Abstract
Endophytic fungi from medicinal plants are a rich source of new biologically active compounds. In the present study, an endophytic fungus Nigrospora sphaerica (Berk. & Broome) Petch was isolated from Dillenia indica L and was characterized morphologically and at molecular levels. The isolated fungus was investigated for antibacterial activity, antifungal activity, antioxidant activity, total phenolics and flavonoids content, and its responsible bioactive molecules. The toxicity test revealed that the crude extract of Nigrospora sphaerica inhibited the growth of pathogenic bacteria, i.e., E. coli, Staphylococcus aureus, Bacillus subtilis and Pseudomonas aeruginosa. The value of MIC (Minimum inhibitory concentration) ranges from 82–115 µg/mL for the selected bacteria. The isolated endophytic fungus exhibited the highest inhibition against the Fusarium oxysporum (72%) and the lowest inhibition against Aspergillus niger (55%). The maximum scavenging activity was 88.1% at 600 µg/mL with an IC50 value of 85 µg/mL. GC–MS (Gas chromatography-Mass spectroscopy) analysis of the ethyl acetate extract revealed the presence of more than 40 compounds. Some of the major compounds present in extract were 1H-Indene, 1-methylene-(3.64%), Dodecane (8.52%), Tetradecane (11.59%), (-)-Mellein (3.85%), Hexadecane (10.13%), 1,2,5-Oxadiazole-3,4-dicarboxamide (5.95%), Octadecane (6.46%) and Benzoic acid, 2-(dimethylamino) ethyl ester. The compounds present in the extracts have various biological activities such as antiviral, antioxidant, insecticidal, cytotoxic, antihyperglycemic, antibacterial, antifungal activity. The compounds present in the extract can be used in clinical trials for further applications. To the best of our knowledge, this is the first report on bioactive molecules produced by Nigrospora sphaerica isolated from Dillenia indica L. having antioxidant and antimicrobial activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interest in the use of biogenic medicines has risen around the world as people become aware of the health risks and toxicity connected with the indiscriminate use of synthetic medications and antibiotics (Nalawade et al. 2003). Over the last two decades, biological resources have provided more than half of the medications in the market (Vuorela 2004).
Medicinal plants are an important source of bioactive molecules and harbour microbes known as endophytes. These microbes live inside the host plant for a part or whole life without any symptoms of diseases to the host (Fernandes et al. 2015). They are an unexplored group of microbes and are a source of novel bioactive compounds. Endophytes benefit the host plant by synthesizing various bioactive compounds that enhance their growth, defend from herbivores, and provide resistance against different biotic and abiotic stress (Schulze-Makuch et al. 2018). It has been reported that the endophytes produce bioactive molecules similar to the host plant. (Ferreira et al. 2017). Endophytic fungi are one of the major sources of new bioactive chemicals with therapeutic value (Newman and Cragg 2016). According to a recent study, natural bioactive molecules or their derivatives account for more than 70% of anticancer and antibacterial drugs derived from endophytic fungi (Newman and Cragg 2020). The phytochemical composition of plants is also influenced by the external environment and internal metabolic balance (Borges et al. 2017). Microorganisms such as endophytes alter the internal environment of plants, increasing the levels of many secondary metabolites.
Due to the increased resistance of bacterial and fungal infections has increased the demand for new antibacterial and antifungal chemicals. Endophytes are considered an important source of novel antibacterial and antifungal chemicals because of their species richness and diverse secondary metabolites (Xu et al. 2015).
The isolation of endophytic fungi and their screening of bioactive compounds from medicinal plants is one of the main areas of endophytic research as endophytic fungi produce secondary metabolites having pharmaceutical applications (Kumar et al. 2004; Tejesvi et al. 2007).
The bioactive compounds derived from most endophytic fungi (Strobel 2006) are regarded as more metabolically active due to their involvement in the environment and they stimulate several metabolic processes to thrive in their host tissues (Strobel and Daisy 2003).
Nigrospora oryzae, a fungal endophyte isolated from Combretum dolichopetalum produces secondary metabolites with antidiabetic activity (Uzor et al. 2017).
Many studies showed the existence of endophytes inside the host plant and their potential bioactive compounds having biotechnological applications (Abdala et al. 2020, Jagannath et al. 2020, Douanla et al., 2012, Hulikere et al. 2019).
Dillenia indica L. is an ethnic medicinal plant commonly known as elephant apple. It has antibacterial (Jaiswal et al. 2014), antidiabetic (Kumar et al. 2011), antioxidant (Singh et al. 2016), anti-inflammatory (Kviecinski et al. 2017), antidiarrheal (Uddin et al. 2012) and anti-cancerous activities (Chowdhary et al. 2013). All the plant parts have lot of biological activities and are used to treat various diseases. Although Dillenia indica L. has many therapeutic importance, as per literature survey, there was no previous report on the characterization of its associated endophytic mycoflora. Keeping in mind the above-mentioned medicinal values, Dillenia indica was selected for the present study.
The current study was initiated to isolate endophytic fungi associated with the ethnomedicinal plant Dillenia indica L. The study describes the isolation and characterization of endophytic fungus Nigrospora sphaerica from Dillenia indica L. and its potential antibacterial, antifungal, total flavonoids, phenolic content and antioxidant properties. Further, GCMS analysis of its crude extract was conducted in order to identify the molecules that provide its antimicrobial and antioxidant properties.
Materials and methods
Plant material collection
The plant selected for the study was identified and authenticated on the basis of Botanical characteristics. Fresh leaves, fruits and stems of Dillenia indica L. were collected from the Botanical Gardens of Panjab University Chandigarh, India, in a zip lock sterilized polythene bags.
Isolation of fungal endophytes
The method given by Hallmann et al. (2007) was used for the isolation of endophytic fungi with slight modifications. The fresh samples were collected and brought to the laboratory and processed within 24 h. The specimens were first rinsed with water and allowed to dry, then cut into pieces (0.5–1.0 cm sections). The chopped pieces were sterilized by employing ethanol (80% ethanol for two minutes), Sodium hypochlorite (3% sodium hypochlorite for 2–3 min) and ethanol (90% ethanol for 2 min). The sterilized samples were then thrice washed with sterilized distilled water and were allowed to dry in the sterilized conditions. The sterilized pieces were placed on PDA (Potato Dextrose Agar) plates supplemented with chloramphenicol to inhibit bacterial growth and incubated at 24ºC. The plates were observed regularly to observe any fungal growth. Sterilization efficiency procedure was ascertained by spreading out last rinse water on Petri plate containing PDA. The purity of cultures was obtained by single hyphal tip isolation and maintained by repeated sub-culturing (Stierle et al. 1993). Stock cultures were maintained by subculturing at monthly intervals. After growing at 25 °C for 7 days, the slants were stored at 4 °C for further use.
Morphological identification of the endophytic fungus
The fungus was preliminarily examined for its morphological characters such as colony colour and texture. For microscopic observations, slides were prepared from the fully grown cultures to observe the morphology of conidia and conidiophores and compare them with the literature.
Molecular identification of the endophytic fungus
For molecular analysis, DNA (Deoxyribonucleic acid) was isolated from the mycelium of fully grown culture by using a fungal DNA extraction kit (HiPurATM SP Fungal DNA Purification kit). The isolated DNA was run in 2% gel electrophoresis and observed DNA band under UV light. ITS (Internal transcribed spacer) region was amplified using ITS1 and ITS4 primers (White et al. 1990). The primers were procured from the Prima- 96, HIMEDIA. The amplified ITS region was sequenced in PGIMER (Post Graduate Institute of Medical Education & Research) Chandigarh, India. The obtained sequence was analyzed in NCBI (National Center for Biotechnology Information) blast, and their closed similar sequenced were obtained. The sequence was submitted in the NCBI database and its accession number was obtained.
Phylogenetic analysis
The obtained Sanger sequence was searched in the NCBI database using the Basic Local Alignment Search Tool (BLAST) at https://www.ncbinlmgov/BLAST. The fungus was described up to species level by comparing sequences to those already submitted to Gene bank. CLUSTAL W was used to perform several sequence alignments of endophytic fungi's ITS regions with reference taxa. The evolutionary relationship and phylogenetic tree of the isolated taxa and its related species were constructed with the help of MEGA 7 software using the neighbor-joining method (Saitou and Nei 1987). The Internal Transcribed Spacer (ITS) consensus sequences were used to frame the phylogenetic tree with 1000 replications bootstrap (Tamaura et al. 2013).
Crude extract preparation
The fungus was grown in an Erlenmeyer flask containing potato dextrose broth for 21 days at 24˚C in a stationary phase. After that, mycelium and broth were separated by filtration through Whatman’s filter paper. Subsequently, an equal amount of ethyl acetate was added to the filtrate and was extracted. The organic phase was evaporated by a rotatory evaporator at 40˚C, and the concentrated crude extract was obtained (Mahmud et al. 2020).
Antibacterial activity
The antibacterial activity of ethyl acetate and the methanolic extract was evaluated by agar well diffusion assay (Singh et al. 2013). The extract was dissolved at the rate of 1 mg/mL in DMSO (Dimethylsulfoxide). The activity was determined against human pathogenic bacteria (both gram-positive and gram-negative) i.e., E. coli (MTCC 82), Staphylococcus aureus (MTCC 87), Bacillus subtilis (MTCC 441), and Pseudomonas aeruginosa (MTCC 424). The bacteria were revived in nutrient agar at 37˚ C for 24 h. The loopful of bacteria from the plates were inoculated in nutrient broth and grow overnight at 37˚ C. From this broth, 100 ul inoculum was taken and spread on the nutrient agar plate using L shape spreader. Wells were made on the plate using a sterile cork borer. 50 µL of the extract was placed in each well. Chloramphenicol (50 µL) and DMSO (50 µL) were used as a positive and negative control. The plates were incubated at 37˚ C for 24 h and the zone of inhibition (ZOI) was recorded in mm. ZOI is a circular area surrounding the antibiotic's active site where bacteria colonies do not develop. The zone of inhibition can be used to determine a bacteria's sensitivity to an antibiotic. The experiment was carried out in triplicate.
Antifungal activity
Antifungal activity of the Nigrospora sphaerica was estimated by dual cultures and poisoned food methods (Singh and Sati 2019; Balouiri et al. 2016). The activity was checked against three pathogenic fungi Aspergillus niger, Altrnaria alternata and Fusarium oxysporum. For the dual culture method, 5-mm fungal discs were cut from the margins of actively growing utilized fungus (as an antagonist) and pathogenic fungi (as test fungi) and placed in the opposite direction in the Petri plate containing PDA medium. Plates were incubated at 24° C for 7 days.
Similarly, for the poisoned food method, various concentrations of crude extract (0.5–4 mg) were used and mixed in autoclaved PDA medium before pouring into the plates. The medium containing Fluconazole was used as a positive control, and plates containing only culture media were negative control. Mycelial disc from 6–7 days old pathogenic fungal cultures was placed to the centre of the culture plates and incubated at 27 °C for 2–5 days, fungal colony diameter measured at the end of the incubation period. The antagonist activity was measured and expressed in percentage.
R1 = Growth of pathogenic fungi in negative control plate. R2 = Growth of pathogenic fungi in a dual culture plate.
Minimum inhibitory concentration (MIC) estimation
Minimal inhibitory concentration was evaluated in Muller Hinton agar broth using the microdilution method as recommended by NCCLS 2009. The bacteria were grown overnight in nutrient broth at 37 °C, having a density of 108 Colony-forming units (CFU)/mL. In the Mueller Hinton Broth medium, the fungal crude extract was added at concentrations of 0, 5, 10, 20, 40, 80, 160, 320, 640 µg/mL. By comparing the inoculum to 0.5 McFarland opacity requirements, the inoculum is prepared. The 0.5 McFarland norm was used to calculate CFU/mL. The turbidity of the bacterial suspensions was set to 105. The extract and the medium dilution were inoculated with 50 µL of bacterial suspension and incubated at 37 °C for 24 h. The extract concentration that inhibited visible microorganism growth was found to be the lowest.
Antioxidant activity
The ethyl acetate crude extract was evaluated for their ability to scavenge free radical 2, 2ʹ-diphenyl-1-picryl-hydrazyl (DPPH) by a standard method with slight modifications (Xie et al. 2010). Various concentration of 100–700 µg/mL were prepared and were mixed with 01 mL of 20 mg% methanolic DPPH (2,2-diphenyl-1-picrylhydrazyl) solution and were mixed properly by vortex. The tubes were incubated in dark at room temperature for 30 min and absorbance was recorded at 517 nm. Alpha-tocopherol was used as a standard (positive control). The negative control contains only DMSO and DPPH solution. The lower the absorbance higher the antioxidant activity. Percentage inhibition was calculated using formula:
where, Ac = absorbance of control, As = absorbance of sample. IC50 was calculated by plotting graph in excel sheet.
Phenolic content determination
The total phenolic content was determined using Folin–Ciocalteau colorimetric based assay (Cicco et al. 2009). For this, in an eppendorf tube, 100 µL of the fungal extract was mixed with 100 µL of Folin–Ciocalteau and left to stand at room temperature for 2 min. The reaction was terminated by mixing 1000 µL of 5% sodium carbonate solution with 800 ul of distilled water, and the volume was measured and adjusted to 5 mL. For 30 min, the mixture was incubated at 45 °C. Similarly, the negative control contains all the solution in the samples mixture except the fungal extract, whereas in the positive control, gallic acid was added in place of fungal extract. The calibration curve was created using gallic acid in various concentrations ranging from 10 to 300 g/mL. The absorbance of the solution was measured at 760 nm. To obtain the mean value of absorbance, the samples were prepared in triplicate for each analysis.
Flavonoids content estimation
The total flavonoids content in the crude extract was determined by using the Aluminium chloride protocol with quercetin as standard (McDonald et al. 2001). For this, a 10 mL of volumetric flask was filled with 1 mL of test material and 4 mL of water. After 5 min, added 0.3 mL of 5% Sodium nitrite and 0.3 mL of 10% Aluminium chloride and incubated at room temperature. After 6 min of incubation, 1 mL of 1 M sodium hydroxide was added to the mixture. With distilled water, the final volume was gradually increased to 10 mL. Similarly, the negative control contains all the solutions in the samples mixture except the fungal extract, whereas in the positive control, quercetin was added in place of fungal extract. The calibration curve was created using quercetin in various concentrations ranging from 10 to 300 g/mL. A spectrophotometer was used to examine the absorbance of samples to that of a blank at 510 nm.
Gas chromatography-mass spectrometry (GC–MS) analysis
GC–MS was carried out on TRACE 1300 GC, TSQ 8000 TRIPLE QUADRUPOLE MS fitted with TG 5MS column and S/SL Injector. The G.C. oven's initial temperature was 60 degrees and then increased at the rate of 10 degrees per minute up to 280 degrees. The injector and detector temperatures were 260 and 280 °C. The mass spectra were carried out at 75 eV. The compounds were identified by comparing their mass spectra with the MS library. The GC–MS spectral analysis was carried out in Sophisticated Analytical Instrumentation Facility (SAIF), Central instrumentation laboratory, Panjab University, Chandigarh, India.
Statistical analysis
All the experiments were conducted in triplicates, and the data were expressed as Mean ± standard error. One-way ANOVA with Tukey's test at p < 0.05as used to examine statistically significant differences between values (using SPSS 16).
Results
Fungal identification
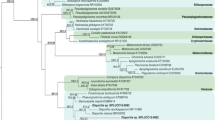
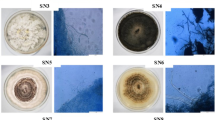
On PDA, colonies were floccose, initially white, becoming black with time. Hyphae were smooth, hyaline, branched, septate, measuring 2.5–6 µm in diameter. Conidiophores micronematous or semi-macronematous, multiseptated, hyaline to pale brown flexuous or straight, 4–7 µm thick. Conidiogenous cells pale brown, subspherical 6–12 µm diam. Conidia were solitary, globose or subglobose, black, shiny, smooth, aseptate, 16–21 µm in diameter, and were formed in abundance (Fig. 1). The species level identification was further confirmed by molecular identification, in which the most conserved Internal Transcribe spacer (ITS) region was sequenced. For this, the fungal DNA was isolated, and the ITS region was amplified by PCR. The PCR product was sequenced, and a 585 bp sequence was obtained. The sequence was then subjected to NCBI blast, and it showed 100% similarity with Nigrospora sphaerica. For phylogenetic analysis, sequences of closely related species were aligned with clustalW, and the phylogenetic tree was constructed using MEGA 7 software (Fig. 2).
The phylogenetic tree was constructed by Mega 7 using neighbor-joining method having bootstraps values 1000 per runs. The constructed tree was based on the ITS rDNA gene sequence of isolated endophytic fungus and its related species. An optimal tree is shown, with a branch length sum of 0.11382017. The branch lengths are in the same units as the evolutionary distances used to estimate the phylogenetic tree, and the tree is drawn to scale. The evolutionary distances were calculated by using the Maximum Composite Likelihood technique and are in base substitutions per site units
Antibacterial activity
The ethyl acetate and methanol extract were evaluated for their antibacterial activity against human pathogenic bacteria. Ethyl acetate extract exhibited activity against all the selected bacteria. The maximum zone of inhibition was observed against E. coli (Zone of inhibition 24 ± 0.6 mm), Staphylococcus aureus (Zone of inhibition 21 ± 0.4 mm), Bacillus subtilis (Zone of inhibition 14 ± 0.6 mm) and Pseudomonas aeruginosa (Zone of inhibition 20 ± 0.5 mm). The methyl extract exhibited the least activity i.e., ranges from 2-6 mm for the selected bacteria (Table 1 and Fig. 3). The lesser activity of methyl extract might be due to the lesser quantity of polar compounds in the extract. The values of MIC ranges from 82 ug/mL to 115 µg/mL. The highest activity was found against E. coli (having MIC 82 ug/mL), followed by Staphylococcus aureus (MIC of 105 ug/mL), Pseudomonas aeruginosa (MIC of 155 ug/mL), and Bacillus subtilis ((MIC of 102 ug/mL).
Antifungal activity
The fungal activity results are presented in Table 2, indicating that the fungus had a high inhibitory capability against the selected pathogenic fungi. In the dual culture method, the highest inhibition was obtained against the pathogenic fungus Fusarium oxysporum (59.76%), followed by Alternaria alternata (55.25%), and the lowest against another test fungus, Aspergillus niger (42.63%). In the food poisoned method, the pathogenic fungus Fusarium oxysporum displayed the maximum inhibition (72.19%), and Aspergillus niger showed the minimum inhibition (55.36%).
Antioxidant activity
The ethyl acetate extract was evaluated for their ability to scavenge free radical DPPH at room temperature. The extract showed free radical scavenging activity against free radical DPPH. The maximum scavenging activity was 88.1% at 600 µg/mL (Fig. 4). The IC50 (value at which 50% scavenging occur) value was found to be 85 µg/mL. From the literature survey, it was found that the antioxidant activity was due to the occurrence of phenolics compounds.
Total phenolics content
Different concentrations of extract were used to evaluate the total phenolic content in the crude extract. The results showed that the phenolic content increased as the concentration of extract increased (Table 3). The highest phenolic content was at 500 µg/mL of crude extract and it was found to be 43.65 ± 0.23 ug GAE/g.
Total flavonoid content
Different concentrations of extract were used to evaluate the total flavonoids content in the crude extract. The results displayed that the flavonoids content increased as the concentration of extract increased (Table 4). The highest flavonoids content was at 500 µg/mL of crude extract and it was found to be.35.21 ± 0.34 mg Quercetin/g.
GC–MS analysis
The chemical compounds present in ethyl acetate extract were identified using National Institute Standard and Technology (NIST), Database, USA. The total ions chromatogram of the crude extract of the fungus is shown below (Fig. 5). The compounds name along with their molecular formula, retention time, percentage area is given below (Table 5). The chromatogram showed the presence of 40 peaks among them 1H-Indene, 1-methylene-(3.64%), Dodecane (8.52%), Tetradecane (11.59%), (-)-Mellein (3.85%), Hexadecane (10.13%), 1,2,5-Oxadiazole-3,4-dicarboxamide (5.95%), Octadecane (6.46%) and Benzoic acid, 2-(dimethylamino) ethyl ester (18.14%) were the predominant compounds. Other compounds show percentage area below 3.
.
Discussion
Endophytic fungi are important sources of novel bioactive molecules having number of therapeutics values such as antibacterial (Hussain et al. 2014; Manganyi et al. 2018; Parthasarathy et al. 2020, Rashid et al. 2021), Antifungal (Zhao et al. 2011; Pan et al. 2016; Erfandoust et al. 2020), anti-cancerous (Arivudainambi et al. 2014; Zhu et al. 2017; Kumar et al. 2019), Antioxidant (Yadav et al. 2014; Ujam et al. 2021), antidiabetics (Kaur et al. 2020), Immunosuppressive (Liu et al. 2016; Wang et al. 2017), antiviral (Guo et al. 2000; Stierle et al. 2001), Anti-inflammatory (Chen et al. 2011), Anti-allergic (Cui et al. 2012).
Medicinal plants harbour endophytic fungi, which produce bioactive molecules similar to that of the host plant (Strobel et al. 2003). Endophytes from medicinal plants are gaining great attention due to their many industrial applications (Janakiraman et al. 2012). They have been identified as a novel source of novel bioactive molecules since the discovery of Taxol from the endophytic fungus Taxomyces andreanae (Stierle et al. 1993). For the last 2–3 decades, many bioactive compounds have been isolated from the endophytic fungi and are classified into alkaloids, flavonoids, terpenoids, phenols, steroids, quinones, lignans, and lactones (Li-jian et al. 2008).
In the present study, endophytic fungi from Dillenia indica L. were isolated and characterized. Nigrospora sphaerica was identified by employing morphological and molecular studies. Previously, Nigrospora spharica was isolated from many host plants such as Moringa oleifera (Zhao et al. 2012), Ginkgo biloba (Pawle and Singh 2014), Adiantum philippense (Ramesha et al. 2020). The Nigrospora is an important source of bioactive molecules having many biological activities such as antibacterial (Tanaka et al. 1997; Ramesha et al. 2020), antifungal (Zhao et al. 2012) herbicidal (Fukushima et al. 1998), Insecticidal (Wu et al. 2009). This is the first-time endophytic fungus Nigrospora sphaerica has been isolated from Dillenia indica.
Ethyl acetate was selected as the crude extracting solvent in this investigation, as it is the most common organic solvent for the extraction of fungal secondary metabolites (Nawaz et al. 2020).
The emergence of multidrug-resistant microorganisms is one of the serious challenges in healthcare. Such life threatening infections are challenging to treat and require many treatment procedures including the use of a more toxic and expensive set of medicines. As a result, more effective, economical and innovative alternatives are required, which are available somewhere in these natural compounds. Crude extract of endophytic fungi isolated from Scletium totosum showed antibacterial activity against numbers of pathogenic bacteria (Manganyi et al. 2019). The ethyl acetate extract exhibited significant antibacterial and antifungal activity against selected pathogenic bacteria and pathogenic fungi reported in the present study.
Excessive production of free radicals in human bodies causes oxidative stress to the biomolecules in our bodies, which can leading to cancer, Alzheimer's disease, aging, and other neurological diseases. Reactive oxygen species (ROS) are produced inside the body during different metabolic activities (Xing et al. 2005). These reactive species play a crucial role in our body, such as protecting from environmental stress and inducing programmed cell death. The antioxidant activity was due to the presence of phenolic compounds (Pandey et al. 2009). The free radical savaging of Nigrospora sphaerica was found 88.1% at 600 µg/mL with IC50 of 285 µg/mL. The total phenolic content was found to be 43.65 ± 0.23 mg GAE/g, and the total flavonoids content was 35.21 ± 0.18 mg quercetin/g. The phenolics compounds in the plants protect them from various biotic and abiotic stresses (Chadra et al. 2021). Extracts with a high phenolic concentration also displayed good antioxidant activity in this investigation. Previous research has found a linear relationship between the relationship between total phenolic content and antioxidant capacity of any representative sample (Sultana et al. 2007).
The phytochemical analysis identifies the major chemical groups in the extract and provides insight into its pharmacological potential. There are many investigations on the phytochemical contents of therapeutic plants and herbs, but only a small number of endophytic fungi are documented for the phytochemical analysis (Singh et al. 2021). The presence of important chemical groups like terpenoids, alkaloids, phenols, and flavonoids, known to impart specific bioactivities, was identified in the phytochemical screening of crude extract. The bioactive compounds present in the extract were analyzed by GC–MS analysis. GC–MS is one of the common and advanced technique to identify chemical compounds. The GC–MS analysis of the ethyl acetate extract of Nigrospora sphaerica revealed the presence of a total of 40 compounds. Among the isolated compounds, some of them were previously reported such as 1-Hexanol, 2-ethyl- as flavour additive, fragrant (Nisha et al. 2018), Maltol, Cetene and Nonadecane, 9-methyl- were reported having antioxidant activity (Wang et al. 2007; Nandhini et al. 2015), Phenylethyl Alcohol, 2,4-Dimethyldodecane, (-)-Mellein, Diethyl Phthalate and Tetradecane, 4-methyl- as antimicrobial activity (Li et al. 2010; Baskaran et al. 2015; Rahbar et al. 2012, Rukachaisiriku et al. 2009, Premjanu et al. 2014), 1H-Indene, 1-methylene- as Antimicrobial, Antitumor anticoagulant potential (Kavitha and Kensa 2020). 6-Tetradecanesulfonic acid, butyl ester as antibacterial and antifungal (Arora et al. 2017). 3-Eicosene, (E)- was reported as antimicrobial and antioxidant activity (Banakar Jayaraj 2018, Ojekale et al. 2013). Hexadecane as antioxidant activity (Padma et al. 2019). Dodecane as antifungal activity (Arora and Meena 2017), 1-Phenoxypropan-2-ol as used in cosmetics and personal care products (Borremans et al. 2004), Nonane, 3-methyl-5-propyl- as antiviral activity (Ibeyaima et al. 2017), 1-Hexadecanol as antimicrobial and anti-inflammatory activity (Mary et al. 2016), 5-Eicosene, (E)- as Antitumor, antifungal, cytotoxic, Antibacterial activity (Abubakar et al. 2016), Hexadecanoic acid, methyl ester as Antioxidant, hypocholesterolemic nematicide, pesticide, anti-androgenic flavor, hemolytic, 5- Alpha reductase inhibitor (Hema et al. 2011), Tetradecane as Antifungal, Antibacterial, Nematicidal activity (Arora et al. 2017). The existence of a variety of antimicrobial and antioxidant chemical components (Tetradecane, Hexadecanoic acid, methyl ester, 5-Eicosene, (E)-, Maltol, Cetene and Nonadecane, 9-methyl-, Phenylethyl Alcohol, 2,4-Dimethyldodecane, (-)-Mellein, Diethyl Phthalate and Tetradecane, 4-methyl- etc.) in the crude extract of Nigrospora sphaerica was thus confirmed by GC–MS analysis, the synergistic effects effect of which can be attributed to the fungus's antimicrobial and antioxidant properties.
Conclusion
Endophytes are important source of bioactive compounds and are being explored for their medicinal values throughout the world. Nigrospora sphaerica was isolated from the medicinal plant Dillenia indica L and was characterised. The crude extract of fungus revealed their antibacterial, antifungal and antioxidant activity. The GC–MS analysis showed the presence of compounds having many biological activities. The present study concludes that the Nigrospora sphaerica possess many bioactive potentials and can be used for the production of bioactive drug having medical and pharmaceutical applications.
Availability of data and material
Data included in this article.
Code availability
Not applicable.
References
Abdalla MA, Aro AO, Gado D, Passari AK, Mishra VK, Singh BP, McGaw LJ (2020) Isolation of endophytic fungi from South African plants, and screening for their antimicrobial and extracellular enzymatic activities and presence of type I polyketide synthases. S Afr J Bot 134:336–342. https://doi.org/10.1016/j.sajb.2020.03.021
Abubakar MN, Majinda RR (2016) GC-MS analysis and preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines 3(1):3. https://doi.org/10.3390/medicines3010003
Arivudainambi UE, Kanugula AK, Kotamraju S, Karunakaran C, Rajendran A (2014) Antibacterial effect of an extract of the endophytic fungus Alternaria alternata and its cytotoxic activity on MCF-7 and MDA MB-231 tumour cell lines. Biol Lett 51(1):7–17. https://doi.org/10.1515/biolet-2015-0002
Arora S, Meena S (2017) GC-MS Profiling of Ceropegia bulbosa Roxb. var. bulbosa, an endangered plant from Thar Desert, Rajasthan. Pharma Innov J 6(11):568–573
Arora S, Kumar G, Meena S (2017) Screening and evaluation of bioactive components of Cenchrus ciliaris L. by GC-MS analysis. Int Res J Pharm 8(6):69–76
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6(2):71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Banakar P, Jayaraj M (2018) GC-MS analysis of bioactive compounds from ethanolic leaf extract of Waltheria indica Linn. and their pharmacological activities. Int J Pharm Sci Res 9(5):2005–2010
Baskaran R, Mohan PM, Madanan MG, Kumar A, Palaniswami M (2015) Characterization and antimicrobial activity of Streptomyces sp. DOSMB-A107 isolated from mangrove sediments of Andaman Island, India. Characterization and antimicrobial activity of Streptomyces sp. DOSMB-A107 isolated from mangrove sediments of Andaman Island 44(5); 714–723.
Borges CV, Minatel IO, Gomez-Gomez HA, Lima GPP (2017) Medicinal plants: Influence of environmental factors on the content of secondary metabolites. In Medicinal Plants and Environmental Challenges (pp. 259–277). Springer, Cham
Borremans M, Van Loco J, Roos P, Goeyens L (2004) Validation of HPLC analysis of 2-phenoxyethanol, 1-phenoxypropan-2-ol, methyl, ethyl, propyl, butyl and benzyl 4-hydroxybenzoate (parabens) in cosmetic products, with emphasis on decision limit and detection capability. Chromatographia 59(1):47–53. https://doi.org/10.1365/s10337-003-0127-2
Chandra H, Kumari P, Prasad R, Gupta SC, Yadav S (2021) Antioxidant and antimicrobial activity displayed by a fungal endophyte Alternaria alternata isolated from Picrorhiza kurroa from Garhwal Himalayas India. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2021.101955
Chen M, Yang L, Li Q, Shen Y, Shao A, Lin S, Huang L (2011) Volatile metabolites analysis and molecular identification of endophytic fungi bn12 from Cinnamomum camphora chvar borneol. China J Chin Mater Med 36(23):3217–3221
Chowdhury MI, Dewan SMR, Ahamed SK, Moghal MMR, Ahmed J (2013) Biological activities of Dillenia indica L. bark growing in Bangladesh. J Biol Sci Opin 1(2):45–49. https://doi.org/10.7897/2321-6328.01202
Cicco N, Lanorte MT, Paraggio M, Viggiano M, Lattanzio V (2009) A reproducible, rapid and inexpensive Folin-Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem J 91(1):107–110. https://doi.org/10.1016/j.microc.2008.08.011
National Committee for Clinical Laboratory Standards [NCCLS]. (2009). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard-Second Edition M27-A2. Wayne, PA: National Committee for Clinical Laboratory Standards.
Cui Y, Yi D, Bai X, Sun B, Zhao Y, Zhang Y (2012) Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia 83(5):913–920. https://doi.org/10.1016/j.fitote.2012.04.009
Douanla-Meli C, Langer E (2012) Diversity and molecular phylogeny of fungal endophytes associated with Diospyros crassiflora. Mycology 3(3):175–187. https://doi.org/10.1080/21501203.2012.705348
Erfandoust R, Habibipour R, Soltani J (2020) Antifungal activity of endophytic fungi from Cupressaceae against human pathogenic Aspergillus fumigatus and Aspergillus niger. J De Mycol Med 30(3):100987. https://doi.org/10.1016/j.mycmed.2020.100987
Fernandes EG, Pereira OL, da Silva CC, Bento CBP, de Queiroz MV (2015) Diversity of endophytic fungi in Glycine max. Microbiol Res 181:84–92. https://doi.org/10.1016/j.micres.2015.05.010
Ferreira MC, Cantrell CL, Wedge DE, Gonçalves VN, Jacob MR, Khan S, Rosa LH (2017) Diversity of the endophytic fungi associated with the ancient and narrowly endemic neotropical plant Vellozia gigantea from the endangered Brazilian rupestrian grasslands. Biochem Syst Ecol 71:163–169. https://doi.org/10.1016/j.bse.2017.02.006
Fukushima T, Tanaka M, Gohbara M, Fujimori T (1998) Phytotoxicity of three lactones from Nigrospora sacchari. Phytochemistry 48(4):625–630. https://doi.org/10.1016/S0031-9422(97)01023-6
Guo B, Dai JR, Ng S, Huang Y, Leong C, Ong W, Carte BK (2000) Cytonic acids A and B: novel tridepside inhibitors of hCMV protease from the endophytic fungus Cytonaema species. J Nat Prod 63(5):602–604. https://doi.org/10.1021/np990467r
Hallmann J, Berg G, Schulz B (2007) Isolation procedures for endophytic microorganisms. Springer, Brelin. https://doi.org/10.1007/3-540-33526-9_17
Hema R, Kumaravel S, Alagusundaram K (2011) GC/MS determination of bioactive components of Murraya koenigii. J Am Sci 7(1):80–83
Hulikere MM, Joshi CG (2019) Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus Cladosporium cladosporioides. Process Biochem 82:199–204. https://doi.org/10.1016/j.procbio.2019.04.011
Hussain H, Kock I, Al-Harrasi A, Al-Rawahi A, Abbas G, Green IR, Krohn K (2014) Antimicrobial chemical constituents from endophytic fungus Phoma sp. Asian Pac J Trop Med 7(9):699–702. https://doi.org/10.1016/S1995-7645(14)60119-X
Ibeyaima A, Dwivedi AK, Saini N, Gupta S, Sarethy IP (2017) Saccharothrix sp. TD-093 from the Thar Desert, India: metabolite fingerprinting of antimicrobial compounds and in silico analysis. Curr Microbiol 74(3):334–343. https://doi.org/10.1007/s00284-016-1183-9
Jagannath S, Konappa N, Lokesh A, Dasegowda T, Udayashankar AC, Chowdappa S, Jogaiah S (2020) Bioactive compounds guided diversity of endophytic fungi from Baliospermum montanum and their potential extracellular enzymes. Anal Biochem 614:114024. https://doi.org/10.1016/j.ab.2020.114024
Jaiswal S, Mansa N, Prasad MP, Jena BS, Negi PS (2014) Antibacterial and antimutagenic activities of Dillenia indica extracts. Food Biosci 5:47–53. https://doi.org/10.1016/j.fbio.2013.11.005
Janakiraman N, Johnson M, Sahaya SS (2012) GC–MS analysis of bioactive constituents of Peristrophe bicalyculata (Retz.) Nees. (Acanthaceae). Asian Pac J Trop Biomed 2(1):S46–S49. https://doi.org/10.1016/S2221-1691(12)60128-2
Kaur J, Sharma P, Kaur R, Kaur S, Kaur A (2020) Assessment of alpha glucosidase inhibitors produced from endophytic fungus Alternaria destruens as antimicrobial and antibiofilm agents. Mol Biol Rep 47(1):423–432. https://doi.org/10.1007/s11033-019-05145-3
Kavitha A, Kensa M (2020) Preliminary phytochemical screening and FTIR analysis on Rivina humilis L. J Inf Comput Sci 10(1):553–559
Kumar DSS, Hyde KD (2004) Biodiversity and tissue-recurrence of endophytic fungi in Tripterygium wilfordii. Fungal Divers 13:69–90
Kumar S, Kumar V, Prakash O (2011) Antidiabetic and antihyperlipidemic effects of Dillenia indica (L.) leaves extract. Braz J Pharm Sci 47(2):373–378. https://doi.org/10.1590/S1984-82502011000200018
Kumar VS, Kumaresan S, Tamizh MM, Islam MIH, Thirugnanasambantham K (2019) Anticancer potential of NF-κB targeting apoptotic molecule “flavipin” isolated from endophytic Chaetomium globosum. Phytomedicine 61:152830. https://doi.org/10.1016/j.phymed.2019.152830
Kviecinski MR, David IMB, Fernandes FDS, Correa MDR, Clarinda MM, Freitas AF, Pedrosa RC (2017) Healing effect of Dillenia indica fruit extracts standardized to betulinic acid on ultraviolet radiation-induced psoriasis-like wounds in rats. Pharm Biol 55(1):641–648. https://doi.org/10.1080/13880209.2016.1266672
Li Q, Ning P, Zheng L, Huang J, Li G, Hsiang T (2010) Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol Technol 58(2):157–165. https://doi.org/10.1016/j.postharvbio.2010.06.003
Li-jian XU, Li-gang ZHOU, Jiang-lin ZHAO, Wei-bo JIANG (2008) Recent studies on the antimicrobial compounds produced by plant endophytic fungi. Nat Product Res Dev 20(4):731–740
Liu H, Chen S, Liu W, Liu Y, Huang X, She Z (2016) Polyketides with immunosuppressive activities from mangrove endophytic fungus Penicillium sp. ZJ-SY2. Mar Drugs 14(12):217. https://doi.org/10.3390/md14120217
Mahmud SN, Sohrab MH, Begum MN, Rony SR, Sharmin S, Moni F, Afroz F (2020) Cytotoxicity, antioxidant, antimicrobial studies and phytochemical screening of endophytic fungi isolated from Justicia gendarussa. Ann Agric Sci 65(2):225–232. https://doi.org/10.1016/j.aoas.2020.12.003
Manganyi MC, Regnier T, Kumar A, Bezuidenhout CC, Ateba CN (2018) Biodiversity and antibacterial screening of endophytic fungi isolated from Pelargonium sidoides. S Afr J Bot 116:192–199. https://doi.org/10.1016/j.sajb.2018.03.016
Manganyi MC, Regnier T, Tchatchouang CDK, Bezuidenhout CC, Ateba CN (2019) Antibacterial activity of endophytic fungi isolated from Sceletium tortuosum L. (Kougoed). Ann Microbiol 69(6):659–663. https://doi.org/10.1007/s13213-019-1444-5
Mary APF, Giri DRS (2016) Phytochemical screening and GC-MS analysis in ethanolic leaf extracts of Ageratum conyzoides (L.). World J Pharm Res 5(7):1019–1029. https://doi.org/10.20959/wjpr20167-6505
McDonald S, Prenzler PD, Antolovich M, Robards K (2001) Phenolic content and antioxidant activity of olive extracts. Food Chem 73(1):73–84. https://doi.org/10.1016/S0308-8146(00)00288-0
Nalawade SM, Sagare AP, Lee CY, Kao CL, Tsay HS (2003) Studies on tissue culture of Chinese medicinal plant resources in Taiwan and their sustainable utilization. Bot Bull Acad Sin 44(2):79–98
Nandhini SU, Sangareshwari S, Lata K (2015) Gas chromatography-mass spectrometry analysis of bioactive constituents from the marine Streptomyces. Asian J Pharm Clin Res 8(2):244–246
Nawaz H, Shad MA, Rehman N, Andaleeb H, Ullah N (2020) Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz J Pharm Sci 56:e17129. https://doi.org/10.1590/s2175-97902019000417129
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79(3):629–661. https://doi.org/10.1021/acs.jnatprod.5b01055
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83(3):770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Nisha RPB (2018) Gas chromatography-mass spectrometry analysis for identification of bioactive compounds in selected genotypes of Trigonella foenum-graecum L. J Pharm Innov 7(4):929–939
Ojekale AB, Lawal OA, Segun AA, Samuel FO, Ismaila AI, Opoku AR (2013) Volatile constituents, antioxidant and insecticidal activities of essential oil from the leaves of Thaumatococcus danielli (Benn.) Benth. From Nigeria. Iosr J Pharm 3(3):01–05
Padma M, Ganesan S, Jayaseelan T, Azhagumadhavan S, Sasikala P, Senthilkumar S, Mani P (2019) Phytochemical screening and GC–MS analysis of bioactive compounds present in ethanolic leaves extract of Silybum marianum (L). J Drug Deliv Ther 9(1):85–89
Pan F, Liu ZQ, Chen Q, Xu YW, Hou K, Wu W (2016) Endophytic fungus strain 28 isolated from Houttuynia cordata possesses wide-spectrum antifungal activity. Braz J Microbiol 47(2):480–488. https://doi.org/10.1016/j.bjm.2016.01.006
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2(5):270–278. https://doi.org/10.4161/oxim.2.5.9498
Parthasarathy R, Chandrika M, Rao HY, Kamalraj S, Jayabaskaran C, Pugazhendhi A (2020) Molecular profiling of marine endophytic fungi from green algae: assessment of antibacterial and anticancer activities. Process Biochem 96:11–20. https://doi.org/10.1016/j.procbio.2020.05.012
Pawle G, Singh SK (2014) Antimicrobial, antioxidant activity and phytochemical analysis of an endophytic species of Nigrospora isolated from living fossil Ginkgo biloba. Curr Res Environ Appl Mycol 4(1):1–9. https://doi.org/10.5943/cream/4/1/1
Premjanu N, Jaynthy C (2014) Antimicrobial activity of diethyl phthalate: an insilico approach. Asian J Pharm Clin Res 7(4):141–142
Rahbar N, Shafaghat A, Salimi F (2012) Antimicrobial activity and constituents of the hexane extracts from leaf and stem of Origanum vulgare L. ssp. Viride (Boiss.) Hayek growing wild in Northwest Iran. J Med Plants Res 6(13):2681–2685. https://doi.org/10.5897/JMPR11.1768
Ramesha KP, Mohana NC, Nuthan BR, Rakshith D, Satish S (2020) Antimicrobial metabolite profiling of Nigrospora sphaerica from Adiantum philippense L. J Genet Eng Biotechnol 18(1):1–9. https://doi.org/10.1186/s43141-020-00080-4
Rashid TS (2021) Bioactive metabolites from tomato endophytic fungi with antibacterial activity against tomato bacterial spot disease. Rhizosphere 17:100292. https://doi.org/10.1016/j.rhisph.2020.100292
Rukachaisirikul V, Arunpanichlert J, Sukpondma Y, Phongpaichit S, Sakayaroj J (2009) Metabolites from the endophytic fungi Botryosphaeria rhodina PSU-M35 and PSU-M114. Tetrahedron 65(51):10590–10595. https://doi.org/10.1016/j.tet.2009.10.084
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Schulze-Makuch D, Wagner D, Kounaves SP, Mangelsdorf K, Devine KG, de Vera JP, Zamorano P (2018) Transitory microbial habitat in the hyperarid Atacama Desert. Proc Natl Acad Sci 115(11):2670–2675. https://doi.org/10.1073/pnas.1714341115
Singh L, Sati SC (2019) Antagonistic activity of isolated root endophytic freshwater fungus Anguillospora longissima against pathogenic fungi. Natl Acad Sci Lett 43(2):201–205. https://doi.org/10.1007/s40009-019-00818-w
Singh D, Rathod V, Ninganagouda S, Herimath J, Kulkarni P (2013) Biosynthesis of silver nanoparticle by endophytic fungi Pencillium sp. isolated from Curcuma longa (turmeric) and its antibacterial activity against pathogenic gram-negative bacteria. J Pharm Res 7(5):448–453. https://doi.org/10.1016/j.jopr.2013.06.003
Singh PA, Brindavanam NB, Kimothi GP, Aeri V (2016) Evaluation of in vivo anti-inflammatory and analgesic activity of Dillenia indica f. elongata (Miq.) Miq. and Shorea robusta stem bark extracts. Asian Pac J Trop Dis 6(1):75–81. https://doi.org/10.1016/S2222-1808(15)60988-4
Singh A, Kumar J, Sharma VK, Singh DK, Kumari P, Nishad JH, Kharwar RN (2021) Phytochemical analysis and antimicrobial activity of an endophytic Fusarium proliferatum (ACQR8), isolated from a folk medicinal plant Cissus quadrangularis L. S Afr J Bot 140:87–94. https://doi.org/10.1016/j.sajb.2021.03.004
Stierle A, Strobel G, Stierle D (1993) Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260(5105):214–216. https://doi.org/10.1126/science.8097061
Stierle AA, Stierle DB, Bugni T (2001) Sequoiatones C− F, Constituents of the Redwood Endophyte Aspergillus parasiticus. J Nat Prod 64(10):1350–1353. https://doi.org/10.1021/np010022e
Strobel G (2006) Harnessing endophytes for industrial microbiology. Curr Opin Microbiol 9(3):240–244. https://doi.org/10.1016/j.mib.2006.04.001
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67(4):491–502. https://doi.org/10.1128/MMBR.67.4.491-502.2003
Sultana B, Anwar F, Przybylski R (2007) Antioxidant activity of phenolic components present in barks of Azadirachta indica Terminalia arjuna, and Eugenia jambolana Lam. trees. Food Chem 104(3):1106–1114. https://doi.org/10.1016/j.foodchem.2007.01.019
Tamaura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Tanaka M, Fukushima T, Tsujino Y, Fujimori T (1997) Nigrosporins A and B, new phytotoxic and antibacterial metabolites produced by a fungus Nigrospora oryzae. Biosci Biotechnol Biochem 61(11):1848–1852. https://doi.org/10.1271/bbb.61.1848
Tejesvi MV, Kini KR, Prakash HS, Subbiah V, Shetty HS (2007) Genetic diversity and antifungal activity of species of Pestalotiopsis isolated as endophytes from medicinal plants. Fungal Diversity 24(3):1–18
Uddin MZ, Dibyajyoti S, Aninda KN, Anowara J, Mycal D, Swati P (2012) Comparative study of antibacterial, antifungal and cytotoxic effects of different extracts of Dillenia indica thunb and Abroma augusta Linn. Bull Pharm Res 2:124–128
Ujam NT, Ajaghaku DL, Okoye FB, Esimone CO (2021) Antioxidant and immunosuppressive activities of extracts of endophytic fungi isolated from Psidium guajava and Newbouldia laevis. Phytomedicine plus 1(2):100028. https://doi.org/10.1016/j.phyplu.2021.100028
Uzor PF, Osadebe PO, Nwodo NJ (2017) Antidiabetic activity of extract and compounds from an endophytic fungus Nigrospora oryzae. Drug Res 67(05):308–311. https://doi.org/10.1055/s-0042-122777
Vuorela P, Leinonen M, Saikku P, Tammela P, Rauha JP, Wennberg T, Vuorela H (2004) Natural products in the process of finding new drug candidates. Curr Med Chem 11(11):1375–1389. https://doi.org/10.2174/0929867043365116
Wang H, Jenner AM, Lee CYJ, Shui G, Tang SY, Whiteman M, Halliwell B (2007) The identification of antioxidants in dark soy sauce. Free Radic Res 41(4):479–488. https://doi.org/10.1080/10715760601110871
Wang LW, Wang JL, Chen J, Chen JJ, Shen JW, Feng XX, Chen FY (2017) A novel derivative of (-) mycousnine produced by the endophytic fungus Mycosphaerella nawae, exhibits high and selective immunosuppressive activity on T cells. Front Microbiol 8:1251. https://doi.org/10.3389/fmicb.2017.01251
White TJ, Bruns T, Lee SJWT, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc: Guide Methods Appl 18(1):315–322
Wu SH, Chen YW, Shao SC, Wang LD, Yu Y, Li ZY, Huang R (2009) Two new solanapyrone analogues from the endophytic fungus Nigrospora sp. YB-141 of Azadirachta indica. Chem Biodivers 6(1):79–85. https://doi.org/10.1002/cbdv.200700421
Xie JH, Xie MY, Nie SP, Shen MY, Wang YX, Li C (2010) Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem 119(4):1626–1632. https://doi.org/10.1016/j.foodchem.2009.09.055
Xing R, Yu H, Liu S, Zhang W, Zhang Q, Li Z, Li P (2005) Antioxidant activity of differently regioselective chitosan sulfates in vitro. Bioorg Med Chem 13(4):1387–1392. https://doi.org/10.1016/j.bmc.2004.11.002
Xu L, Meng W, Cao C, Wang J, Shan W, Wang Q (2015) Antibacterial and antifungal compounds from marine fungi. Mar Drugs 13(6):3479–3513. https://doi.org/10.3390/md13063479
Yadav M, Yadav A, Yadav JP (2014) In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac J Trop Med 7:S256–S261. https://doi.org/10.1016/S1995-7645(14)60242-X
Zhao LJ, Yang XN, Li XY, Wei MU, Feng LIU (2011) Antifungal, insecticidal and herbicidal properties of volatile components from Paenibacillus polymyxa strain BMP-11. Agric Sci China 10(5):728–736. https://doi.org/10.1016/S1671-2927(11)60056-4
Zhao JH, Zhang YL, Wang LW, Wang JY, Zhang CL (2012) Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J Microbiol Biotechnol 28(5):2107–2112. https://doi.org/10.1007/s11274-012-1015-4
Zhu X, Zhou D, Liang F, Wu Z, She Z, Li C (2017) Penochalasin K, a new unusual chaetoglobosin from the mangrove endophytic fungus Penicillium chrysogenum V11 and its effective semi-synthesis. Fitoterapia 123:23–28. https://doi.org/10.1016/j.fitote.2017.09.016
Acknowledgements
The authors acknowledge Department of Botany, Panjab University Chandigarh, India for providing infrastructure and instrumentation. Vijay Kumar is also thankful for Senior Research Fellowship (File No. 09/135(0854)/2019-EMR-I) by Council of Scientific and Industrial research (CSIR), India during research work.
Funding
The research work is not funded by any agency.
Author information
Authors and Affiliations
Contributions
Vijay Kumar, carried out experimental work and prepared manuscript. I.B. Prasher supervised the work and refined manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Vijay Kumar has no conflict of interest. I. B. Prasher has no conflict of interest.
Ethical approval
Not applicable.
Consent for publication
The work is original; there is no plagiarism, and it has not been published anywhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, V., Prasher, I.B. Phytochemical analysis and antimicrobial potential of Nigrospora sphaerica (Berk. & Broome) Petch, a fungal endophyte isolated from Dillenia indica L.. ADV TRADIT MED (ADTM) 23, 525–537 (2023). https://doi.org/10.1007/s13596-021-00619-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-021-00619-x