Abstract
Diseases of the kidney and urinary tract including urolithiasis became a major cause of global illness and death. There are approximately 7.85 million people suffering from chronic renal failure in India. Further, urolithiasis is also a common global phenomenon of renal disorders. Unani Medicine (UM) is a famous kind of conventional medicine practised throughout South Asia that relies on the ancient conventional medicinal systems of India, Egypt, China, Iraq, Syria, and Persia. Berberis vulgaris (BV), Physalis alkekengi (PA) and Boswellia serrata (BS) are very common drugs against urinary disorders in Unani medicine. But, to establish their role to treat in urinary disorders are still obscure. HPTLC fingerprints confirmed the presence of signature compounds according to pharmacopeia. Polyphenolic acids and flavonoids in the test drugs have been quantified by HPLC. BS showed maximum inhibitory action on DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals, nitric oxide and superoxide scavenging than other two test drugs, while, PA have highest hydrogen peroxide scavenging action. In diuretic activity, maximum urination occurred in BS group than other two test drugs BV and PA. The highest excretion of sodium–potassium ratio was noted in PA groups followed by BS and BV. BV showed maximum efficacy in crystal formation in test tubes followed by BS and PA.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of renal failure throughout the world has doubled over the last 15 years (Bellomo 2006). There are approximately 7.85 million people suffering from chronic renal failure in India. In this country 90% patients who suffer from renal disease are not able to afford the cost of treatment (Loh and Cohen 2009). Moreover, there are very limited treatments and perhaps, no safe modern drug in renal disorders is available in the market. Regrettably, recent medicines origin almost twenty percent of public and hospital learnt incidents of acute renal failure. Immune suppressants (Cyclosporine-A), aminoglycosides (Gentamicin) and aminonucleoside (Puromycin) are alleged for nephrotoxicity during the course of therapeutic regimen. Amphotericin-B, Cisplatin, ACE inhibitors are also taking major role in the treatment of renal disorders. Acquaintance to chemical substances similar to sodium oxalate, carbon tetra chloride, ethylene glycol and heavy metals such as cadmium, arsenic, lead and mercury also persuades nephrotoxicity (Naughton 2008; Lopez-Novoa et al. 2011). Further, urolithiasis is also a common global phenomenon of renal disorders. The maximum highest risk was reported in Saudi Arabia (20%) (Ahmed et al. 1993). Urolithiasis describes the development of stones in any of the urinary organs such as the kidney, ureter, urinary bladder, or urethra. The major cause of morbidity due to any major disease of urinary tract is Urolithiasis (Soundararajan et al. 2006). Even though maximum patients suffer from only one stone occurrence but 25% of total population face persistent stone production (Hesse et al. 2000). Urinary stone illness is still uncommon in kids, with the cumulative rate remaining constant in major studies. In children, variables linked to the metabolic syndrome complex, such as obesity, raise the danger of urinary stone development, just as they do in adults (Sarica et al. 2009). For all stone types, urolithiasis represents a disease with a definite male preponderance. There is different mechanism to form Urinary stone in the body. Unlike uric acid or cystine calculi, which are caused by oversaturation, infection stones are caused by bacterial metabolism (Moe 2006). The development of the most prevalent portion, calcium-containing calculi, is more complicated and, unexpectedly, remains a mystery. Therefore, searching of new and safer drugs for prevention and treatment of urinary ailments is utmost urgent.
Unani Medicine (UM) is a widespread system of alternative treatment extensively proficient across Southern Asia that is based upon the primordial conventional medicinal systems of India, Iraq, China, Syria, Egypt and Persia. The finest conceivable methods of development of health by this holistic medicinal system are perfection of Tabiyat by immune modulators, self-control of Asbaab-e-Sitta Zarooriya (6 vital aspects for life) and acceptance of Ilaj Bil Tadabeer (regimental therapeutic), Ilaj-bil-ghiza (dieto-therapy) and Munzijwa Mushil (concoctive and purgative) therapeutic. Natural unani treatments include suitable herbal medicines, minerals, additional food, the promotion of health-promoting norms of behaviour and adequate rest as preventive and cure. According to Ibn Sina, in Unani Medicine, urolithiasis is a disorder where stone is produced in Kuliya (kidney), in Masana (urinary bladder) and Qanat-e-Bauliyah (urinary tract). The major reasons for these ailments describe as Sue-Mizaj-e-Haar (impairment of hot temperament), Zof-e-Quwwat-e-Dafia (weakness of power of expulsion), Zof-e-Kuliya (weakness of kidney), Iltihab-e-Kuliya (inflammation of kidney), Ehtibas-e-Mawaadfasida (retention of excreta), Qurooh-e-Kuliya (renal sepsis). Many plants and natural products were identified for the prevention and the therapy of urinary maladies in UM throughout the ecosphere. Most informative and promising plants so far used in UM for urinary ailments are Aerva lanata Juss (Bisehribooti), Rheum officinalis (Revandchini), Withania somnifera (Asgand), Tribulus terrestris (Kharekhasak), Ferula foetida (Hing), Berberis vulgaris (Zereshk), Boswellia serata (Luban), Physalis alkekengi (Kaknaj), Banadequl buzoor (Polyherbal formulation) etc. Scientific reports revealed their chemical constituents and pharmacological activities (Soundararajan et al. 2006; Hesse et al. 2000; Sarica et al. 2009; Moe 2006; https://upnrhm.gov.in/assets/site-files/gogl/fy2014-15/Essential_Unani_Medicines.pdf). But, to establish their role to treat in urinary disorders are still obscure. Hereafter, in this research work an effort was taken to compute the experimental protocol against urinary disorders by taking three plants Berberis vulgaris, Boswellia serrata and Physalis alkekengi.
About the plants
Berberis vulgaris (Family: Berberidaceae) is a shrub, instinctive to central and southern Europe, northwest Africa and western Asia. It is used in all traditional medical systems, like Ayurveda, Unani, Homeopathy, Iranian and Chinese medicine. All portions of the plant have been claimed to have medicinal qualities, including antipyretic, antibacterial, antipruritic, tonic and cholagogue effects. Cholecystitis, jaundice, gall stones, cholelithiasis and dysentery have also been treated with it. For the treatment of kidney and urinary bladder stone it is useful. B. vulgaris fruit juice has the abilities to precipitate out calcium oxalate. The anticancer property of B. vulgaris has also been reported (https://ccrum.res.in/writereaddata/UploadFile/The%20Science%20of%20Health%20and%20Healing01076_1861.pdf; Syeda et al. 2004; Lone et al. 2012). Despite its wide range of uses and many characteristics, the mechanism of action for the majority of its effects remains unknown.
Boswellia serrata (Family: Burseraceae) is a deciduous tree with ash-colored papery bark that grows to be moderate to big in size. Resin from this is prevalently recycled in Indian Traditional Systems of medication for the many epochs in several disorders specifically, cardiac problems, asthma, rheumatism, urinary disorders etc. Boswellic acid and other volatile oils are present in resin. It is also an ingredient of certain compound formulations viz. Majoonkundur, Murawwah-ul-Arwah, Dawa-ul-Kibrit in Unani medicine for treating different urinary problems. It’s also beneficial in dysuria and is useful in urinary or renal dysfunctions (Seyyed and Fallah 2012; Arayne et al. 2007; Tomosaka et al. 2008; Fatehi-Hassanabad et al. 2005). More scientific knowledge are required to establish its precise role in urinary disorders.
Physalis alkekengi (Family: Solanaceae) is an important medicine used frequently by Unani physicians in the management at different urinary diseases. It has anti-inflammatory, diuretic and reno-protective properties. It is useful in renal stone and urinary tract and bladder infections. Presence of glycosidic alkaloids, physalins and histonin are found in this plant. Other studies confirm its anti-neoplastic activity (Alam et al. 2011, 2012; Shah et al. 2008). But, its actual role in urinary disorders is still doubtful.
Materials and methods
Plant materials
The fruits of Berberis vulgaris and Physalis alkekengi were collected, identified and processed following Indian Pharmacopeia’s stated guidelines, while standardized Boswella serrata resin were procured from registered vendor in powder form (Fig. 1). Botanical Survey of India, West Bengal, authenticated all three plant materials. The shed dried fruits of Berberis vulgaris and Physalis alkekengi were extracted with hydro-ethanol (50% v/v) and evaporated to dryness.
Phytochemical standardization
The shade dried fruits were percolated in hydro-alcohol (50:50 v/v) for 3 days. The solvents were filtered and dried. The code names used for the test drugs were BV (Berberis vulgaris), BS (Boswellia serrata) and PA (Physalis alkekengi).
The presence of bioactive phytoconstituents in test extracts (BV, BS & PA) were analysed by qualitative processes (Asif et al. 2014). In densiometric HPTLC analysis, the test samples were marked like bands on a pre-coated silica gel plates (Merck, 60F254, 10 × 10 cm) using Camag Linomat 5 applicator. The solvent system used for the development of this chromatogram was Toluene, Ethyl acetate and Formic acid (3.5:4:1). The densitometric scanning were completed on Camag TLC Scanner 3 at absorbance 280 nm. It was operated by multi-level winCATS planar chromatography manager (Ahmad et al. 2010).
The test samples were then passed through the HPLC analyses. It was executed using Dionex Ultimate 3000 liquid chromatograph (Germany) with quaternary pump including a diode array detector. The separations were reached by a reversed-phase Nova pac C18 column and one percent acetic acid aqueous solution with acetonitrile were in the mobile phase (rate of flow 0.7 ml/min). Gradient fractionation was carried out by changing the ratio of solvent B to A. Different standard solutions were produced individually with methanol and mobile phase (1:1v/v) at a concentration of 1 mg/ml. The sample was quantified via measuring the integrated peak area over the concentration of the respective standards at 272 nm (Domberger 1981).
Gas chromatography with electrochemical detection (GC-ECD) studies had been carried out utilising gas chromatograph, Agilent Technologies (USA) and electrochemical detector (MSD 5977A). An Agilent Wax capillary column with a length of 30 m, an internal diameter of 0.25 mm, and a thickness of 0.25 m was utilized. The column's initial temperature was set to 50 °C (5 min), with a 5 °C per min rise up to 250 °C (10 min). 99.9% helium has been utilized as a carrier gas. The injector as well as detector were both 250 °C. A 1 µl injector volume has been utilized. The samples were prepared utilising a standard protocol (Masao et al. 1988). The elements have been detected by comparing the mass and molecular structure utilising Max-Planck Institute Library (Germany) software search programmer.
In vitro antioxidant activities of test drugs
DPPH radical scavenging
The molecule α,α-diphenyl-β-picrylhydrazyl (DPPH) is classified as a stable free radical due to the delocalization of the spare electron throughout the whole molecule, which prevents dimerization, as is the case with most other free radicals. The deep violet coloration is also caused by electron delocalization. When a DPPH solution is combined with a test material that may donate a hydrogen atom, the reduced form is formed with the loss of the violet coloration.
The stable free radical DPPH has been utilised to evaluate the radical scavenging assay of plant extracts by monitoring change in optical density of DPPH radicals. 0.1 ml of herbal extracts in methanol (80%) at various concentrations (known) has been combined with 3.9 ml of 0.135 mM DPPH solution and kept in the dark for 30 min. At 517 nm, the absorbance values were measured (Harbone 2002). The percentage of DPPH radical scavenging was computed by the equation mentioned below. The IC50 values were determined using linear regression analysis and were utilized to determine antioxidant capability.
where Abr and Aar are the absorbance before and after the reaction respectively.
Hydrogen peroxide scavenging
Humans are exposed indirectly to H2O2 from the atmosphere at a rate of around 0.28 mg/kg/day, with the majority of their consumption coming from leafy cereals. H2O2 might permeate the mammalian by inhaling vapour or mist, as well as through eye or skin contact. H2O2 is rapidly degraded into oxygen and water, which can result in the formation of hydroxyl radicals (OHֺ•), which can trigger lipid peroxidation and damage DNA in the body. The approach may be used to determine the capacity of plant extracts to scavenge hydrogen peroxide are mentioned below.
A spectrophotometer was utilized to study the scavenging of hydrogen peroxide by plant extracts. The reaction mixture contains 2 ml of different concentration of test extracts in 50 mM phosphate buffer pH 7.4 and 0.6 ml of 40 mM H2O2. After 10 min, this mixture was thoroughly mixed and absorbance was measured at 532 nm (Senguttuvan and Subramaniam 2011). Phosphate buffer without H2O2 considered as blank. Then the proportion of H2O2 scavenging is computed as follows. The IC50 values were determined using linear regression analysis and were utilized to determine antioxidant capability.
where Ai and At are the absorbance of control and test respectively.
Nitric oxide radical scavenging
NOֺ• is produced in biologic tissues by certain nitric oxide synthases, which convert arginine to citrulline, resulting in the creation of NO via a five-electron oxidative process. At physiological pH (7.2), the chemical sodium nitroprusside is known to breakdown in aqueous solution, releasing NO•. Under aerobic circumstances, NO• reacts with oxygen to form stable compounds (nitrate and nitrite), the amounts of which may be measured using the Griess reagent.
The test extracts were mixed with 0.5 ml of 1 M phosphate buffer saline and 2 ml of 10 mM sodium nitroprusside and incubated at 25 °C for 150 min. Afterward, 0.5 ml of the nitrite-comprising reaction mixture was combined with 1 ml of 0.33% sulphanilic acid reagent and left standing for 5 min. Thereafter, 1 ml of 1% naphthyl ethylene di-amine dihydrochloride was added, mixed and left standing for another 30 min (Harbone 2002). At 546 nm, the absorbance was detected. The quantity of nitric oxide radical inhibition is estimated using the following equation.
where A0 and A1 are the absorbances before and after the reaction with Griess reagent respectively.
Superoxide anion radical scavenging
Although superoxide anion is a mild oxidant, it eventually forms harmful hydroxyl radicals and singlet oxygen, both of which lead to oxidative stress. The superoxide anion scavenging activity can be determined in the manner described below.
Three millilitres of Tris–HCl buffer (0.1 M, pH7.4) comprising 0.75 ml of NADH (936 µM) solution, 0.75 ml of nitro blue tetrazolium (300 µM) solution, and 0.3 ml of test solution at different concentrations were used to produce the super-oxide radical mixture. To initiate the reaction, 0.75 ml of 120 µM phenazine methosulphate was added to that composition. The resultant mixture solution was exposed to light at room temperature for 5 min. Thereafter, the absorbance was taken at 560 nm (Harbone 2002). The percentage inhibition of generating super oxide anion was calculated and represented as IC50.
Pharmacological actions of test drugs
Animals
Swiss mice (25 to 30 gm) of either sex and adult Wistar rats (180 to 200 gm) were utilized in this experiment. All experiments have been performed in compliance with the criteria for the care and management of laboratory animals established by The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA 2003), Govt. of India (Serra et al. 2021). Group housed (maximum 4) in stainless steel grill top polypropylene cage, having water bottle and food facilities, and clean husk bedding for mice and rats. Air-conditioned rooms, temperature between 20–25°C, 65% relative humidity and an artificially fluorescent illumination cycle programmed to 12 h of light and darkness. The mice and rats were fed the same standard pellet diet. Purified water was delivered twice a day to the food. Food was withdrawn according to study protocol. Institutional animal ethical committee (R/N 959/c/06/CPSEA) approved the proposal and given their consent for animal use.
Acute toxicity studies
Single dose toxicity limit test was done in compliance with the Organisation for Economic Co-operation and Development (OECD) guideline number 423 (Ronald Hites 1997). Limit tests were performed with three animals for each test substance, BV, BS, and PA, at a single dose level of 2000 mg per kg body weight (b.w). If test substance-associated mortality was seen, additional testing at a lower threshold might well be required. The studies were conducted on Swiss albino female mice (25 to 30 gm b.w) with a single fixed dose (2 g/kg) given orally. Foods were withheld overnight before administering the test drugs; however, water were available during this period. The mice were then observed for incidence of mortality and signs of intoxication for 14 days.
Selection of doses
The test drugs, BV, BS and PA were administered through oral gastric tube once a day in at three dose levels, 50 mg/kg, 100 mg/kg and 200 mg/kg. The doses were selected depending upon the results of LD50 or based on pilot studies. In pilot study, 25 mg/kg–400 mg/kg dose was taken. The dose of 50, 100, and 200 mg per kg b.w were found to be most effective as per the dose response relationship.
Diuretic activity
Diuretics are substances that induce diuresis, or the increased output of urine. This involves forced diuresis. The diuretic property of the test extracts in rat model were also done by placing the rat in the metabolic cage and estimating the parameters in urine. All the parameters, like, sodium, potassium, chloride was standardized. The primary findings depicted that all three-test drug have to some extent diuretic properties.
The standard method (Mahmud et al. 2012; Andreae 1955) has been utilized to determine diuretic action. The diuretic activities of individual test drugs in rats weighing 180–200 g were evaluated. The animal groups were made according to the Table 1.
Prior to the experiment, eleven groups of six rats were starved and deprived of water for eighteen hours. A priming dose of 25 ml per kg b.w. normal saline solution was administered to all of the animals. The rats were immediately placed in metabolic cages designed to separate faeces and urine and were maintained at 25 ± 0.5 °C throughout the trial. During this time, animals were not provided food or water. Furosemide was administered at a dosage of 20 mg per kg. The urine was acquired in measuring cylinders up to 4 h after dosing. The urine volume was measured by graduated glass measuring cylinder. The routine urinalysis, particularly colour, pH, specific gravity, glucose, protein, blood, ketone, bilirubin was done by strip test (Siemens HealthCare Diagnostics Limited, India). Potassium, sodium, and chloride present in rat urine were measured spectrophotometrically using commercially ready kits (Coral, India). The diuretic activities of individual test drugs in rats were assessed.
Antiurolithiatic activity
Earlier research found that male rat’s ammonium chloride and ethylene glycol led in the production of renal calculi primarily constituted of CaC2O4. Hyperoxaluria, that causes increased renal retention and excretion of oxalate, induces stone development in ethylene glycol fed rats (Green et al. 1982). Renal CaC2O4 deposition in rats by ammonium chloride and ethylene glycol is widely used to simulate human urinary stone formation. Ammonium chloride has been found to hasten lithiasis (Rodrigo et al. 2002). Effective urolithiasis prevention medications are now necessary. However, despite significant advances in medical care, there is no effective medicine to treat kidney stones.
Because plant extracts and their constituents have antiurolithiatic actions, herbal remedies could be a source of potential antiurolithiatic therapies. These might have an impact on pH, urine volume, urinary magnesium and oxalate levels, creatinine, reactive oxygen species (ROS), serum urea and uric acid levels, and inflammatory reactions. The anti-urolithiatic effects of the three test drugs were evaluated in this study by using both in-vitro and in-vivo methods.
In vitro crystallisation techniques have been extensively employed in urolithiasis investigation for a variety of applications. Kidney stone production is a complicated process that involves a series of physicochemical events such as super saturation, nucleation, growth, aggregation, and retention within renal tubules. The first positive (+ ive slope) slope of the turbidity curve is usually noted, that is primarily owing to an increasing particle count caused by crystal nucleation. Following the achievement of a plateau, a gradual drop in absorbance (-ve slope) shows the reduction in particle count caused by crystal aggregation. Calcium stone inhibitors are substances that prevent the production of calcium stones. Prevent crystal formation and aggregation by covering the surface of developing calcium crystals with calcium and oxalate or by treating with calcium and oxalate.
The test drugs were assessed using both in vitro and in vivo methods.
In vitro method
Nucleation & aggregation assay
The production of a stone in the urinary tracts or kidneys is the characteristic of urolithiasis. Stones forms as a result of a phase transition wherein dispersed salts condensed to solids, which is influenced by super saturation (SS). Because of the simplicity and reliability, nucleation assay is the standard model for studying oxalate crystallisation. This model comprises the investigation of crystallisation without and with inhibitor in order to evaluate the inhibitory potential of any chemical species utilized. Calcium chloride (CaCl2) and sodium oxalate (NaC2O4) solutions were prepared with respective proportion of 5 mmol per lit and 7.5 mmol per lit in a buffer comprising NaCl 0.15 mol per lit and Tris 0.05 mol per lit at pH 6.5. 100 μl of individual plant extracts at different concentrations (40, 80, 100, 200 and 400 µg/ml) were combined with CaCl2 solution (950 μl). The addition of sodium oxalate (950 µl) solution started the crystallisation process. Crystals of substance will dissolve if SS is less than one, while crystals can form and expand if SS is greater than 1. If the urine SS is greater than 1 then it is metastable and excess dissolved substances are precipitated. As a control, distilled water (100 µl) without extract has been utilized. The samples have been kept at 37 °C. The turbidity of the solutions was measured at 620 nm after incubation. The rate of nucleation was calculated by matching the induction times for CaC2O4 crystal formation in the absence and presence of the individual test plant extracts.
The approach aggregation assay was used to determine the rate of aggregation of CaC2O4 crystals. The CaC2O4 monohydrate crystals were synthesized by combining 50 mmol per lit solutions of CaCl2 and NaC2O4. Both solutions were then equilibrated in a water bath at 60 °C for 1 h. The solutions were then cooled to 37 degrees Celsius and evaporated. At pH 6.5, the CaC2O4 monohydrate crystals were dissolved with Tris 0.05 mol per lit and NaCl 0.15 mol per lit to a final concentration of 0.8 mg per ml. Experiments were carried out at 37 °C in presence of the 100 µl plant extracts after the stirring was stopped. The absorbance was measured at 620 nm (Sharma et al. 2017; Lipschitz et al. 1943). The percentage aggregation inhibition was then estimated by comparing the turbidity in the presence of extract to that obtained in the control using the formula below. The 50% inhibitory concentrations (IC50) were calculated by probit analysis and assessed using the chi-squared test.
In vivo methods
Urinary super-saturation with regard to stone-forming components is often regarded as a causal element in calculogenesis. The antilithiatic effect in albino rats was evaluated using an ethylene glycol-induced hyperoxaluria model. In the present investigation, urolithiasis was produced in all groups except the normal group by adding ammonium chloride (0.75%, w/v) to drinking water for three days and ethylene glycol (1%, v/v) for the remaining 33 days (Al-Saikhan and Ansari 2016). The test drugs with fixed doses were given simultaneously with ethylene glycol (Table 2).
On day 36, blood was drawn from the animal’s heart that had been anaesthetized with thiopentone sodium (45 mg/kg, body wt., i.p.) and thereafter sacrificed and weighed. The kidney index/coefficients were calculated using organ to body weight ratio.
Albumin levels, serum protein, SGPT, SGOT, alkaline phosphatase, urea, creatinine and uric acid was estimated spectrophotometrically using commercial kits (Hennequin et al. 1993). Renal tissues were homogenized in phosphate buffer saline to measure protein (Hess et al. 1989), lipid peroxides (LPO) (Aggarwal et al. 2010) reduced glutathione (GSH) (Divakar et al. 2010) superoxide dismutase (SOD) (Mitchell and Rosner 2006) and nitric oxides (NO) (Lowry et al. 1951).
Moreover, histological section of kidney was stained (H & E) and microscopically determined the number of CaC2O4 deposits were counted microscopically at ten random fields (× 1000 magnification). The following scores were assigned to renal crystal deposits: no crystals deposition = 0; crystals deposition in the papillary tip = 1; crystals deposition in the cortico-medullary junction = 2 and crystals deposition in the cortex = 3. If the crystals were spotted in various regions, the points were summed altogether to determine the total score (Ohkawa et al. 1979).
Nephro-protective activity
The detection of early kidney injury utilizing urinary biomarkers is crucial for assessing adversity in preclinical toxicology investigations, which will support in the reduction of lead candidate attrition in drug development. Gentamicin (GM) is an efficient aminoglycoside antibiotic that is extensively recommended for the treatment of infections, but its long-term therapeutic use is limited due to the accompanying adverse effects of oxidative stress and renal injury. Earlier research clearly suggests that ROS have a role in the renal effects of GM. Furthermore, ROS are involved in proximal tubular necrosis. Earlier it was observed the roles of test drugs to protect the renal tissues caused by oxidative stress.
This current investigation was intended to assess protective and curative efficacies of BS, BV and PA on GM-mediated nephrotoxic effects in rats. The preventive and curative aspects of test drugs were assessed in GM mediated nephrotoxicity in rats. GM at the dosage 100 mg per kg per day intraperitoneally was given to Wistar rats for consecutive five days to produced nephrotoxicity (Ellman 1959; Kono 1978). Thereafter, the individual test drugs at different doses were given from day 6 and continued to day 15. Induction of GM-mediated nephrotoxic effects in rats were standardized following the estimation of all biochemical parameters such as sodium, potassium, chloride, urea, uric acid, creatinine and also determination of all antioxidant biomarkers in renal tissues like, lipid peroxides, reduced glutathione, superoxide dismutase, catalase and nitric oxides. Histopathological evaluation in renal tissues was also conducted.
Preventive study of nephro-protective activity
The individual test extracts at different doses (50, 100 and 200 mg per kg, single/day) have been given orally for 5 consecutive days. 24 h after the last treatment (at day 6) all rats were examined for their protective actions.
Curative study of nephro-protective activity
The individual test drugs at multiple doses (50, 100 and 200 mg per kg, single/day) were started from day 6 and continued for day 15. At day 15 all animals were examined for their curative actions as Nephro-protective drug. The following parameters were assessed to find out the efficacy of individual test drugs and compared to control.
Urine analysis
After the last dose, individual animal was kept in distinct metabolic cages for 4 h for collecting urine. The urine output, urinary albumin, urea, creatinine and electrolytes (sodium, potassium, and chloride) were measured (Hennequin et al. 1993).
Blood analysis
At the final moment of the investigation (24 h of last dose), blood samples were collected to assess blood protein, albumin, uric acid, urea nitrogen (BUN), creatinine, potassium, sodium, and chloride using commercial kits (Hennequin et al. 1993).
Antioxidants analysis
Renal tissues were homogenized in phosphate buffer to measure protein (Hess et al. 1989), LPO (Aggarwal et al. 2010), GSH (Divakar et al. 2010), SOD (Mitchell and Rosner 2006) and NO (Lowry et al. 1951).
Histological analysis
A portion of the kidney were processed for histological studies and stained with hematoxylin–eosin for examining histomorphometry of glomeruli, proximal and distal tubules of kidney (Ohkawa et al. 1979).
Statistical analysis
The data were given in mean ± standard deviation. Percent change was calculated if necessary. Utilizing software (SPSS v20), the groups were statistically analyzed through ANOVA and post hoc Dunnett’s test. p value < 0.05 was deemed significant.
Results
Phytochemical standardization
All three test drugs (extracts) have bioactive or pharmacologically active groups like, tannins, triterpenoids, phenolics, flavonoids and sterols. Besides that, BV and PA also contain glycosides, BS and PA contain saponins. Previous reports supported that plant derived components tannins, triterpenoids, phenolics, flavonoids and sterols have several known therapeutic properties like, inflammation, vascular damage, metabolic disorders, urinary and renal dysfunctions and microbial infections (https://ccrum.res.in/writereaddata/UploadFile/The%20Science%20of%20Health%20and%20Healing01076_1861.pdf; https://iris.who.int/bitstream/handle/10665/340393/9789290228295-eng.pdf?sequence=1; Syeda et al. 2004; Lone et al. 2012; Seyyed and Fallah 2012; Arayne et al. 2007; Tomosaka et al. 2008; Fatehi-Hassanabad et al. 2005; Alam et al. 2011; Shah et al. 2008). From the leads, further chemical analyses were directed.
HPTLC fingerprints of the test drugs were showed characteristics bands of individual plant derived components. The identifications and characterizations were matched batch to batch to confirm the quality of test drug. It is interesting to note that all chromatograms were showed distinct features at 280 nm instead of 254 and 266 nm. In 280 nm small phenolics and flavonoids were usually detected. The chromatographic fingerprints of all three test drugs are given below.
Table 3 represents HPTLC chromatogram of BS contains 9 distinct peaks. The maximum area 36.92% was observed in peak 6. Other 4 peaks, i.e., peak 5 (12.49%), peak 7 (10.78%), peak 8 (11.99%) and peak 9 (10.94%) were covered 46.2%. The HPTLC fingerprint of BV have 13 peaks and maximum area noted in peak 7 (23.74%) and confirms the presence of gallic acid in BV. The Rf of gallic acid was 0.44. HPTLC chromatogram also exhibits that PA confirms 11 distinct peaks and out of them peak 4 to peak 11 showed clear separations of 7 smaller area or components.
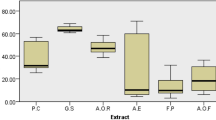
In phytochemical screening, all three test drugs have showed presence of flavonoids and phenolics. Based on these findings, quantitative analyses of small molecular phenolic acids present in these test drugs were done using HPLC methods. The standard chromatogram is given below. Figure 2 indicates that the BS bioactive phenolics and flavonoids like, catechin, quercetin, chlorogenic acid, caffeic acid, syringic acid and sinapic acid. Among all these phenolics, chlorogenic acid shows maximum content (1.6 µg/ml) in BS. Previous studies reported, chlorogenic acid has renoprotective properties (Kono 1978). It exhibits syringic acid, caffeic acid, p-coumeric acid, rutin, sinapic acid, coumarin and quercetin presents in BV. Among all these phenolics acids, sinapic acid shows maximum (1.25 µg/ml) in BV. It also represents chlorogenic acid, catechin, syringic acid, caffeic acid, p-coumeric acid, and sinapic acid presents in PA. Among all these phenolics acids, sinapic acid shows maximum (1.2 µg/ml) in PA. Finally, structure elucidations of bioactive compounds in test drugs were confirmed by Gas chromatography with electrochemical detection (GC-ECD) method. In this phase, BV has been analysed (Fig. 3). The results exhibited that BV contains three important bioactive and pharmacologically known compounds, viz., β-carotene, β-stigmasterol and β-sitosterol. These confirmatory finding may be helpful to clarify the biological actions of the test drug.
In vitro antioxidant activity
In vitro antioxidant properties of the test drugs were carried out using DPPH scavenging activity, hydrogen peroxide-, nitric oxide- and superoxide radical-scavenging activity. Antioxidants are therapeutically used to combat oxidative stress occurring in signalling pathways of many deadly diseases, viz., cardiovascular, neurodegenerative, diabetes, arthritis, nephritis etc. Table 4 represents the outcome results of antioxidant capacities of test drugs. Lower IC50 value means higher antioxidant capacity. BS showed maximum inhibitory action on DPPH radicals, superoxide and nitric oxide scavenging than other two test extracts. PA showed maximum hydrogen peroxide scavenging action than other test drugs. Moreover, BV and PA have also radical scavenging activities. In comparisons, it may be reported that BS, BV and PA have antioxidant capacities on different mechanistic pathways. From the above observations, it may therefore be concluded that all three-test extracts have strong antioxidant capacity and protective role in oxidative stress. Therefore, it also be postulated that the test drugs might have renoprotective actions, particularly on oxidative stress induced renal damage.
Acute toxicity studies
Single dose oral toxicity studies are done on Swiss mice according to OECD guideline number 423 for acute toxicity in animals weighing upto 2 gm per kg (limit test). Single oral administration of three test drugs do not show mortality and evident toxicity up to the dose of 2 g/kg orally in adult Swiss female mice. Hence, the 50% lethal dose of the test drugs was not possible to determine. The test compounds have no toxicity and mortality up to the oral dose of 2 g/kg.
Selection of curative doses
The doses were chosen based on the results of a pilot trial. The test drugs, BV, BS and PA were administered through oral gastric tube once a day. In pilot study, 25 mg/kg–400 mg/kg dose was taken. In diuretic activity and renoprotective activity, the dose of 50, 100, and 200 mg per kg was found to be most effective as per the dose response relationship. Finally, three dose levels (low, medium and high) were selected. For better comparison, all the test compounds were tested for these three dose levels, which were 50, 100 and 200 mg per kg. The test drugs were administered through oral gastric tube once a day. The comparable volume of distilled water (0.5 ml per 100 gm rat) was administered orally to the control rats.
Extrapolating animal dose to human dose conversions, these would be as 8.1 mg/kg human or 486 mg/day; 16.2 mg/kg human or 972 mg/day and 32.4 mg/kg human or 1944 mg/day respectively.
Diuretic activity
The physical parameters of urine in diuretic study did not show any significant changes (Table 5). Urine volumes were significantly changed after treatment with test extracts. Other than standard drug Furosemide, among these three test extracts, BS showed maximum urination than other two BV and PA (Table 6).
On the basis of diuretic index, BS at the dosage 200 mg per kg b.w exhibited maximum diuretic action followed by BV and PA (Table 7). From the above data, it may therefore be concluded, that all three test drugs have diuretic actions. BV exhibited maximum elimination of water through urine, while PA showed maximum elimination of Na+/K+ through urine, better than standard prototype Furosemide at dosage 20 mg per kg b.w.
In vitro antiurolithiatic activity
Nucleation is the formation of a solid crystal phase in a solution. In the present study, all three test drug showed dose dependent inhibition of crystal formation in test tubes within 3 h (Table 8; Fig. 4). BV was maximum effective in the inhibition of urinary stone followed by BS and PA. Stone crystals attach to each other by aggregation or agglomeration, which is aided by strong chemical and electrical forces. Adhered crystals remain retained in place and are difficult to detach, which is significant in lithiasis. Particles that are too big to move freely through renal tubules could form as a result of crystal growth and agglomeration. Small crystals could also be preserved by attaching to the surface of the urothelial lining and growing to a bigger size.
Furthermore, solid bridges generated by crystalline materials linking 2 particles can help to stabilize aggregation. The repulsive electrostatic surface charge, known as Zeta potential, is the principal force that prevents aggregation. Crystal aggregation is a more essential element than nucleation and development in several stages of stone formation since it occurs in seconds. In the present study, all three test drugs showed dose dependent inhibition in (in vitro) renal stone formation. Inhibitors are substances which enhance the SS necessary to facilitate nucleation, slow crystal development and aggregation, and prevent secondary nucleation. In this study, all the three test extracts showed significant effectiveness in the process of aggregations of renal stones (in vitro). Maximum efficacy was observed in PA followed by BV and BS (Table 9).
The anti-urolithiatic effects of the three test drugs were evaluated by considering all the parameters like pH, urine volume, urinary magnesium and oxalate levels, creatinine, ROS, serum urea and uric acid levels, and inflammatory reactions. All these three test extracts significantly decreased renal index or coefficient which mighty be due to repressing renal inflammatory conditions (Table 10). In the comparative efficacies of three test samples BS showed better result than BV and BV showed better result than PA. So, BS showed best result in between these extracts.
In the present study, it has also been noticed that all three test extracts have significantly altered these parameters (SGOT, SGPT and alkaline phosphatase) and considering their roles in the prevention of urolithiasis caused by ethylene glycol and also confirm their renoprotective action (Table 10).
Renal dysfunction observed in calculi-induced rats was caused by higher serum levels of creatinine, urea, and uric acid. Howsoever, treating with all the three test extracts significantly ameliorated the elevations of creatinine, urea, and uric acid in serum (Table 11).
It has also been found that the three extracts BS, BV, and PA shown powerful antioxidant activity thereby inhibit crystal deposition in renal tissue by interrupting crystal formation at an early stage. BS, BV, and PA therapy efficiently reduce ROS formation by boosting antioxidants SOD and GSH (Table 12) in dysregulated oxidative antioxidant balance related stone disease.
Renal tissue was significantly affected in control urolithiatic rats. In urolithiatic rats, histological damages such as tubular cell necrosis, tubule dilation, tubular necrosis, larger renal tubules with crystal deposits in the lumen, and inflammation were found (Table 13; Fig. 5). Treatments with BS, BV, and PA significantly reduced the quantity of CaC2O4 crystal formations in different portions of kidney sections, as well as the crystal deposition score, histological damage score, and inflammation score in the treated groups' kidneys.
This current investigation was intended to assess protective and curative efficacies of BS, BV and PA on GM-mediated nephrotoxic effects in rats. Induction of GM-mediated nephrotoxic effects in rats were standardized following the estimation of all biochemical parameters such as sodium, potassium, chloride, urea, uric acid, creatinine and also determination of all antioxidant biomarkers in renal tissues like, lipid peroxides, reduced glutathione, superoxide dismutase, catalase and nitric oxides. Histopathological evaluation in renal tissues was also conducted. Further, the efficacy of three test drugs on the prevention as well as curative action of GM-induced nephrotoxicity were done in rats. The obtained data were tabulated and statistically analyzed for pharmacological efficacy. In the present study, body and kidney weight did not significantly alter in GM-induced animals and also after treatment with test drugs (Table 14).
Acute renal damage is defined as a rise in serum creatinine of 0.3 mg/dl or more in 48 h or a rise in serum creatinine of at least 1.5-fold from baseline in 7 days. Creatinine formation is defined by the quantity of creatinine produced in the liver, kidneys, and pancreas, creatinine ingested and muscle function. In the present study, blood creatinine was enhanced in GM-induced animals than normal groups. Interestingly, BS, BV and PA significantly prevent the enhancement of blood creatinine levels in rats. BS showed maximum potency than BV and followed by PA (Table 15). In the same study, the blood urea levels also enhanced 4.5 folds in GM-induced acute kidney injury in rats. All three test extracts dose dependently and significantly lowered the blood urea levels. Similar findings were also noted in blood uric acid levels (Table 15). Among these three test extracts, BS exhibited best performances than other two. Apart from that the test extracts BS, BV and PA dose dependently and significantly restores the elevated electrolyte balance in kidney, which confirmed their preventive roles in the protection of renal tissues from damage (Table 16).
In this antiurolithiatic activity study, it has been seen that GM enhances lipid peroxides and nitric oxide generation, and simultaneously lowered the levels of glutathione, catalase and superoxide dismutase. But, all the three test extracts showed potent renoprotective properties which were reflected in blood and tissue parameters (Table 17).
Histological findings were also supported the biochemical parameters of renal protection. Furthermore, portions from the control group had normal histology. Light microscopy revealed no significant alterations in the glomeruli of the GM groups, but there was evidence of tubular injury, such as loss of brush boundaries, tubular debris, and extensive cellular vacuolations. All three-test drug-treated groups, i.e., BS, BV, and PA, had significantly reduced histological damages than the GM-treated rats (Fig. 6).
During studying GM-induced nephrotoxicity, it was found that the test extracts individually at different doses when given to rats from day 6 and continued to day 15, it has been found that GM had no impact on rat b.w while considerably increasing kidney weight (Table 18). After treatment, the test drugs significantly and dose dependently reduced the kidney weight.
In the present animal model for studying nephroprotective action, within 15 days GM enhances serum urea and creatinine level more than 7 and 19 folds respectively and indicated nephrotoxicity. Treatments with test extracts at three different doses were started from day 6 and continued up to day 15. All three test extracts dose dependently and significantly lowered the serum urea and creatinine levels (Table 19). Maximum potential was observed in BV followed by BS and PA. It has also been observed that all three test extracts have preventive role in GM-induced nephrotoxicity (Table 20). Now the present study clearly supported that all three test drugs have also curative role in the renal damage.
The maintenance of electrolytes balances in the body is highly disturbed in renal dysfunctions. Blood sodium, potassium and chloride concentrations were significantly higher in nephrotoxic animals than normal. Sodium excretion is one of the prime targets of electrolytes balances in renal dysfunction. The treatments with test drugs showed significant reductions in all three electrolytes (sodium, potassium and chlorides) in bloods. BV exhibited maximum potentialities than BS and PA (Table 21).
Discussion and conclusion
In this work, we established phytochemical standardization, antioxidant, diuretic, and antiurolithiatic properties of BS, BV, and PA in experimentally induced animal models. Initially the phytoconstituents present in the test extracts were screened by means of HPTLC, HPLC and lastly Gas chromatography, where the phyto components were detected quantitative as well as qualitatively.
This study also recognizes the antioxidant activity. Many research in recent years have suggested that ROS play an important role in GM-mediated nephrotoxicity. Antioxidants and ROS scavengers are employed to treat GM-mediated nephrotoxicity. The chemical nature of all three test drugs exhibited presence of polyphenolic acids and flavonoids as their constituents and all of them have strong radical scavenging antioxidant properties. Furthermore, all of them have preventive actions on renal tissue damages. In the present context, all three test extracts ameliorated oxidative stress related parameters like, lipid peroxides, glutathione, catalase, superoxide dismutase and nitric oxides in renal tissues. Maximum ROS scavenging action was noted in BV, followed by BS and PA.
GM-related nephrotoxic effects were linked with tubular and glomerular damage, as evidenced by tubular lumen destruction. Congested proximal tubules, glomerulus and bowman capsule degeneration, tubular necrosis and leucocytes infiltration were noted in GM nephrotoxicity (Fig. 7). Treatments with test samples reversed these conditions and corroborate other biochemical findings.
Toxicity study was done on Swiss mice according to OECD guideline number 423 for acute toxicity in animals at a single oral dose 2 gm per kg (limit test). Three test extracts didn’t exhibit any mortality in adult Swiss female mice. Hence, after considering all results, it could be concluded that those test extracts have no toxicities and mortality up to the oral dose of 2 g/kg and these are safe for oral use as drugs.
The diuretics are employed for the treatment of oedema, hypertension etc. Three important blood electrolytes mainly sodium, potassium and chloride are balanced by urine. The present study demonstrated that all three test drugs have significant diuretic actions. Table 6 exhibited the excretion of electrolytes through urinalysis. The maximum elimination of sodium–potassium ratio was noted in PA followed by BS and BV and acts like Furosemide (loop diuretic). Diuretic Index (Na+/K+) of test drugs were also supported the diuretic actions, like loop diuretics.
Kidney stone development is caused by urine salt supersaturation and crystal deposition in the urinary system. Because the human nephron is an adynamic system, in vitro crystal behaviour is too simple to explain renal calculus production completely. A complex combination of inhibitors and promoters seems to be at operation. Stone disease is almost always predisposed by a lack of inhibitors and/or a surplus of promoters in the urine. The importance of cellular damage in the promotion and progression of kidney stones may be an even more essential determinant. Possibly the existence of the stone actually triggers an inflammatory response, resulting in additional epithelial disruptions and stone production. Inflammation modulation might result towards intriguing new therapeutic methods for the treatment of stone disease. Hence, in vivo experimental studies were conducted to confirm the anti-lithiatic properties of test compounds.
Serum protein and albumin are the important markers of renal dysfunction. Urolithiasis reduced the serum protein and albumin levels, whereas markedly enhanced the liver enzymes, particularly SGOT, SGPT and alkaline phosphatase. This is due to the cells' broken structural integrity, which causes the enzyme located in the cytoplasm to be discharged into the circulation. In this experiment, it has been found that all three test extracts confirm their renoprotective action by showing significant impact on the parameters mentioned above and prevent ethylene glycol induced urolithiasis.
The glomerular filtration rate (GFR) drops in urolithiasis because of stones in the urinary tract obstructing urine outflow. As a result, waste products, notably nitrogenous compounds like creatinine, urea, and uric acid, build in the blood. Renal dysfunction was observed in calculi-induced rats, as evidenced by higher serum levels of creatinine, urea, and uric acid. All the three test extracts significantly ameliorated the elevation levels of creatinine, urea, and uric acid in serum, which are the important markers of renal tissue damage.
It has been demonstrated that oxalate crystal exposure causes alterations in the renal epithelium, which serves as a nidus and improves crystal retention inside the kidney as well as the formation of ROS. Renal tissue injury causes nidus by offering a location for the preservation of CaC2O4 crystals, that develop to stone by a series of events (Ali et al. 2009). BS, BV, and PA might interface with CaC2O4 crystals, and its potential to be a powerful antioxidant could inhibit crystal deposition in renal tissue by interrupting crystal formation at an early stage. Stone disease has been linked to a dysregulated oxidative antioxidant balance, and BS, BV, and PA therapy efficiently reduce ROS formation by boosting antioxidants SOD and GSH.
The effect of BS, BV, and PA could've been maintained by striking a balance among stone promoters and inhibitors, enhancing liver functions, changing serum and urine parameters such as Ca, citrate, Mg, and oxalate levels, sustaining the antioxidant environment, lowering CaC2O4 deposits, and reducing the chance of small particles of CaC2O4 being retained in the urinary tract. As a result, BS, BV, and PA have been proven to have a preventative and protective impact against the formation of renal stones.
Acute kidney injury (AKI) is a phenomenon caused by a variety of factors that results in a severe renal dysfunction and ailed to uphold electrolyte, fluid, and acid–base balance. The cornerstone of AKI management remains supportive, with specific therapy reserved for the rarer causes. Therefore, the prevention of AKI and minimization of its severity and duration are essential aspects of its management. GM is one among the most efficient antibiotics for treating gram-(-ve) bacterial infection in both animals and humans. GM and other aminoglycosides are commonly utilized to treat gram-(-ve) infections. Nephrotoxicity is a significant side effect of these medicines. The aetiology of aminoglycoside neurotoxic effects occurs in 2 stages. The 1st stage is for renal proximal tubular cells to transport and accumulate antibiotics in higher levels. The 2nd stage involves an unfavourable interaction among these polycationic medications, which causes cell damage. One of the most significant and well-validated models for studying the renoprotective potential of medicines and phytopharmaceuticals is the GM-induced nephrotoxicity model. During nephrotoxicity, direct tubular necrosis is also evident. Antioxidants derived from natural sources are essential medical reservoirs.
The kidneys perform a critical function in electrolyte and acid–base balance management. An electrolyte disturbance is characterized by an imbalance of various ionized salts in the blood (for example, calcium, bicarbonate, chloride, phosphate, magnesium, sodium, and potassium). Derangements in electrolytes and acid–base balance are unavoidable as kidney function declines, contributing to poor patient outcomes. The kidneys are largely in charge of excreting potassium from the body and adjust the extent of excretion based on the present concentration in the blood.
Hyperkalemia is one of the most prevalent and potentially fatal electrolyte abnormalities in acute renal failure. Other than potassium, sodium and chloride imbalances are also noted in renal diseases (Pandey and Rizvi 2009). GM treatment in rats resulted in a reduction in glomerular filtration rate, as seen by decreased creatinine clearance and increased serum creatinine. This deterioration in glomerular function was followed by an increase in serum potassium and sodium. This indicates proximal tubular dysfunction. In the present study, both sodium and potassium concentrations were elevated. The existence of tubular disruption was supported by a rise in serum chloride and it indicates direct toxic injury. The test drugs, BS, BV and PA dose dependently and significantly restores these electrolytes balance confirmed their preventive roles in the protection of renal tissues from damage.
Even though the mechanism causing GM-induced renal cellular damage isn’t fully understood, it has been linked to the production of hydrogen peroxide, superoxide anion, and hydroxyl radicals. Many experimental models have demonstrated a link between nephrotoxicity and oxidative stress (Karadi et al. 2006). The possible involvement of the L-arginine-Nitric oxide (NO) pathway in GM-induced nephrotoxicity (Atmani et al. 2003; Veena et al. 2008; Piccoli et al. 2010; Walker et al. 1999; Rivas-Cabanero et al. 1994). These researchers discovered enhanced glomerular NO production in mice with GM-mediated renal failure (Atmani et al. 2003; Veena et al. 2008; Piccoli et al. 2010; Walker et al. 1999; Rivas-Cabanero et al. 1994). Oxidative stress is mainly regulated by the cellular enzymatic (catalase, superoxide dismutase, glutathione peroxidase) and nonenzymatic (glutathione, ascorbic acid, α-tocopherol) factors. In the present study, GM enhances lipid peroxides and nitric oxide generation, but lowered the levels of glutathione, catalase and superoxide dismutase. All those three extracts showed potential renoprotective function.
Kidney damage can be acute (a sudden decrease in renal functions or urine output) or chronic (consistent structural and functional abnormalities) and is generally detected utilizing serum biomarkers such as urea and creatinine levels, which rise only after considerable (about 30%) kidney problems. AKI is described as occurring over 7 days, whereas CKD begins when kidney disease has been present for longer than 90 days. In the present animal model, within 15 days GM enhances serum urea and creatinine level more than 7 and 19 folds respectively and indicated nephrotoxicity. Treatments with test drugs at three different doses were started from day 6 and continued up to day 15. All three test drugs dose dependently and significantly lowered the serum urea and creatinine levels, where BV exhibited maximum potentialities followed by BS and PA. Moreover, it has been observed that all three test samples have preventive role in GM-induced nephrotoxicity. Now the present study clearly supported that all three test drugs have also curative role in the renal damage.
Finally, after performing all the tests it could be stated that the three extracts BV, BS and PA exhibited multiple potent activities like diuretics, antioxidant, antiurolithiatic. It has also impact over levels of urea, creatinine etc. in the body. Those three test extracts could be used in therapeutic treatment.
Availability of data and material
All data and material are available upon request.
Code availability
Not applicable for this article.
References
Aggarwal A, Tandon S, Singla KS, Tandon C (2010) Diminution of oxalate Induced renal tubular epithelial cell injury and inhibition of calcium oxalate crystallization in vitro by aqueous extract of Tribulus terrestris. Int Braz J Urol 36:480–489
Ahmad W, Khan NA, Ahmad G, Ahmad S (2010) Effect of Kaknaj (Physalis alkekengi Linn fruit) on gentamicin induced acute renal impairment in rats. Hippocratic J Unani Med 5:107–117
Ahmed A, Pendse AK, Sarana SS, Sing PP (1993) Effectiveness of a unani therapy (Sange sarmahi) in management of urinary stone disease. Indian J Exp Biol 31:20–264
Alam M, Javed K, Jafri MA (2011) Effect of oleo gum resin Boswellia serrata (Kundur) on renal functions in albino rats. Indian J Trad Knowl 10:736–740
Alam M, Khan H, Samiullah L, Siddique KM (2012) A review of phytochemical and pharmacological studies of Kundur (Boswellia serrata): A Unani drug. Appl Pharm Sci 2:148–156
Ali BH, Al Salam S, Al Husseini I, Nemmar A (2009) Comparative protective effect of N-acetyl cysteine and tetramethylpyrazine in rats with gentamicin nephrotoxicity. J Appl Toxicol 29:302–307
Al-Saikhan FI, Ansari MN (2016) Evaluation of the diuretic and urinary electrolyte effects of methanolic extract of Peganum harmala L. in Wistar albino rats. Saudi J Biol Sci 23(6):749–753.
Andreae WA (1955) A sensitive method for the estimation of hydrogen peroxide in biological materials. Nature 175:859–860
Arayne MS, Sultana N, Bahadur SS (2007) The Berberis story: Berberis vulgaris In therapeutics. Pak J Pharm Sci 20:83–92
Asif M, Jabeen Q, Majid AMS, Atif M (2014) Diuretic activity of Boswellia serrata Roxb. oleo gum extract in albino rats. Pak J Pharm Sci 27:1811–1817
Atmani F, Slimani Y, Mimouni M, Hacht B (2003) Prophylaxis of calcium oxalate stones by Herniaria hirsute on experimentally induced nephrolithiasis in rats. BJU Int 92:137–140
Bellomo R (2006) The epidemiology of acute renal failure: 1975 versus 2005. Curr Opin Crit Care 12:557–560
CPCSEA guidelines for laboratory animal facility (2003) Committee for the purpose of control and supervision of experiments on animals. Indian J Pharmacol 35:257–274. https://ijp-online.com/article.asp?issn=0253-7613;year=2003;volume=35;issue=4;spage=257;epage=274;aulast=CPCSEA;type=0. Accessed 15 April 2020
Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G (2010) Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol 48:1013–1018
Domberger K (1981) Potential antineoplastic acting constituents of Physalis alkekengi Linn. Pharmazie 41:265–268
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70
Essential Drug List: Unani Medicine. Department of AYUSH, 2013, Ministry of Health & Welfare, Govt. of India, New Delhi. https://upnrhm.gov.in/assets/site-files/gogl/fy2014-15/Essential_Unani_Medicines.pdf. Accessed 5 March 2020.
Fatehi-Hassanabad Z, Jafarzadeh M, Tarhini A, Fatehi M (2005) The antihypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive rats. Phytother Res 19:222–225
Green LC, Wagner DA, Glogowski J (1982) Analysis of nitrate, nitrite, and (15N) nitrate in biological fluids. Anal Biochem 126:131–138
Harbone JB (2002) Phytochemical methods. Chapman and Hall, London
Hennequin C, Lalanne V, Daudon M, Lacour B, Drueke T (1993) A new approach to studying inhibitors of calcium oxalate crystal growth. Urol Res 21:101–108
Hess B, Nakagawa Y, Coe FL (1989) Inhibition of calcium oxalate monohydrate crystal aggregation by urine proteins. Am J Physiol 257:99–106
Hesse A, Brandle E, Wilbert D, Kohrmann K-U, Alken P (2000) Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol 44:709–713
Karadi RV, Gadge N, Alagawadi KR, Savadi RV (2006) Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 105:306–311
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Lipschitz WL, Hadidian Z, Kerpesar A (1943) Bioassay of diuretics. J Pharmacol Exp Ther 79:97–110
Loh AHL, Cohen AH (2009) Drug-induced kidney disease – pathology and current concepts. Ann Acad Med Singapore 38:240–250
Lone AH, Ahmed T, Anwar MD, Sofi GH, Imam H (2012) Perception of health promotion in Unani medicine. Med J Islamic World AcadSci 20:1–5
Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ (2011) New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int 79:33–45
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J BiolChem 193:265–275
Mahmud Z, Bachar S, Qais N (2012) Antioxidant and hepatoprotective activities of ethanolic extracts of leaves of Premna esculenta Roxb. against carbon tetrachloride-induced liver damage in rats. J Young Pharm 4(4):228–234
Masao K, Toichi O, Masaki N, Taketoshi M, Yasuo B, Yuji M, Kenichi H (1988) Structure of Physelin and isolated from Physalis alkekengi var. francheti. Bull Chem Soc Japan 61:2696–2698
Mitchell H, Rosner W (2006) Renal function testing. Am J Kidney Dis 47:174–183
Moe OW (2006) Kidney stones: pathophysiology and medical manage-ment. Lancet 367:333–344
Naughton CA (2008) Drug-induced nephrotoxicity. Am Fam Phys 78:743–750
OECD No. 423: Guideline for the testing of chemicals. Acute oral toxicity in animals. Adopted 21st September. 1998. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2001)4&doclanguage=en. Accessed 30 April 2020.
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2(5):270–278
Piccoli GB, Capobianco M, Odetto L, Deagostini MC, Consiglio V, Radeschi G (2010) Acute renal failure, severe sodium and potassium imbalance and sudden tetraplaegia. Neprol Dial Transpl plus 3:247–252
Rivas-Cabanero L, Montero A, Lopez-Novoa JM (1994) Increased glomerular nitric oxide synthesis in gentamicin-induced renal failure. Eur J Pharmacol 270:119–121
Rodrigo R, Rivera G, Orellana M, Araya J, Bosco C (2002) Rat kidney antioxidant response to long term exposure to flavonol rich red wine. Life Sci 71:2881–2895
Ronald Hites A (1997) Gas chromatography mass spectrometry: handbook of instrumental techniques for analytical chemistry. In: Watson JT (ed) Introduction to mass spectrometry, 2nd edn. Raven Press, New York, pp 609–626
Sarica K, Eryildirim B, Yencilek F, Kuyumcuoglu U (2009) Role of over-weight status on stone-forming risk factors in children: a prospec-tive study. Urology 73:1003–1007
Senguttuvan J, Subramaniam P (2016) HPTLC fingerprints of various secondary metabolites in the traditional medicinal Herb Hypochaeris radicata L. J Bot 5429625:11
Serra V, Salvatori G, Pastorelli G (2021) Dietary polyphenol supplementation in food producing animals: effects on the quality of derived products. Animals (basel) 11(2):401
Seyyed MJ, Fallah SR (2012) Therapeutic application of different parts Berberis vulgaris. Int J Agric Crop Sci 4:404–408
Shah SA, Rathod IS, Suhagia BN, Pandya SS, Parmar VK (2008) A simple high performance liquid chromatographic method for the estimation of Boswellic acids from the market formulations containing Boswellia serrata extract. J Chromatogr Sci 46:735–738
Sharma I, Khan W, Ahmad S (2017) In vitro and ex vivo approach for anti-urolithiatic potential of bioactive fractions of gokhru with simultaneous HPLC analysis of six major metabolites and their exploration in rat plasma. Pharm Biol 55(1):701–711
Soundararajan P, Mahesh R, Ramesh T, Hazeena Begum V (2006) Effect of Aerva Lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol 44(981986):2
Syeda A, Rughooputh S, Greenwe P (2004) The Unani system of medicine: does it have a scientific basis? Biomed Sci 48:971–972
Tomosaka H, Chin YW, Salim AA, Keller WJ, Chai H, Kinghorn AD (2008) Antioxidant and cytoprotective compounds from Berberis vulgaris (barberry). Phytother Res 22:979–981
Traditional medicine in the WHO South-East Asia Region. 2020. https://iris.who.int/bitstream/handle/10665/340393/9789290228295-eng.pdf?sequence=1. Accessed 21 December 2021.
Unani System of Medicine: The Science of Health & Healing.2013, Department of AYUSH, Ministry of Health & Welfare, Govt. of India, New Delhi. https://ccrum.res.in/writereaddata/UploadFile/The%20Science%20of%20Health%20and%20Healing01076_1861.pdf. Accessed 15 March 2020.
Veena CK, Josephine A, Preetha SP, Rajesh NG, Varalakshmi P (2008) Mitochondrial dysfunction in an animal model of hyperoxaluria: a prophylactic approach with fucoidan. Eur J Pharmacol 579:330–336
Walker PD, Barri Y, Shah SV (1999) Oxidant mechanisms in gentamicin nephrotoxicity. Ren Fail 21:433–442
Acknowledgements
The authors are thankful to Central Council for RESEARCH IN UNANI MEDICINE (CCRUM) for approving fund for this project under EMR scheme. The authors are also thankful to Department of Pharmacology, R. G. Kar Medical College, Kolkata-700004 for allowing to conduct the project work.
Funding
This work was supported by Central Council for RESEARCH IN UNANI MEDICINE (CCRUM), Under EMR scheme (Project ID: Z.2015/122/2014-HPC(EMR)-AYUSH-C dated 21st September 2015), Ministry of AYUSH, Government of India, New Delhi.
Author information
Authors and Affiliations
Contributions
A.A. has received the project from Central Council for RESEARCH IN UNANI MEDICINE (CCRUM). S.B., M.R. and R.I. have performed different parts of the project work. S.D.B. has written the manuscript. S.S.B. edited the manuscript.
Corresponding authors
Ethics declarations
Ethical statement
All experiments have been performed in compliance with the criteria for the care and management of laboratory animals established by The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India. Institutional animal ethical committee (R/N 959/c/06/CPSEA) approved the proposal and given their consent for animal use.
Conflict of interest
Anjan Adhikari has no conflict of interest. Sangita Bhattacharya has no conflict of interest. Sankhadip Bose has no conflict of interest. Moumita Ray has no conflict of interest. Rania Indu has no conflict of interest. Sabyasachi Banerjee has no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adhikari, A., Bhattacharya, S., Bose, S. et al. Prospective experimental studies of some herbs used in urinary disorders in Unani medicine. ADV TRADIT MED (ADTM) 23, 753–775 (2023). https://doi.org/10.1007/s13596-022-00639-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-022-00639-1