as a brain tonic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by a gradual loss of cognitive abilities, affecting nearly 47.5 million people worldwide (Pahwa and Goel 2016). It is an accelerating health crisis in developing countries with an increasing population of the elderly (Mathuranath et al. 2012). Many factors contribute to the etiology of AD, such as amyloid-beta plaque formation, hyperphosphorylation of tau protein, variation in cholinergic transmission, formation of reactive oxygen species (ROS) due to oxidative stress, inflammation, monoaminergic disturbances, and apoptotic processes (Odubanjo et al. 2018).
The extent of cognitive decline and memory impairment in an Alzheimer’s patient is directly related to the loss of cholinergic function (Jeong et al. 2009). It has been proven that patients with AD exhibit a decrease in acetylcholine (ACh) and cholineacetyltransferase and an increase in acetylcholinesterase (AChE) levels (Rubio et al. 2007). Additionally, Keller et al. (2005) have demonstrated the presence of oxidative damage in the initial stages of AD. Reactive oxygen species (ROS) cause neurodegeneration by initiating lipid peroxidation in the central cholinergic system (Ngoupaye et al. 2017). This shows that oxidative stress coupled with cholinergic hypofunction might be one of the underlying causes of cognitive dysfunction in Alzheimer’s disease.
The therapeutic agents currently used in the management of AD are AChE inhibitors such as donepezil, galantamine, rivastigmine, and tacrine. However, these drugs have limited efficacy, poor bioavailability, narrow therapeutic windows, and cause adverse effects like cholinergic side effects in the periphery and hepatoxicity; thus restricting their usage (Ghumatkar et al. 2015). There is a need to search for alternative therapy, as conventional anti-AD agents offer only symptomatic relief. There is a growing interest in herbal drugs as they are considered to be more ‘natural’ and ‘gentle’ alternatives to synthetic drugs (Ishola et al. 2013), and are easily available and cost effective (Foyet et al. 2015). Herbal drugs are also preferred as they have a ‘built-in poly-pharmacology’ due to the presence of numerous secondary metabolites in a single plant, and synergism among those phytoconstituents could provide a remedy to the multifactorial pathogenesis of AD (Ngoupaye et al. 2017; Dey et al. 2017).
Mesua ferrea Linn. (Calophyllaceae) is a perennial tree which is indigenous to parts of India, Sri Lanka, southern Nepal, Thailand, and a few other South-Asian countries (Lim 2016). In the Indian system of medicine, the plant, commonly known as Nagakesara, is traditionally used for its antiemetic, anthelminthic, aphrodisiac and diuretic effects, and as an antidote (Anandakumar et al. 1986). Nagakesara has been used as a brain tonic in Ayurveda (Anandakumar et al. 1986), and is an ingredient of ayurvedic formulations such as Brahma Rasayana and Chyawanprash (Chahar et al. 2012), which are manufactured commercially till date. Brahma Rasayana is primarily used for brain-specific disorders in the elderly. Daily consumption of Brahma Rasayana is believed to improve memory, learning and cognition (Baliga et al. 2013). Chyawanprash is a generic household cure used widely in India. It has been used as a health food in the country for over 2000 years, which aims at the maintenance of the physique and vigor, while delaying ageing. It rejuvenates and nourishes the brain cells, thereby enhancing memory and learning (Vasudevan and Parle 2007). Nagakesara is also used in the preparation of Kalyanaka Ghrita, an ayurvedic ghee-based formulation which is given to improve memory and concentration. This preparation is used in the treatment of disorders wherein the mental capabilities and intellect of a patient are weakened (Rajput 2018).
Extracts of Mesua ferrea flowers are known to contain fractions of bioactive compounds including a biflavonone (mesuaferrone-b), triterpenoid (β-amyrin), sterol lipids (β-sitosterol), cyclohexadione compound (mesuaferrol), and eighteen 4-phenyl-5,7-dihydroxycoumarins which include mesuol, mammeisin, and mesuagin (Raju et al. 2016, Dennis and Kumar 1988, Verotta et al. 2004). Moreover, Mesua ferrea flowers have been reported to demonstrate antioxidant activity in ABTS (Wessapan et al. 2007) and DPPH assays (Makchuchit et al. 2010). However, no studies have justified the use of Mesua ferrea as a brain tonic or delved into its effects on memory and learning.
Scopolamine, a well-known anticholinergic, is often used in preclinical studies of new drugs which are designed to treat cognitive dysfunction. Studies have shown that scopolamine also causes oxidative stress through its actions on the cholinergic system, leading to memory impairment (Klinkenberg and Blokland 2010). This model was chosen as it is a quick and simplified method to test the nootropic potential of drugs. Nagakesara is mostly attributed to the stamens or flowers of Mesua ferrea (Anandakumar et al. 1986), and therefore the flowers of this tree were selected for the study. Based on the traditional claim and reported in-vitro antioxidant effects of Mesua ferrea, the aim of this study was to investigate the pharmacological effects of the ethanolic extract of its flowers on learning and memory in scopolamine-induced memory deficit models of disease. Piracetam, an important nootropic agent, acts by improving cholinergic function, stimulating oxidative glycolysis, and increasing cerebral oxygen consumption (Hitzenberger 1998). Since the interest of this experiment was to determine if the herbal extract had anticholinesterase and antioxidant properties, piracetam was chosen as the standard drug for comparison.
Materials and methods
Plant collection, extract preparation and phytochemical analysis
Mesua ferrea flowers were collected from Ratnagiri, Maharashtra, India. The flowers were identified and authenticated at the Agharkar Research Institute, Pune. The ethanolic extract (Batch No.: 11/2016, Ref.: SE/EQ/2016-17/11) of Mesua ferrea flowers (MFE) was prepared by M/S Shamantak Enterprises (B/II/293/0206776, Pune, Maharashtra, India). A voucher specimen (No. 4/2016) was stored for future reference. The extraction procedure was carried out as described previously by Garg et al. (2009) with slight modifications. Dried and powdered flowers of Mesua ferrea (500 gms) were extracted with 90% ethanol (5 L) for 72 hours using a Soxhlet apparatus (temp: 55 °C). The residual solvent in the crude extract was then evaporated under vacuum to produce MFE (yield 23.7% w/w). The extract was an aromatic, viscous, chocolate brown paste with 10.2% w/w moisture content and 3.02% w/w ash content, the latter complying with the Indian Pharmacopoieal standards (Quality Report No. II 19). The presence of various phytochemical constituents were analysed using standard procedures (Khandelwal 2008). MFE tested positive for the presence of phenolics, tannins, flavonoids, saponins and alkaloids.
Quantitative phytochemical estimation
Total phenolic content (TPC) and total flavonoid content (TFC)
Folin-Ciocalteu reagent was used to determine the total phenolic content of the plant extract. The plant extract (0.1 ml) was mixed with 0.5 ml of Folin-Ciocalteu reagent and 1.5 ml of 20% w/v sodium carbonate. The solution was then mixed thoroughly and the volume was made up to 10 ml with distilled water. After allowing the mixture to stand for 2 hours, the absorbance was read at 765 nm. The data was used to estimate the total phenolic content using a standard curve obtained for various concentrations of gallic acid (Abozed et al. 2014). The total flavonoid content was determined using the procedure described in Kumaran and Joel Karunakaran (2007). 1 ml of plant extract in methanol (10 mg/ml) was added to 1 ml aluminum trichloride in ethanol (20 mg/ml) followed by a drop of acetic acid. This mixture was then diluted with ethanol to 25 ml. The absorption was recorded at 415 nm after 40 minutes. For the blank samples, 1 ml of plant extract was mixed with a drop of acetic acid, and diluted to 25 ml with ethanol. The standard used was rutin solution (0.5 mg/ml) in ethanol, and the absorption was measured at the same conditions mentioned above. All the determinations were performed in duplicates. The amount of flavonoids in the extract in rutin equivalents (RE) was calculated using the formula:
\({\text{X}} = \left( {{\text{A}} \times {\text{m}}_{0} } \right)/({\text{A}}_{0} \times {\text{m}})\)where X is the flavonoid content, mg/mg plant extract in rutin equivalents, A is the absorption of the plant extract solution, A0 is the absorption of the standard rutin solution, m is the weight of the plant extract (mg) and m0 is the weight of rutin in the solution (mg).
Drugs and chemicals
Scopolamine hydrobromide and Griess’ reagent were purchased from Sigma-Aldrich, USA. Piracetam (Nootropil ®) was a product of Dr. Reddy’s Laboratories, India. Acetylthiocholine iodide (ATC), trichloroacetic acid (TCA) and thiobarbituric acid (TBARS) were manufactured by Loba Chemie Pvt. Ltd., India. 5,5’-dithio-bis (2-nitrobenzoic acid) (DTNB) and all other reagents were of analytical grade and were obtained from Analab Fine Chemicals, India.
Experimental animals
Thirty-six male Wistar rats, weighing about 180-220 g were used for the nootropic study. The animals were kept under ambient conditions at a temperature of 25 ± 2 °C, 45-55% relative humidity and a 12-hour light/dark cycle. Animals were fed with standard diet (Amrut Feed, Chakan Oil Mills, Pune) and water ad libitum. The research protocols used in this study complied with the requirements of Institutional Animal Ethics Committee (IAEC) of AISSMS College of Pharmacy, Pune, constituted under the Committee for Purpose of Control and Supervision of Experiments on Animals (CPCSEA), approval no. CPCSEA/IAEC/PC-01/02-2K16. All efforts were taken to reduce animal suffering and to minimize the number of animals used in the experimental tasks.
Acute oral toxicity study
The determination of acute toxicity was conducted in accordance to the Organization for Economic Cooperation and Development (OECD) guideline 420 (OECD 2001). Female rats were used in this study as females are generally more sensitive to toxic effects as compared to males (OECD 2000). Six rats, aged 8-10 weeks were divided into two groups comprising three animals each. The animals were fasted overnight with access to water, weighed and marked prior to administration. The first group received the MFE at a dose of 2000 mg/kg and the second group received only distilled water orally. The rats were observed for individually for 24 h for signs of toxicity and death. For the remaining 14 days, the animals were monitored daily for any behavioral changes or clinical signs of toxicity and the weekly body weight was recorded. On the last day, the animals were sacrificed by cervical dislocation under inhaled diethyl ether anesthesia and the organs and tissues were macroscopically examined for any toxic abnormalities. The administration of a single dose of MFE (2000 mg/kg) did not induce mortality or any toxic effects in the animals after treatment. The changes in body weights were normal and there were no visible abnormalities in the tissues examined.
Experimental groups and drug treatments
The animals were randomly divided into groups (n=6) and treated as follows for 14 days:
-
Group I (Control) - normal control group (vehicle; p.o.)
-
Group II (SCOP) - amnesic control (vehicle, p.o.)
-
Groups III-V (MFE groups) - test groups (MFE - 100, 200 and 400 mg/kg respectively, p.o.)
-
Group VI (PIR) - standard group (Piracetam - 200 mg/kg, p.o.)
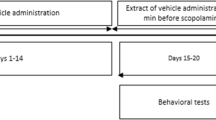
Freshly prepared solutions of MFE and piracetam in distilled water, and scopolamine in saline were administered to the rats. Fig. 1 depicts the pictorial representation of the experimental paradigm. On the 14th day, scopolamine was administered subcutaneously at a dose of 1 mg/kg (Bhattamisra et al. 2012, Shin et al. 2018, Kumar et al. 2000) to groups II-VI, one hour after the administration of distilled water/MFE/piracetam. Cognitive tests were conducted 30 minutes after scopolamine injection on the 14th day. The animals were examined daily for any signs of inactivity or lethargy. All the experiments were conducted during the diurnal phase (9:00 am to 6:00 pm). The animals were shifted to the laboratory one hour prior to the experiment to allow habituation and were deprived of food 12 hours before the behavioral testing. However, all the animals were allowed to drink water ad libitum. On the 13th day, the rats were subjected to a habituation trial for the NORT, wherein they were allowed to explore the open area freely for 2 minutes. The same set of animals were subjected to the T-CAT and NORT. The T-CAT was conducted 1 hour after the NORT, and the rats were sacrificed immediately after the behavioral tests to collect the brains for the assessment of biochemical parameters. During the experiments, the odor cues were moderated by cleaning the maze with a damp cloth dipped in 70% alcohol before each new animal started its session.
Behavioral cognition tests
T-maze continuous alternation task (T-CAT)
The T-maze (VJ Instruments, India) made of black colored plywood, consisted of two open arms (40 cm × 10 cm × 20 cm) and the starting arm (70 cm × 10 cm), elevated to a height of 25 cm. The experiment was conducted according to the procedure described in Spowart-Manning and van der Staay (2004) with slight modifications. Briefly, the experiment protocol consisted of one single session, which started with one ‘forced-choice’ trial, followed by 14 ‘free-choice’ trials. In the ‘forced-choice’ trial, entry to either of the maze arms were obstructed by a black-colored guillotine door. After a 5 s confinement period in the start box, the rat would navigate the maze, in due course enter the open goal arm, and return back. For the subsequent 14 ‘free-choice’ trials, post confinement, the rat was free to choose between the left and right open goal arms (no guillotine doors lowered). After the rat entered either one open goal arm, the other arm was closed. The rat then eventually returned to the start box, and the next free-choice trial started 5 s after restriction in the start box. A session was termed completed as soon as 14 free-choice trials were performed or 10 minutes had passed, whichever occurred first. Spontaneous alternations are defined as entry in a different arm of the T-maze over successive trials (i.e., left-right-left, etc.). The percentage of alternations over the free 14 free-choice trials were determined for each rat accordingly:
The total time elapsed until completion of each session was also recorded. Throughout the sessions, the animals were not once handled by the experimenter, which is a key modification of the usual T-maze task. Continuous handling of the animals might induce fear, and the anxious animal would react either by long freezing responses or refusal to run in the maze. This would cause disruptions in the continuation of the experiment, and also increase the duration of the trials (Gerlai 1998; Wu et al. 2018).
Novel object recognition test (NORT)
The object recognition apparatus (VJ Instruments, India) was made of an enclosed plexiglass box with a black colored floor (70 × 60 × 30 cm). The experiment was performed according to the procedure described in Ozarowski et al. (2013). Post the habituation trial on the 13th day, the test conducted the next day was divided into three phases – acquisition, inter-trial interval and retention trials. In the acquisition trial on the test day, two identical objects (A1 and A2; biologically inert substance – plastic, sufficiently weighted to stay put) were placed in two opposite corners of the box. The animals were allowed to explore the objects for a period of 3 minutes. The behavior was termed as exploration when the animals’ nose was directed towards the object at a distance less than 2 cm and/or touching the object with the nose or sniffing at the object. However, leaning against, standing/sitting on the object and turning round were not noted as exploratory behavior. The animals were then returned to their cages for a period of 30 minutes. At the retention trial, one of the objects used in the acquisition trial was replaced with a novel object (B), and the rats were then allowed to explore the box individually for 3 minutes. The time spent in exploring the familiar (A) and novel (B) object were recorded separately, and the discrimination index (DI) was calculated as \(({\text{B}} - {\text{A}}) - /({\text{B}} + {\text{A}})\). A higher DI is reflective of a higher memory capacity.
Biochemical assays
Preparation of tissue homogenates
On completion of the behavioral tests on the 14th day, the rats were anesthetized and sacrificed by cervical dislocation. The brain tissue was rapidly excised and weighed. The tissue samples were then rinsed with cold normal saline, and then homogenized in 0.03 M sodium phosphate buffer (pH 7.4). The homogenates were used in the determination of acetylcholinesterase (AChE) and in the antioxidant assays of malondialdehyde (MDA), reduced glutathione (GSH), catalase (CAT) and nitrite estimation.
Estimation of brain AChE level
The quantitative measurement of AChE levels in the brain was performed according to the method of Ellman et al. (1961). The assay mixture containing 0.4 ml of brain homogenate, 2.6 ml of phosphate buffer (0.1 M, pH 8.0) and 100 μl DTNB was mixed by bubbling air and placing it in a spectrophotometer. Once the reaction mixture was stable, absorbance was noted at 412 nm for the basal reading followed by addition of 5.2 μl of acetylcholine iodide to the cuvette. Any change in absorbance was recorded from zero time until 10 minutes at 25 °C. The rates were calculated as follows:
where R is rate, in moles substrate hydrolyzed per minutes per g of tissue; ∆A is change in absorbance per minute; C0 is the original concentration of the tissue (mg/ml). Acetylcholinesterase activity was expressed as nmol of acetylthiocholine iodide hydrolyzed/min/mg of tissue.
Determination of MDA
Malondialdehyde levels were estimated using the thiobarbituric acid (TBARS) assay procedure. The supernatant (200 μl) was briefly mixed with 1 ml of 50% trichloroacetic acid in 0.1 M HCl and 1 ml of 26 mM thiobarbituric acid. After mixing on a vortex, the samples were maintained at 95 °C for 20 minutes, after which they were centrifuged at 960×g for 10 minutes. The supernatants recovered after centrifugation were read at 532 nm. The results were expressed as U/mg protein (Ishola et al. 2018).
Determination of GSH
GSH levels were determined using the method described by Ellman (1959). Equal amounts of the brain homogenate and 10% TCA were mixed and centrifuged at 2000×g for 10 minutes at 4 °C. The supernatant was collected, and to 300 μl of the supernatant, 0.5 ml of phosphate buffer (0.1 M, pH 8.4) and 0.2 ml of DTNB were added. This mixture was shaken vigorously on a mixer, after which the absorbance was read at 412 nm within 15 minutes. The results were expressed as U/mg protein.
Determination of catalase activity
The measurement of catalase activity in the brain was performed according to the colorimetric assay described by Sinha (1972). 100 μl of the brain homogenate was mixed with 2 ml of 0.01 M phosphate buffer (pH 7.0) and 0.4 ml of 0.2 M hydrogen peroxide. The reaction was stopped by addition of 2 ml of dichromate acetic acid reagent. The decomposition of hydrogen peroxide was directly estimated by the net decrease in absorbance at 570 nm. The results were expressed as U/mg protein.
Nitrite estimation
Nitrite estimation was performed using Griess’ reagent which is an indicator of nitric oxide production. Briefly, 100 μl of Griess’ reagent was mixed with equal volume of the supernatant and vortexed. The absorbance was read at 542 nm. Nitrite concentration was calculated using standard curve for sodium nitrite (0.01–0.1 mg/ml) (Ishola et al. 2018).
Statistical analysis
All statistical analysis were performed using GraphPad Prism Version 7 (GraphPad Software, CA, USA). The results were expressed as mean ± SEM (n=6). Two-way ANOVA followed by Bonferroni’s multiple comparisons test was used to determine the difference in exploration time between groups in the NORT. One-way ANOVA followed by Tukey’s post hoc multiple comparisons test was used to determine the discrimination index in the NORT, percent spontaneous alternations and mean session durations in the T-CAT, and the biochemical assay results. A P < 0.05 was considered statistically significant.
Results
Quantitative phytochemical estimation
Total phenolic content (TPC) and total flavonoid content (TFC)
The total phenolic content of MFE obtained from the calibration curve (y= R2= 0.9973) was 118.35 ± 2.08 mg/g GAE (gallic acid equivalents). The total flavonoid content of MFE was found to be 29 ± 3.46 mg/g RE (Rutin equivalent). The values were taken in triplicates.
Behavioral cognition tests
T-maze continuous alternation task (T-CAT)
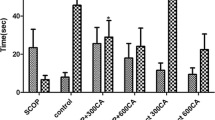
The effects of MFE on spatial memory were investigated by assessing the spontaneous alternation behavior exhibited in the T-maze task (Fig. 2). One way ANOVA showed a significant difference between the treatment groups (P < 0.001). Post hoc test demonstrated the significant deficit in percent spontaneous alternations caused by scopolamine in the amnesic control group, when compared with the control group (P < 0.001). However, pre-treatment with MFE and piracetam ameliorated the effect of scopolamine on spontaneous alternations. The lowest dose of MFE (100 mg/kg) significantly (P < 0.01) alleviated the memory impairment caused by scopolamine when compared with the amnesic control group. The other groups, i.e., MFE (200 and 400 mg/kg) and piracetam (200 mg/kg) also showed a significantly higher percent of spontaneous alternation behavior when compared to the amnesic control group (P < 0.001). The highest dose of MFE (400 mg/kg) produced similar effects as that of the standard piracetam group. However, there was no visible difference seen in the time taken to complete the sessions between each of the groups (data not shown). The difference in mean session duration between the groups was statistically insignificant (P < 0.05).
Effect of MFE on percent spontaneous alternations in the T-CAT (n = 6). ###P < 0.001 when compared to control, **P < 0.01; ***P < 0.001 when compared to SCOP (vehicle-scopolamine) group; one-way ANOVA followed by post-hoc Tukey’s multiple comparison test. SCOP, scopolamine; MFE, Mesua ferrea ethanolic extract; PIR, Piracetam
Novel object recognition test (NORT)
The effects of MFE on non-spatial memory were assessed using the novel object recognition test. Fig. 3 depicts the results of the exploration time of the groups between the novel (B) and familiar (A) object. The control group spent a significantly longer time (P < 0.001) exploring the novel object (B). However, the amnesic control group (vehicle + scopolamine) spent lesser time exploring the novel object and explored the familiar object (A) for a significant amount of time (P < 0.001), suggesting cognitive impairment. The rats in this group had lost the ability to distinguish between the familiar and novel objects. The group treated with the lowest dose of MFE (100 mg/kg) did spend more time exploring the novel object, however, this was statistically insignificant when compared to the time spent exploring the familiar object. The other groups, treated with MFE (200, 400 mg/kg) and piracetam (200 mg/kg) showed a higher preference toward novel object exploration, and spent a significant time (P < 0.001) exploring the same, when compared to the amnesic control (vehicle + scopolamine) group.
Effect of MFE on exploration time in the NORT (n = 6). The graph represents the exploration time in seconds of the familiar (A*) and novel (B) objects within 3 min of the retention trial. Each animal serves as its own control. ###P < 0.001 scopolamine group when compared with exploration time of the familiar object; ***P < 0.001 when compared with exploration time of the familiar object; two-way ANOVA followed by post-hoc Bonferroni’s test. SCOP, scopolamine; MFE, Mesua ferrea ethanolic extract; PIR, Piracetam
The results depicting the discrimination index (DI) data are shown in Fig. 4. The amnesic control rats showed impaired cognitive ability as evident by the decreased discrimination index (P < 0.001) as compared to the control group. Administration of the lowest dose of MFE (100 mg/kg) significantly (P < 0.01) prevented the memory impairment caused by scopolamine, based on the DI value when compared with the amnesic control group. Post hoc analysis demonstrated that the groups treated with MFE (200 and 400 mg/kg) and piracetam (200 mg/kg) remembered the familiar object better compared to the amnesic control group (P < 0.001) as evidenced by their preference to explore the novel object. This indicates amelioration of memory impairment caused by scopolamine and improved cognitive ability in the above-mentioned groups.
Effect of MFE on discrimination index in the NORT (n = 6). ###P < 0.001 when compared to control, **P < 0.01; ***P < 0.001 when compared to SCOP (vehicle-scopolamine) group; one-way ANOVA followed by post-hoc Tukey’s multiple comparison test. SCOP, scopolamine; MFE, Mesua ferrea ethanolic extract; PIR, Piracetam
Biochemical assays
Estimation of brain AChE level
Administration of scopolamine (1 mg/kg) produced a significant increase (P < 0.001) in brain AChE levels as compared to the control group. Administration of MFE (100, 200 and 400 mg/kg) produced a significant decrease (P < 0.01, P < 0.001) in AChE levels when compared to the amnesic control group. Furthermore, piracetam (200 mg/kg) showed a significant decrease (P < 0.001) in the brain AChE levels when compared with the amnesic control group (Fig. 5).
Effect of MFE on acetylcholinesterase (AChE) activity in the rat brains (n = 6). ###P < 0.001 when compared to control, **P < 0.01; ***P < 0.001 when compared to SCOP (vehicle-scopolamine) group; one-way ANOVA followed by post-hoc Tukey’s multiple comparison test. SCOP, scopolamine; MFE, Mesua ferrea ethanolic extract; PIR, Piracetam
Determination of MDA
Scopolamine administration (1 mg/kg) significantly increased (P < 0.001) malondialdehyde (MDA) content when compared with the control group. Pretreatment of the rats with MFE and piracetam for 14 days significantly decreased (P < 0.001) MDA levels when compared to amnesic control (Fig. 6).
Effect of MFE on concentration of malondialdehyde (MDA) in the rat brains (n = 6). ###P < 0.001 when compared to control; ***P < 0.001 when compared to SCOP (vehicle-scopolamine) group; one-way ANOVA followed by post-hoc Tukey’s multiple comparison test. SCOP, scopolamine; MFE, Mesua ferrea ethanolic extract; PIR, Piracetam
Determination of GSH
As revealed in Fig. 7, scopolamine injection (1 mg/kg) produced a significant decrease (P < 0.001) in GSH levels, as compared to the control group. The lowest dose of MFE (100 mg/kg) significantly (P < 0.05) mitigated this effect compared with the amnesic control group. Pretreatment with MFE (200 and 400 mg/kg) and piracetam (200 mg/kg) prior to scopolamine administration significantly increased (P < 0.001) GSH levels in the brain.
Effect of MFE on concentration of reduced glutathione (GSH) in the rat brains (n = 6). ###P < 0.001 when compared to control, *P < 0.05; ***P < 0.001 when compared to SCOP (vehicle-scopolamine) group; one-way ANOVA followed by post-hoc Tukey’s multiple comparison test. SCOP, scopolamine; MFE, Mesua ferrea ethanolic extract; PIR, Piracetam
Determination of catalase activity
Scopolamine (1 mg/kg) significantly decreased (P < 0.001) catalase levels in the amnesic control group, when compared with control. MFE (200 and 400 mg/kg) and piracetam significantly increased (P < 0.001) the levels of catalase when compared with amnesic control group. However, pretreatment with 100 mg/kg of MFE did not yield significant results (Fig. 8).
Effect of MFE on catalase (CAT) activity in the rat brains (n = 6). ###P < 0.001 when compared to control; ***P < 0.001 when compared to SCOP (vehicle-scopolamine) group; one-way ANOVA followed by post-hoc Tukey’s multiple comparison test. SCOP, scopolamine; MFE, Mesua ferrea ethanolic extract; PIR, Piracetam
Nitrite estimation
Nitrite levels in the amnesic control group were significantly increased (P < 0.001) after scopolamine administration, when compared with control. The first group pre-treated with MFE (100 mg/kg) significantly (P < 0.01) decreased nitrite levels as compared to amnesic control. Pretreatment with MFE (200 and 400 mg/kg) and piracetam (200 mg/kg) significantly decreased (P < 0.001) nitrite levels in the brain (Fig. 9).
Effect of MFE on nitrite levels in the rat brains (n = 6). ###P < 0.001 when compared to control, **P < 0.01; ***P < 0.001 when compared to SCOP (vehicle-scopolamine) group; one-way ANOVA followed by post-hoc Tukey’s multiple comparison test. SCOP, scopolamine; MFE, Mesua ferrea ethanolic extract; PIR, Piracetam
Discussion
This study was conducted to assess the nootropic effects of the ethanolic extract of Mesua ferrea flowers (MFE) in scopolamine-treated rats. We decided to explore the possible mechanisms of the reported ethnomedicinal claim, and have justified the use of this plant as a brain tonic scientifically. The results of this study proved that pre-treatment with MFE at the doses of 100, 200 and 400 mg/kg successfully ameliorated scopolamine-induced memory deficits.
According to the ‘cholinergic hypothesis’, deficiency of ACh levels in the brain is a significant factor in the prognosis of AD. Amelioration of AD, therefore, is focused on the enhancement of the cholinergic function of the brain (Yassin et al. 2013). Scopolamine, a non-selective muscarinic receptor antagonist, is used to cause cognitive disruption and is a widely-used paradigm for evaluating potential nootropic agents (Vasileva et al. 2016). Scopolamine produces cognitive impairment including lack of focusing attention, and processing and learning of new information; both in rodents and humans (Ozarowski et al. 2013). Studies have shown that scopolamine causes oxidative stress through its actions on the cholinergic system, leading to memory impairment. Oxidative stress is an imbalance between free radicals and antioxidative defense, both of which are important factors in age-related neurodegeneration (Rabiei et al. 2015). Increase in oxidative stress after scopolamine administration, coupled with disruption of cholinergic function, makes it a suitable model for testing potential nootropic agents (Alibabaei et al. 2014). This study was designed to investigate if MFE had a positive effect on cholinergic function, and also alter the oxidative stress indices caused by scopolamine administration.
The first model employed to test cognitive activity was the T-maze; a test which is highly sensitive to numerous pharmacological manipulations which affect memory formation (Andriambeloson et al. 2014). The role of ACh in spontaneous alternations has been previously established, and is used in assessment of drug effects. Scopolamine adversely effects alternation rate in rats and mice (Spowart-Manning and van der Staay 2004). In agreement with previous studies, scopolamine administration impaired spatial working memory, evidenced by the significant reduction in spontaneous alternation behavior. Pre-treatment of rats with MFE at all the selected doses significantly improved the relative proportion of spontaneous alternation percentage when compared to the scopolamine-treated group. These results suggest that MFE could enhance short-term or working memory. On the contrast, scopolamine had no effect on the time taken by the rats to complete the sessions. This resonates with the findings of Thouvarecq et al. (2001), where subcutaneous administration of scopolamine hydrobromide did not affect motor skills of the animals. There was no significant difference noticed in the mean session durations between any of the groups.
The novel object recognition test is a task based on a non-rewarded model, which is centered around the spontaneous exploratory behavior of rodents (Pitsikas 2015). The test employs both an exploratory behavior and a memory retention paradigm, as the animal must first spend considerable time exploring the familiar object in the acquisition trial, before it is able to distinguish between the familiar and novel objects in the retention trial (Foyet et al. 2015). MFE significantly increased the preference of the rats towards exploring the novel object as compared to the amnesic control group, and subsequently increased the discrimination index, inferring that MFE has a positive effect on recognition memory.
In order to understand the underlying mechanism of action of MFE, we measured various biochemical parameters, i.e., AChE and antioxidant enzyme levels in the brain. Evidence suggests an extensive loss of cholinergic function in AD, and alteration of AChE activity is a hallmark of AD pathophysiology. Therefore, AChE inhibitors have been a first choice of treatment of AD, with an aim to increase the endogenous acetylcholine levels and relieve symptoms, thereby reversing cognitive impairment (Lima et al. 2009; Khan 2012). In this study, pre-treatment with MFE showed a significant reduction in AChE activity, with the highest dose of MFE producing effects similar to that of the standard drug. Thus, it can be inferred that the nootropic effect of MFE could be due to the alteration of the cholinergic neuronal system.
Agents with more than one pharmacological activity can be more beneficial in the treatment of AD, since they can act on multiple targets simultaneously (Barai et al. 2018). Healthy neural cells contain high concentrations of enzymes such as GSH peroxidase, catalase; and small molecules such as glutathione and ascorbic acid. These substances act as antioxidant defenses which protect the cells from damage caused by ROS. Oxidative stress occurs due to an excessive production of ROS, loss of antioxidant defenses, or a combination of both (Schulz et al. 2000). Since the brain consumes a high amount of oxygen, it is therefore highly predisposed to the detrimental effects of oxidative stress (Ngoupaye et al. 2017). Lipid peroxidation is a process caused by ROS-mediated damage to cellular membranes, which usually produce a number of stable end-products like malondialdehyde (MDA); which can in turn be used as a biomarker to determine oxidative stress (Sultana et al. 2013). Increased nitrite levels are indicative of nitrosative stress in the brain (Ishola et al. 2016), and increased nitric oxide production is observed in neurodegenerative diseases (Ma et al. 2010). Scopolamine administration caused an increase in oxidative and nitrosative stress shown by elevated MDA and nitrite levels. Pre-treatment with MFE significantly lowered the elevated levels of MDA and nitrite in the brain. Glutathione is a tripeptide thiol antioxidant which is involved in cellular detoxification of ROS. Intracellular presence of GSH indicates oxidative stress. (Naik et al 2017; Rahman et al. 2007). Catalase is an enzyme which reduces hydrogen peroxide and prevents hydroxyl radical generation, thereby exerting a protective effect on cells. The activity of CAT is highly impacted by oxidative alterations (Khan 2012). Treatment of the amnesic rats with MFE significantly preserved the activities of GSH and catalase, which were adversely affected by scopolamine. These results denote that MFE possesses potent antioxidant activity by scavenging free radicals, thereby exerting a protective effect against SCOP-mediated oxidative damage.
Quantitative phytochemical estimation revealed MFE to be a rich source of phenolic compounds, primarily, of flavonoids. Reports have established a link between phenolic compounds and antioxidant activity (Habu and Ibeh 2015). Out of the 8000 naturally occurring phenolics, about a half of them are flavonoids; which are present in plants either in their free state or as glycosides. Flavonoids acts as antioxidants, an activity highly dependent on their free hydroxyl group (Sulaiman and Balachandran 2012). There is strong evidence that flavonoids have a positive impact on memory and learning with possible mechanisms including synaptic signal regulations, which affect synaptic plasticity and produce an elongated long-term potentiation (LTP) in the hippocampus (Rendeiro et al. 2012). The results obtained in this study confirm the flavonoid-rich extract of Mesua ferrea to possess significant nootropic activity.
Increase in cholinergic neurotransmission is achieved by inhibiting the metabolizing enzyme AChE, therefore making cholinesterase inhibitors an important therapeutic regimen in the treatment of AD (Saxena et al. 2007). This mechanism combined with an antioxidant effect of a drug, would effectively inhibit the progression of AD; therefore making Mesua ferrea a potential candidate for the treatment of dementia.
Conclusion
To our best knowledge, this is the first report with scientific evidence on the memory-enhancing effect of Mesua ferrea flowers. The extract improved scopolamine-induced spatial and non-spatial memory deficits in the T-maze continuous alteration and novel object recognition paradigms/tasks/models, decreased brain acetylcholinesterase activity, and demonstrated potent antioxidant capability. However, this can be confirmed by further studies on the identification and isolation of the active constituent/s of this plant responsible for this activity.
Availability of data and material
To be made available on request.
Code availability
Not applicable.
Abbreviations
- ABTS:
-
2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- ACh:
-
Acetylcholine
- AChE:
-
Acetylcholinesterase
- AD:
-
Alzheimer’s disease
- ANOVA:
-
Analysis of variance
- ATC:
-
Acetylthiocholine iodide
- CAT:
-
Catalase
- CPCSEA:
-
Committee for purpose of control and supervision of experiments on animals
- DI:
-
Discrimination Index
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- DTNB:
-
5,5’-dithio-bis (2-nitrobenzoic acid)
- GAE:
-
Gallic acid equivalents
- GSH:
-
Glutathione
- IAEC:
-
Institutional Animal Ethics Committee
- LTP:
-
Long-term potentiation
- MDA:
-
Malondialdehyde
- MFE:
-
Mesua ferrea flowers ethanolic extract
- NORT:
-
Novel object recognition test
- PIR:
-
Piracetam
- RE:
-
Rutin equivalents
- ROS:
-
Reactive oxygen species
- SEM:
-
Standard error of mean
- SCOP:
-
Scopolamine
- TBARS:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- T-CAT:
-
T-maze continuous alternation task
- TFC:
-
Total flavonoid content
- TPC:
-
Total phenolic content
References
Abozed SS, El-kalyoubi M, Abdelrashid A, Salama MF (2014) Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Ann Agric Sci 59(1):63–67. https://doi.org/10.1016/j.aoas.2014.06.009
Alibabaei Z, Rabiei Z, Rahnama S, Mokhtari S, Rafieian-Kopaei M (2014) Matricaria chamomilla extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Biomed Aging Pathol 4(4):355–360. https://doi.org/10.1016/j.biomag.2014.07.003
Anandakumar A, Balasubramanian M, Muralidharan R (1986) Nagakesara - a comparative pharmacognosy. Anc Sci Life 5(4):263–268
Andriambeloson E, Huyard B, Poiraud E, Wagner S (2014) Methyllycaconitine- and scopolamine-induced cognitive dysfunction: differential reversal effect by cognition-enhancing drugs. Pharmacol Res Perspect 2(4):1–8. https://doi.org/10.1002/prp2.48
Baliga SM, Sharake M, Vaishnav LK, Rao S, Palatty PL (2013) Rasayana drugs from the ayurvedic system of medicine as possible radioprotective agents in cancer treatment. Integr Cancer Ther 12(6):455–463. https://doi.org/10.1177/1534735413490233
Barai P, Raval N, Acharya S, Acharya N (2018) Bergenia ciliata ameliorates streptozotocin-induced spatial memory deficits through dual cholinesterase inhibition and attenuation of oxidative stress in rats. Biomed Pharmacother 102:966–980. https://doi.org/10.1016/j.biopha.2018.03.115
Bhattamisra SK, Singh PN, Singh SK (2012) Effect of standardized extract of Marsilea minuta on learning and memory performance in rat amnesic models. Pharm Biol 50(6):766–772. https://doi.org/10.3109/13880209.2011.632421
Chahar MK, Sanjaya Kumar DS, Lokesh T, Manohara KP (2012) In-vivo antioxidant and immunomodulatory activity of mesuol isolated from Mesua ferrea L. seed oil. Int Immunopharmacol 13(4):386–391. https://doi.org/10.1016/j.intimp.2012.05.006
Dennis TJ, Akshaya Kumar K (1998) Constituents of Mesua ferrea. Fitoterapia 69:291–304
Dey A, Bhattacharya R, Mukherjee A, Pandey DK (2017) Natural products against Alzheimer’s disease: pharmaco-therapeutics and biotechnological interventions. Biotechnol Adv 35(2):178–216. https://doi.org/10.1016/j.biotechadv.2016.12.005
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Foyet HS, Ngatanko Abaïssou HH, Wado E, Asongalem Acha E, Alin C (2015) Emilia coccinae (SIMS) G Extract improves memory impairment, cholinergic dysfunction, and oxidative stress damage in scopolamine-treated rats. BMC Complement Altern Med 15(1):1–12. https://doi.org/10.1186/s12906-015-0864-4
Garg S, Sharma K, Ranjan R, Attri P, Mishra P (2009) In vivo Antioxidant activity and hepatoprotective effects of methanolic extract of Mesua ferrea linn. Int J PharmTech Res 1(4):1692–1696
Gerlai R (1998) A new continuous alternation task in T-maze detects hippocampal dysfunction in mice: a strain comparison and lesion study. Behav Brain Res 95(1):91–101. https://doi.org/10.1016/S0166-4328(97)00214-3
Ghumatkar PJ, Patil SP, Jain PD, Tambe RM, Sathaye S (2015) Nootropic, neuroprotective and neurotrophic effects of phloretin in scopolamine induced amnesia in mice. Pharmacol Biochem Behav 135:182–191. https://doi.org/10.1016/j.pbb.2015.06.005
Habu JB, Ibeh BO (2015) In vitro antioxidant capacity and free radical scavenging evaluation of active metabolite constituents of Newbouldia laevis ethanolic leaf extract. Biol Res 48:1–10. https://doi.org/10.1186/s40659-015-0007-x
Hitzenberger G (1998) Pharmacological properties of piracetam: rationale for use in stroke patients. CNS Drugs 9(SUPPL. 1):19–27. https://doi.org/10.2165/00023210-199809001-00003
Ishola IO, Tota S, Adeyemi OO, Agbaje EO, Narender T, Shukla R (2013) Protective effect of Cnestis ferruginea and its active constituent on scopolamine-induced memory impairment in mice: a behavioral and biochemical study. Pharm Biol 51(7):825–835. https://doi.org/10.3109/13880209.2013.767360
Ishola IO, Awoyemi AA, Afolayan GO (2016) Involvement of antioxidant system in the amelioration of scopolamine-induced memory impairment by grains of paradise (Aframomum melegueta KSchum.) extract. Drug Res (Stuttg) 66(9):455–463. https://doi.org/10.1055/s-0042-109391
Ishola IO, Ikuomola BO, Adeyemi OO (2018) Protective role of Spondias mombin leaf and Cola acuminata seed extracts against scopolamine-induced cognitive dysfunction. Alexandria J Med 54(1):27–39. https://doi.org/10.1016/j.ajme.2016.08.001
Jeong EJ, Ma CJ, Lee KY, Kim SH, Sung SH, Kim YC (2009) KD-501, a standardized extract of Scrophularia buergeriana has both cognitive-enhancing and antioxidant activities in mice given scopolamine. J Ethnopharmacol 121(1):98–105. https://doi.org/10.1016/j.jep.2008.10.006
Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR (2005) Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 64(7):1152–1156. https://doi.org/10.1212/01.WNL.0000156156.13641.BA
Khan RA (2012) Effects of Launaea procumbens on brain antioxidant enzymes and cognitive performance of rat. BMC Complement Altern Med 12(1):1. https://doi.org/10.1186/1472-6882-12-219
Khandelwal KR (2008) Preliminary Phytochemical Screening. In: Practical pharmacognosy. Nirali Prakashan, Pune, India, pp. 149-157.
Klinkenberg I, Blokland A (2010) The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev 34(8):1307–1350. https://doi.org/10.1016/j.neubiorev.2010.04.001
Kumar V, Singh PN, Muruganandam AV, Bhattacharya SK (2000) Effect of Indian Hypericum perforatum Linn on animal models of cognitive dysfunction. J Ethnopharmacol 72(1–2):119–128. https://doi.org/10.1016/S0378-8741(00)00216-6
Kumaran A, Joel Karunakaran R (2007) In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci Technol 40(2):344–352. https://doi.org/10.1016/j.lwt.2005.09.011
Lim TK (2016) Mesua ferrea, In: Edible medicinal and non-medicinal plants. Volume 7, Flowers. Springer, Netherlands, pp: 641-652. https://doi.org/10.1007/978-94-017-7276-1
Lima JA, Costa RS, Epifânio RA, Castro NG, Rocha MS, Pinto AC (2009) Geissospermum vellosii stembark. Anticholinesterase activity and improvement of scopolamine-induced memory deficits. Pharmacol Biochem Behav 92(3):508–513. https://doi.org/10.1016/j.pbb.2009.01.024
Ma N, Sasoh M, Kawanishi S, Sugiura H, Piao F (2010) Protection effect of taurine on nitrosative stress in the mice brain with chronic exposure to arsenic. J Biomed Sci 17(SUPPL. 1):1–6. https://doi.org/10.1186/1423-0127-17-S1-S7
Makchuchit S, Itharat A, Tewtrakul S (2010) Antioxidant and nitric oxide inhibition activities of thai medicinal plants. J Med Assoc Thai 93(7):S227–S235
Mathuranath PS, George A, Ranjith N, Justus S, Kumar MS, Menon R, Sarma PS, Varghese J (2012) Incidence of Alzheimer’s disease in India: A 10 years follow-up study. Neurol India 60(6):625–630. https://doi.org/10.4103/0028-3886.105198
Naik B, Nirwane A, Majumdar A (2017) Pterostilbene ameliorates intracerebroventricular streptozotocin induced memory decline in rats. Cogn Neurodyn 11(1):35–49. https://doi.org/10.1007/s11571-016-9413-1
Ngoupaye GT, Pahaye DB, Ngondi J, Moto FCO, Bum EN (2017) Gladiolus dalenii lyophilisate reverses scopolamine-induced amnesia and reduces oxidative stress in rat brain. Biomed Pharmacother 91:350–357. https://doi.org/10.1016/j.biopha.2017.04.061
Odubanjo VO, Ibukun EO, Oboh G, Adefegha SA (2018) Aqueous extracts of two tropical ethnobotanicals (Tetrapleura tetraptera and Quassia undulata) improved spatial and non-spatial working memories in scopolamine-induced amnesic rats: Influence of neuronal cholinergic and antioxidant systems. Biomed Pharmacother 99:198–204. https://doi.org/10.1016/j.biopha.2018.01.043
Organization for Economic Cooperation and Development (OECD) (2000) Guidance document on acute oral toxicity. Environmental Health and Safety Monograph Series on Testing and Assessment No.24. Paris, France: OECD.
Organization for Economic Cooperation and Development (OECD) (2001) Draft updated test guidelines 420: Acute Oral toxicity – Fixed Dose Procedure. OECD, Paris
Ozarowski M, Mikolajczak PL, Bogacz A, Gryszczynska A, Kujawska M, Jodynis-Liebert J, Piasecka A, Napieczynska H, Szulc M, Kujawski R, Bartkowiak-Wieczorek J, Cichocka J, Bobkiewicz-Kozlowska T, Czerny B, Mrozikiewicz PM (2013) Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia 91:261–271. https://doi.org/10.1016/j.fitote.2013.09.012
Pahwa P, Goel RK (2016) Asparagus adscendens root extract enhances cognition and protects against scopolamine induced amnesia: an in-silico and in-vivo studies. Chem Biol Interact 260:208–218. https://doi.org/10.1016/j.cbi.2016.10.007
Pitsikas N (2015) The role of nitric oxide in the object recognition memory. Behav Brain Res 285:200–207. https://doi.org/10.1016/j.bbr.2014.06.008
Rabiei Z, Mokhtari S, Asgharzade S, Gholami M, Rahnama S, Rafieian-kopaei M (2015) Inhibitory effect of Thymus vulgaris extract on memory impairment induced by scopolamine in rat. Asian Pac J Trop Biomed 5(10):845–851. https://doi.org/10.1016/j.apjtb.2015.07.006
Rahman I, Kode A, Biswas SK (2007) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1(6):3159–3165. https://doi.org/10.1038/nprot.2006.378
Rajput PV (2018) Mode of action of Kalyanak Ghrita. World J Pharm Res 7(13):1273–1277
Raju MS, Srimannarayana G, Pa NVS (2016) Tetrahedron Letters NO. 49, DP 4509 - 4512, 1976. (49):6–9
Rendeiro C, Guerreiro JDT, Williams CM, Spencer JPE (2012) Postgraduate symposium: flavonoids as modulators of memory and learning: molecular interactions resulting in behavioural effects. Proc Nutr Soc 71(2):246–262. https://doi.org/10.1017/S0029665112000146
Rubio J, Dang H, Gong M, Liu X, Chen S, lin, Gonzales GF, (2007) Aqueous and hydroalcoholic extracts of Black Maca (Lepidium meyenii) improve scopolamine-induced memory impairment in mice. Food Chem Toxicol 45(10):1882–1890. https://doi.org/10.1016/j.fct.2007.04.002
Saxena G, Singh SP, Pal R, Singh S, Pratap R, Nath C (2007) Gugulipid, an extract of Commiphora whighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol Biochem Behav 86(4):797–805. https://doi.org/10.1016/j.pbb.2007.03.010
Schulz JB, Lindenau J, Seyfried J, Dichgans J (2000) Glutathione, oxidative stress and neurodegeneration. Eur J Biochem 267:4904–4911
Shin CY, Kim HS, Cha KH, Won DH, Lee JY, Jang SW, Sohn UD (2018) The effects of donepezil, an acetylcholinesterase inhibitor, on impaired learning and memory in rodents. Biomol Ther 26(3):274–281. https://doi.org/10.4062/biomolther.2017.189
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394. https://doi.org/10.1016/0003-2697(72)90132-7
Spowart-Manning L, Van Der Staay FJ (2004) The T-maze continuous alternation task for assessing the effects of putative cognition enhancers in the mouse. Behav Brain Res 151(1–2):37–46. https://doi.org/10.1016/j.bbr.2003.08.004
Sulaiman CT, Balachandran I (2012) Total phenolics and total flavonoids in selected indian medicinal plants. Indian J Pharm Sci 74(3):258–260. https://doi.org/10.4103/0250-474X.106069
Sultana R, Cenini G, Butterfield DA (2013) Biomarkers of oxidative stress in neurodegenerative diseases. In: Villamena FA (ed) Molecular basis of oxidative stress: chemistry, mechanisms, and disease pathogenesis. John Wiley and Sons, Inc., New York. pp 359–376. https://doi.org/10.1002/9781118355886.ch14
Thouvarecq R, Protais P, Jouen F, Caston J (2001) Influence of cholinergic system on motor learning during aging in mice. Behav Brain Res 118(2):209–218. https://doi.org/10.1016/S0166-4328(00)00330-2
Vasileva LV, Getova DP, Doncheva ND, Marchev AS, Georgiev MI (2016) Beneficial effect of commercial Rhodiola extract in rats with scopolamine-induced memory impairment on active avoidance. J Ethnopharmacol 193:586–591. https://doi.org/10.1016/j.jep.2016.10.011
Vasudevan M, Parle M (2007) Memory enhancing activity of Anwala churna (Emblica officinalis Gaertn.): an Ayurvedic preparation. Physiol Behav 91(1):46–54. https://doi.org/10.1016/j.physbeh.2007.01.016
Verotta L, Lovaglio E, Vidari G, Finzi PV, Neri MG, Raimondi A, Parapini S, Taramelli D, Riva A, Bombardelli E (2004) 4-Alkyl- and 4-phenylcoumarins from Mesua ferrea as promising multidrug resistant antibacterials. Phytochemistry 65:2867–2879. https://doi.org/10.1016/j.phytochem.2004.07.001
Wessapan C, Charoenteeraboon J, Wetwitayaklung P, Limmatvapirat C, Phaechamud T (2007) Antimicrobial activity of some edible flowers in Thailand. Planta Med 73(09):P_201. doi: https://doi.org/10.1055/s-2007-986982
Wu CYC, Lerner FM (2018) e Silva AC, Possoit HE, Hsieh TH, Neumann JT, Minagar A, Lin HW, Lee RHC (2018) Utilizing the modified T-maze to assess functional memory outcomes after cardiac arrest. J Vis Exp 131:1–7. https://doi.org/10.3791/56694
Yassin NAZ, El-Shenawy MA, Mahdy KA, Gouda NAM, Razik A, Marrie H, Farrag H, Ibrahim BMM (2013) Effect of Boswellia serrata on Alzheimer’s disease induced in rats. J Arab Soc Med Res 8:1–11. https://doi.org/10.7123/01.JASMR.0000429323.25743.cc
Acknowledgements
The authors would wish to thank the Principal and the administration of AISSMS College of Pharmacy, Pune, Maharashtra for providing the necessary facilities and equipment for the conduction of the studies.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Pallavi Shirsat-John – planned and performed experiments and biochemical analysis, data collection, statistical analysis, and drafting of the article; Tina Saldanha – data analysis and interpretation, critical revision of the article; Swati Kolhe – project planning and supervision; Ziyaurrahman A.R. – supervision of the research work and in-charge of overall direction and planning.
Corresponding author
Ethics declarations
Ethical statement
The guidelines established by the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), Govt. of India, were followed for the animal experiments. The protocols were approved by the Institutional Animal Ethics Committee of AISSMS College of Pharmacy, Pune (Reg. No. – 257/PO/ReBi/S/2000/CPCSEA).
Conflict of interest
Shirsat‑John Pallavi has no conflict of interest. Tina Saldanha has no conflict of interest. Swati Kolhe has no conflict of interest. A. R. Ziyaurrahman has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shirsat-John, P., Saldanha, T., Kolhe, S. et al. Antiamnesic effect of Mesua ferrea (L.) flowers on scopolamine-induced memory impairment and oxidative stress in rats. ADV TRADIT MED (ADTM) 23, 1109–1121 (2023). https://doi.org/10.1007/s13596-022-00654-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-022-00654-2