Abstract
Root bark preparations of the Gabonese plant Tabernanthe iboga (T. iboga) has long been used in traditional medicine in Central and West African regions for the management of type 2 diabetes (T2D). This study is the first investigation of in vivo hypoglycaemic activity in healthy rats and anti-hyperglycaemic activity of T. iboga in a 10% fructose-fed (40 mg/kg i.p.) streptozotocin (STZ) injected type 2 diabetic rat model. T. iboga at 50 to 200 mg/kg induced hypoglycaemia activity over 3 h fasted glucose tolerance in healthy Wistar rats and anti-hyperglycaemic effects on non-fasted and fasted blood glucose in fructose-fed STZ T2D rats with no toxicity. Fructose-fed STZ T2D rats developed characteristic type 2 diabetic complications over 6 weeks exhibiting significantly elevated fasting and non-fasting blood glucose, polydipsia, reduced body weight gain and glucose and insulin tolerance compared with STZ alone and normal control rats. T. iboga (50 mg/kg and 200 mg/kg bw) administered p.o. once daily for 4 weeks significantly improved diabetic symptoms of polydipsia, reduced body weight, hyperglycaemia, glucose and insulin tolerance (as AUC) compared with fructose-fed STZ T2D rats. T. iboga aqueous extract (50 mg/kg and 200 mg/kg) also significantly reversed altered actions of marker enzymes of liver including alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), creatinine, HbA1c and elevated triglycerides in fructose-fed STZ type 2 diabetic rats. Our outcomes show that daily oral provision of T. iboga improves type 2 diabetes complications, superior to glibenclamide, in rat fructose-fed STZ model and offers the potential for safe clinical management of T2D in Gabon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is the most prevalent form of diabetes, which results largely from the inadequate use of insulin by the body, insulin resistance, excess of body weight and physical inactivity (Motala and Ramaiya 2010). T2DM is leading to glucose intolerance, hyperglycaemia and overt diabetes causing blindness, end-stage renal disease as a consequence of microvascular pathology, numerous debilitating neuropathies increasing mortality and morbidity in diabetics (Tripathi and Srivastava 2006; Domingueti et al. 2016).
Tabernanthe iboga (T. iboga) root bark from the family of Apocynaceae is generally used in traditional Gabonese medicine for the management of T2DM (Bading-Taika et al. 2018a, b). Although, to date, there is a lack of scientific knowledge about the appropriate range of concentrations for T. iboga extract to be used safely for diabetes management in animals and in patients (Bading-Taïka et al. 2018a, b).
We have previously shown that the aqueous extract of T. iboga has sulfonylureas-like action on insulin release (Souza et al. 2011). Precedent in vitro studies have demonstrated that T. iboga aqueous extract at 1 µg/ml significantly potentiates the secretion of insulin in response to glucose stimulatory concentrations (11.1 and 16.7 mM) via the K+-ATP channels closure and the increase of the influx of Ca2+ in isolated pancreatic islets triggering the secretion of insulin induced by glucose, in the same way as the medicine tolbutamide (200 µM) (Souza et al. 2011).
Animal models of diabetes are widely used to study in vivo the efficacy of anti-diabetic plants, their side effects and routes of action (Eddouks et al. 2012). Wilson and Islam (2012) have characterised a non-genetic, cost effective, model for T2D in rats with a fast induction time and a stable pathogenesis of insulin resistance, T2D and incomplete dysfunction of pancreatic ß-cell over an experimental period of 11 weeks. Given the insulin potentiation effects reported in vitro of T. iboga (Souza et al. 2011), here we aim to establish the effect of a range of concentrations of T. iboga on oral test of tolerance to glucose and intraperitoneal fasting test of tolerance to insulin in vivo in 10% fructose-fed STZ (40 mg/kg i.p.) injected rats (Wilson and Islam 2012; Guarino et al. 2013; Sadeghi et al. 2017).
Evidence has shown the main constituent in T. iboga, ibogaine, exhibits toxicity with LD50 ranging from 145 to 175 mg/kg via i.p. and 263 to 327 mg/kg p.o. in rats (Kubiliene et al. 2008). Despite the neurotoxic and cardio toxic reported properties of ibogaine, there may be other compounds in T. iboga aqueous extract with hypoglycaemic properties that prevent or reduce the progress of T2D (González-Castejón and Rodriguez-Casado 2011; Bading-Taïka et al. 2018a, b).
To determine a safe, non-toxic range of concentrations of T. iboga aqueous extract in vivo, we first evaluated the toxicity in healthy rats. The animal doses were calculated based on the reported quantity of T. iboga administered daily by traditional healers to their patients. This dose was considered as the equivalent human dose (HED) (100 ml of the aqueous extract of T. iboga per day) which was used to calculate back the range of doses for toxicological and in vivo studies (Hosseini et al. 2018).
Materials and methods
Plant collection
T. iboga root barks were collected by botanist from the institute of Pharmacopoeia and Traditional Medicine (IPHAMETRA) at Lambaréné forest. The plant authentication was at the National Herbarium of Gabon (no 20358), the root barks were grounded in a traditional big grounder to have a fine powder. Root barks powder (75 g) was macerated for 24 h first in n-hexane (500 ml), at room temperature and under magnetic stirrer. The solid material obtained was then macerated with distilled water (500 ml) at room temperature and under magnetic stirrer for 24 h. The macerate was filtered using cotton and Whatman number 1 filter paper placed in a Buchner funnel, and freeze-dried in a lyophilisator (Alpha 1-2 LDplus). The powder was stored at room temperature in a desiccator to avoid humidity.
Animals and environment for in vivo experiments
All study protocols were approved by the National Committee of Ethics in Scientific Research of Gabon (0013/2019/MESRSTT/USTM/VR). Male and female Wistar rats 9 weeks age (180–250 g) from the Animal Unit of IPHAMETRA, were used for toxicological and pharmacological studies. The animals were housed in group of same genders (3 each) and similar weight in standard cages (Techniplast 2000P) with sawdust and shredded paper wool bedding with free access to water and food (Labdiet 5001).
Toxicological studies: acute and sub-acute toxicities (dosing schedule) of T. iboga aqueous extract in Wistar rats
Acute toxicity in male Wistar rats over 24 h
Acute oral toxicity was carried out in accordance with adjusted Lorke’s method (Lorke 1983). Phase 1 required 12 animals, separated into 4 lots of 3 animals each. Each lot was given a single oral (p.o.) dose of the plant aqueous extract tested (10, 100, 1000 mg/kg b.w.). The animals were placed under observation for their gross behavioural, neurologic (such as tremors, seizures), autonomic and toxic effects up to 24 h, as well as mortally (Table 1).
In phase 2, animals were administered a single higher dose 1600, 2900, and 5000 mg/kg b.w. of tested aqueous plant extract followed by an observation of 24 h to establish the correct medium Lethal Dose (LD50: the dose that kills 50% of test population) value. On the day of treatment, animals were observed frequently and signs of acute toxicity such as autonomic and toxic effects, gross behavioural, neurologic trouble, as well as mortality in surviving animals, were monitored. At 2 h the consumption of food, the faeces and urine were also assessed, followed by an assessment at 6 h intervals for 24 h. For the surviving animals, food consumption and body weight were monitored for up to 3 weeks to established recovery from any toxicity.
The calculation of LD50 used the following:
D0 = Highest dose leading to no mortality, D100 = Lowest dose leading to mortality.
The evaluation of toxicity associated with the LD50 used the scale on Table 2 (Hodge and Sterner 2005).
Sub-acute oral toxicity in male and female Wistar rats over 28 days
Sub-acute oral toxicity test was conducted on Wistar rats (females and males) separated into 8 lots of 3 animals per gender, according to Adewale et al. (2016). The doses were selected regarding the acute toxicity results in rats. The highest subacute dose represented the 1/5th of the largest dose used in the acute toxicity study (200 mg/kg). Daily treatment for 28 days was applied to all animals p.o. (Dose volume = Xml/kg). Animals were monitored twice daily for any toxicity signs or adverse effects such as behavioral alterations, morbidity and mortality until the end of the study. Extract or distilled water were administered by oral gavage. Body weights (weekly), water and food consumption (daily) were recorded. Groups were distributed as followed:
-
Group 1 and 2: Female and Male Control group (vehicle p.o. equivalent dose volume)
-
Group 3 and 4: Female and male 50 mg/kg b.w. of T. iboga p.o.
-
Group 5 and 6: Female and male 100 mg/kg b.w of T. iboga p.o.
-
Group 7 and 8: Female and male 200 mg/kg b.w. of T. iboga p.o.
On the last day of the experiment (29th day), animals were terminated by cervical dislocation after increasing CO2 exposure following overnight fasting. Animal weight was recorded, organs such as liver, heart, kidneys, spleen, lungs, testis and ovaries were dissected out with care and weighed. Serum biochemical parameters were measured (AST, ALP, ALT, TC, Urea, TG, Blood Glucose, Creatinine).
Experimental design for the assessment of hypoglycaemic activity of T. iboga aqueous extract
20 healthy Wistar rats were separated and treated in the following Jaiswal et al. (2009) pattern: N = 5 per group (4 treatment groups).
An oral glucose tolerance test (OGTT) was completed to evaluate hypoglycaemic activity of T. iboga. Fasting blood glucose (FBG) was checked initially (overnight fast 12 h), then blood glucose level (BGL) was taken 90 min before treatment, which was considered as Time “0 h” value followed by Time 1 h; T2: Time 2 h; T3: Time 3 h; post treatment.
-
Group 1: Normal Control (NC)
-
Group 2: T. iboga (50 mg/kg)
-
Group 3: T. iboga (100 mg/kg)
-
Group 4: T. iboga (200 mg/kg)
Experimental treatment design for the effect of T. iboga on OGTT (FBG: Fasting blood glucose; dh2O: distilled water; BG: Blood glucose; BGL: Blood glucose level; T0: Time 0 h; T1: Time 1 h; T2: Time 2 h; T3: Time 3 h; b.w.: body weight)
Doses of T. iboga were calculated according to the subacute toxicity test results. We used values below the 10% of oral DL50 (1442.77 mg/kg b.w.) in rats, we obtained a value of 144.28 mg/kg b.w. value that gave no adverse effect and good recovery after a long-term treatment with T. iboga aqueous extract.
Experimental design of the assessment of anti-hyperglycaemic activity of T. iboga aqueous extracts on 10% fructose-fed STZ (40 mg/kg i.p.) type 2 diabetic model adapted from Wilson and Islam (2012)
42 male Wistar rats (180–250 g) were used. Water supplemented with 10% fructose (FR10) for an initial 2 weeks’ period was provided followed by normal water regimen and Labdiet 5001 ad libitum throughout the period of investigation. Normal control group (NC, group 1) was provided with standard water throughout the study.
On the day of STZ injection, STZ was dissolved in a citrate buffer (pH 4.4) at 4 mg/ml of concentration and allowed to stand in the fridge for 30 min prior to injection (De la Garza-Rodea et al. 2010). After an overnight fasting, all animals were injected intraperitoneally (i.p., DV 10 ml/kg) with STZ at 40 mg kg b.w., except for group 1 (normal control, NC) and group 8 (Fructose 10% control, FC) animals that were injected with citrate buffer, pH 4.4. A droplet of blood was withdrawn from the tail vain and blood glucose measured with glucometer (Accu-Check active, @Roche, Germany). Rats with Non-Fasting Blood Glucose (NFBG) level ≥ 300 mg/dl were considered as diabetic and selected for the study.
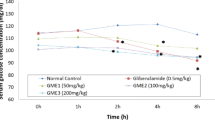
Weekly FBG and NFBG were measured throughout the experiment. In the study, 42 rats, including 10% of possible non-diabetic rats were used. Animals were randomly separated based upon mean body weight into 8 groups of 5 rats each after the confirmation of diabetes (blood glucose ≥ 300 mg/dl) and drugs/aqueous extracts were administered as in Fig. 1. Doses were selected according to the sub-acute toxicity test results and hypoglycaemic efficacy in healthy rats (Fig. 2).
Hypoglycaemic effect of graded doses of aqueous extract of T. iboga on blood glucose of normal male Wistar rats during OGTT. Data is presented as mean ± SEM (n = 5) and were analysed by two-way ANOVA repeated measures followed by Dunnett’s multiple comparison test. Statistical significance is represented as ***p < 0.001 and ****p < 0.0001 compared with NC (no control, no extract)
-
Group 1: Normal Control: no Fructose, no STZ
-
Group 2: STZ control: STZ (40 mg/kg i.p.), no Fructose
-
Group 3: STZ/Fructose 10% Control STZ/FR10 (40 mg/kg i.p.)
-
Group 4: T. iboga (50 mg/kg p.o.) + Fructose (10%, + STZ (40 mg/kg i.p.)
-
Group 5: T. iboga (100 mg/kg p.o.) + Fructose (10% + STZ (40 mg/kg i.p.)
-
Group 6: T. iboga (200 mg/kg p.o.) + Fructose (10% + STZ (40 mg/kg i.p.)
-
Group 7: Glibenclamide 5 mg/kg b.w. + Fructose (10%), + STZ (40 mg/kg i.p.)
-
Group 8: Fructose control: Fructose (10%), no STZ
Treatments (T. iboga, Glibenclamide, Vehicle) were orally administered once daily (8 am every day) for 4 weeks (28 days).
Blood glucose was measured at time 0, 30, 60 min then 120 min following the first administration of treatment and then once daily (NFBG) during the treatment period.
OGTT and intraperitoneal insulin tolerance test (i.p.ITT) were performed at the 2nd and 4th weeks of treatment.
Stock of Humulin R solution (100 U/ml) was mixed with saline to 0.5 U/ml (1/200 dilution) by adding 10 μl stock (100 U/ml) to 1990 μl 0.9% (w/v) sterile saline for i.p.ITT.
Working insulin solution (0.075 U/ml) of 12 ml was prepared by adding 1.8 ml of 0.5 U/ml insulin solution to 10.2 ml of sterile physiological saline (0.9% NaCl) in a sterile 15-ml centrifuge tube. Rats were fasted overnight before OGTT and i.p.ITT. Blood glucose was measured prior to insulin treatment (Time 0) followed by an injection intraperitoneally of 10 µl of 0.075 U/ml insulin solution per gram body weight (dose of 0.75 U/kg, 10 ml/kg) with a 28 G × ½” needle.
Blood glucose was measured before the insulin injection (Time 0) and at 30, 60, 90 and 120 min’ post injection.
Water and food intake, and body weights were recorded every morning during the 28 days’ experiment. At the end of 4 weeks’ study, rats were euthanised by cervical dislocation after increasing CO2 exposure. Blood was withdrawn from abdominal aorta and collected in EDTA tubes, centrifuged (CYAN CL008, Cypress Diagnostics, Belgium) at 3000 rpm for 10 min to obtain plasma.
Organs (heart, lungs, liver, kidneys, testis) were collected, weighed and serum biochemical parameters measured (HDL, LDL, Hba1c, AST, ALP, ALT, TC, Urea, TG, Blood Glucose, Creatinin).
Biochemical analyses
The plasma obtained was used for biochemical analyses using the biochemistry analyser, CYANsmart (Cypress Diagnostics, Belgium). Hepatic enzymes (ALT, AST, ALP), lipids profiles, proteins and glycated haemoglobin concentrations were assessed using kits (Biomed-Gabon, Cypress Diagnostics, Belgium). A glucometer (Accu-Check active, @Roche, Germany) was used to measure blood glucose.
Data analysis
All experimental data are presented as mean ± standard error mean SEM. Data were analysed by GraphPad Prism 7.0 software. One-way ANOVA was performed to assess the effect of a single independent variable on more than two groups followed by Dunnett’s test for comparison between treatment groups. Two-way repeated measures ANOVA was performed to evaluate the effect of two independent variables (e.g. treatment, time) in specific experiments followed by Tukey’s tests. All data including food intake, body weight, OGTT, and i.p.ITT were analysed using a repeated-measures-in-time design ANOVA. Repeated measures within animals were modelled using a first-order autoregressive variance–covariance structure (body weight and food intake over time) or an unstructured variance–covariance structure (OGTT and i.p.ITT over time). Baseline values were used as linear covariate for body weight and food intake data.
Results
Toxicological studies: acute and sub-acute toxicities
Acute toxicity of T. iboga in healthy rats
The maximum oral dose of the extract that killed 0% of the group (LD0) was 784.71 mg/kg b.w. and the lowest dose of the extract that killed 100% of the population (LD100) was 2000 mg/kg b.w. LD50 by oral route was 1442.77 mg/kg b.w.
T. iboga aqueous extract was found to be slightly toxic by oral route, according to Hodge and Sterner scale.
Sub-acute toxicity study in healthy rats: mortality and general behaviour
No mortality in rats of both gender was induced by the oral consumption of the aqueous extract of T. iboga at 50, 100 and 200 mg/kg b.w. once daily for 28 days. In treated and control groups, no significant difference in food and water consumption was noticed (Table 3).
Male rats in control group gained weight throughout the study. However, female rats in control group lost weight during the first 2 weeks and then did not gain or lose weight (Table 4). In group 1, treated with 50 mg/kg b.w. of T. iboga, male rats gained significantly more weight from the first week until the end of the study compared to control (p < 0.05) group. Similarly, female rats of the same group gained weight throughout the study except after 28 days. In groups treated with 100 mg/kg, male and female rats gained weight throughout the 28 days experimental period, whilst only male rats gained weight over the first 14 days at the 200 mg/kg b.w. dose.
The Table 5 shows that male lungs weights in groups which received 200 mg/kg of T. iboga were significantly higher than control after 28 days (p < 0.0001). Male liver weights in group which received 100 mg/kg of T. iboga significantly diminished compared to control (p < 0.01). Similarly, female heart and lungs weights in group which received 200 mg/kg and 100 mg/kg of T. iboga were significantly elevated comparing to control (p < 0.05, p < 0.01 respectively) and female liver weights in group which received 200 mg/kg of T. iboga were significantly increased compared to control (p < 0.0001). Kidneys (left), Ovaries and uterus weights were also significantly increased in female rats which received 100 mg/kg and 200 mg/kg of T. iboga aqueous extract compared to control (p < 0.05 and p < 0.01, respectively). No change in tissue weights were seen in groups treated at 50 mg/kg (p > 0.05) in both males and females.
The Table 6 indicates a significant drop of ALP and AST levels in male and female groups treated with T. iboga at 100 mg/kg (p < 0.0001) and ALT levels only in females group. Creatinine levels in male and female groups treated with T. iboga at 50 and 200 mg/kg were significantly lowered compared to control groups, and at the 100 mg/kg dose in females only (p < 0.0001).
Hypoglycaemic effect of T. iboga doses (50, 100 and 200 mg/kg b.w.) during a 3 h glucose tolerance test in healthy rat
Figure 2 illustrates the effect of daily oral administration for 28 days with variable doses of aqueous extract of T. iboga (50, 100 and 200 mg/kg) on OGTT of normal male Wistar rats. There was a significant increase in blood glucose at 50 mg/kg of T. iboga between 1 and 2 h after glucose load, followed by a significant fall in blood glucose by 3 h. At higher doses of T. iboga (100 and 200 mg/kg), blood glucose was elevated at 2 h after glucose load and significantly dropped at 3 h. All doses showed the same temporal hypoglycaemic effect after 3 h’ post dose, where the dose of 50 mg/kg showed a maximum fall of 48.2% at 3 h after glucose load, whereas fall of 44.4% and 38.5% was observed with 100 and 200 mg/kg respectively at the corresponding time.
Effect of T. iboga doses (50, 100 and 200 mg/kg) on 10% fructose-fed (FR10)/streptozotocin (40 mg/kg i.p., STZ) type 2 male diabetic rats: body weight, food and fluid intake, and blood glucose
Figure 3 a, b shows that all experimental groups gained weight during the 2 first weeks of the study (Fig. 1). However, from 3 weeks onwards, weight gain significantly decreased in group 3 (STZ/FR10) compared to normal control (p < 0.05) group 2 treated with STZ control and group 8 (FR10, no STZ) (p < 0.05, Fig. 3a). Group 8 (FR10, no STZ) gained weight significantly from week 3 onward compared to group 2 (STZ control), group 3 (STZ/FR10) and normal control (p < 0.05). Groups treated with STZ/FR10 and T. iboga at 50, 100 and 200 mg/kg gained significantly less weight than normal control, while groups treated glibenclamide gained significantly more weight than group 3 (STZ/FR10 control, Fig. 3b).
Mean body gain weight over 6 weeks of experimental period. a Control groups N = 4 and b treated groups N = 6—Fructose 10% treatment from week 0 to week 2, STZ injection at week 2, T. iboga treatment from week 2 to week 6. Data are presented as mean ± SEM (n = 5 per group), and were analysed by two-way ANOVA with post hoc Tukey’s multiple comparison test. Statistical significance is represented as *p < 0.05 versus NC, #p < 0.05 versus STZ/FR10 control, †p < 0.05 versus STZ control, +p < 0.05 versus FR10, no STZ
Food intake did not fluctuate throughout the experimental period Fig. 4, however, the fluid intake increased significantly in group 3 (STZ/FR10 control) compared to normal control and group 2 (STZ control) (p < 0.05). Also, fluid intake increased significantly in group 2 (STZ control) and group 8 (FR10, no STZ) compared to normal control (p < 0.05).
Mean food and fluid intake over 6 weeks of experimental period. Data are presented as mean ± SEM (n = 5 per group), and were analysed by two-way ANOVA with post hoc Tukey’s multiple comparison test. Statistical significance is represented as ****p < 0.0001 and ***p < 0.001 versus NC, ##p < 0.01 and #p < 0.05 versus STZ/FR10 and ††p < 0.01 versus STZ control
FBG in all groups did not fluctuate during the 2 first weeks of the experimental period (Fructose treatment period). However, from week 4, after STZ injection, NFBG increased significantly in all groups compared to normal control. In group 3 (STZ/FR10) NFBG levels increased steadily during the remaining experimental period compared to normal control group and group 2 (STZ control) group (p < 0.05) (Fig. 5a). However, in group 2 (STZ control) NFBG decreased significantly compared to group 3 (STZ/FR10) and remained low until the end of experimental period (p < 0.05) group 8 (FR10, no STZ) NFBG remained low throughout the experimental period (Fig. 5a). Also, NFBG significantly increased in groups treated with T. iboga (50 to 200 mg/kg) and group 7 (Glibenclamide) compared to normal control (NC). From week 3, in group 4 (T. iboga 50 mg/kg) NFBG levels decreased significantly during the remaining experimental period compared to group 3 (STZ/FR10 control) and group 2 (STZ control) (p < 0.05) to reach levels comparable to those of NC group (Fig. 5b). Also, in group 6 (T. iboga 200 mg/kg), NFBG levels followed the same pattern as group 4 (T. iboga 50 mg/kg), although NFBG levels remained significantly higher than those of NC (p < 0.05) in a similar way of group 3 (STZ/FR10) (Fig. 5b). In group 5 (T. iboga 100 mg/kg), NFBG levels remained significantly higher than NC, (STZ/FR10 control) and (FR10, no STZ) groups from week 4 up to week 6 after STZ injection at week 2 (p < 0.05) (Fig. 5 b). In group 7 (Gli), NFBG levels increased significantly after STZ injection (at week 2) compared to NC (p < 0.05) and decreased gradually from week 3 up to the end of experimental period, however, NFBG levels remained significantly higher than those of NC (p < 0.05) (Fig. 5 b).
Mean blood glucose over 6 weeks of experimental period. a Control groups N = 4 and b treated groups N = 6—(FR10) Fructose 10% treatment was indicated from week 0 to week 2 with Fasting Blood Glucose (FBG), STZ injection was indicated at week 2 with Non-Fasting Blood Glucose(NFBG) levels, T. iboga treatment from week 2 to week 6. Data are presented as mean ± SEM (n = 5 per group), and were analysed by two-way ANOVA with post hoc Tukey’s multiple comparison test. Statistical significance is represented as *p < 0.05 versus NC, #p < 0.05 versus STZ/FR10 control, †p < 0.05 versus STZ control, +p < 0.05 versus Gli (5 mg/kg)
Effect of T. iboga (50 to 200 mg/kg p.o.) and Glibenclamide on glucose tolerance and insulin tolerance in STZ/FR10 type 2 diabetic rats
The prime objective of this experiment was to establish a correlation between the ingestion of T. iboga aqueous extract and the improvement of glucose and insulin tolerance in T2D induced by STZ/FR10 in rats. The results of the glucose tolerance tests conducted following 2 weeks of treatment with T. iboga showed that groups treated with the plant aqueous extract removed blood glucose slower after glucose load compared to the negative control group (Fig. 6a). At week 2, group 3 (STZ/FR10 control) showed significantly higher levels of blood glucose compared with NC and group 2 (STZ control) (p < 0.05). Similarly, in group 7 (Gli), blood glucose levels were significantly higher than NC and STZ control group 2 up to 60 min after glucose load and decreased significantly after 120 min compared to group 3 (STZ/FR10 control) (p < 0.05) (Fig. 6a). The area under the curve (AUC) of groups 3 (STZ/FR10 control showed marked glucose intolerance compared to NC, STZ control and FR10, no STZ groups (p < 0.0001).
Effect of T. iboga aqueous extract on glucose tolerance in T2D rats at 2 weeks (a, b) and 4 weeks (c, d). Data are presented as mean ± SEM (n = 5 per group), and were analysed by two-way ANOVA with post hoc Tukey’s multiple comparison test. Statistical significance is represented as ****p < 0.0001, *p < 0.05 versus NC, ####p < 0.0001, ###p < 0.001, ##p < 0.01 versus STZ/FR10 control, ††††p < 0.0001, †††p < 0.001, †p < 0.05 versus STZ control and +p < 0.05 versus Gli (5 mg/kg)
At 4 weeks of T. iboga treatment, the glucose tolerance tests showed that group treated with 50 mg/kg and 200 mg/kg of T. iboga aqueous extract cleared glucose in the same manner of NC group. However, groups treated with 100 mg/kg of T. iboga cleared slower glucose compared to the NC group (Fig. 6c, d). Group 5 (T. iboga 100 mg/kg), exerted significantly higher blood glucose than NC and group 2 (STZ control) at 30 min (p < 0.05) in the same pattern than group treated with Glibenclamide (Fig. 6c, d). However, group 4 exhibited significant lower blood glucose levels compared to group 3 after 60 min (p < 0.05) but these levels remained significantly higher than those of NC (p < 0.05) (Fig. 6c, d). Also, group 6 (T. iboga 200 mg/kg) exerted significantly lower levels of blood glucose compared to group 3 (STZ/FR10 control) from 30 to 120 min after glucose load (p < 0.05) (Fig. 6c, d). The AUC results show marked glucose intolerance in groups 3 (STZ/FR10, control), 5 (T. iboga 100 mg/kg, STZ/FR10) and 7 (Gli) (p < 0.0001) compared to NC rats whilst group 4 and 6 (T. iboga 50 and 200 mg/kg, STZ/FR10 cleared glucose in the same manner as NC rats (Fig. 6d).
The outcome of the insulin tolerance tests performed at 2 weeks of treatment with T. iboga showed that the plant extract did not improve insulin sensitivity compared to negative control group (Fig. 7a, b) at 2 weeks and 4 weeks of plant extract treatment. Also, the group treated with glibenclamide did not either improve insulin sensitivity during this experiment (Fig. 7a–d). At 2 weeks, group 5 (T. iboga 100 mg/kg) at 30 min after insulin injection exerted significantly higher levels of blood glucose compared to NC, group 2 (STZ control) and group 3 (STZ/FR10 control) (p < 0.05) (Fig. 7a, b). Similarly, at week 4, blood glucose levels in group 5 (T. iboga 100 mg/kg) significantly increased at 30 min after insulin injection compared to NC and group 2 (STZ control) (p < 0.05) and decreased rapidly to reach levels close to those of NC at 120 min (Fig. 7c, d). In group 7, at 60 min after insulin injection, blood glucose levels remained significantly higher than those of group 2 (STZ control) and NC (p < 0.05), to increase again after 60 min to levels significantly higher than those of group 3 (STZ/FR10 control), group 2 (STZ control) and NC (p < 0.05) (Fig. c, d). In contrast T. iboga 50 and 200 mg/kg (groups 4 and 6) exhibited blood glucose significantly lower than group 3 (STZ/FR10 control) throughout the insulin tolerance time (p < 0.05) (Fig. 7 c, d).
Effect of T. iboga aqueous extract on insulin tolerance at 2 weeks (a, b) and 4 weeks (c, d). Data are presented as mean ± SEM (n = 5 per group), and were analysed by two-way ANOVA with post hoc Tukey’s multiple comparison test. Statistical significance is represented ****p < 0.0001, *p < 0.05 versus NC, ##p < 0.01, #p < 0.05 versus STZ/FR10 control, ††††p < 0.0001, †††p < 0.001, ††p < 0.01, †p < 0.05 versus STZ control and +p < 0.001 versus Gli (5 mg/kg)
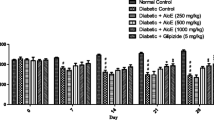
Effect of T. iboga doses (50, 100 and 200 mg/kg b.w.) on organs weights and biochemical parameters of STZ/FR10 T2D rats
As shown on Table 7, T. iboga (50 to 200 mg/kg) did not influence tissue weight during the 4 weeks’ experimental period except for group 6 at 200 mg/kg in which liver weight was significantly increased compared to group 3 (STZ/FR10 control) (p < 0.05). However, in group 3 (STZ/FR10 control), liver weight was significantly decreased compared to NC (p < 0.05) and increased in group 8 (FR10, no STZ) compared to NC, group 2 (STZ control) and group 3 (STZ/FR10 control) (p < 0.05).
The Table 8 shows the group 3 (FR10/STZ control) and group 5 (T. iboga 100 mg/kg) have significantly higher glucose levels compared to NC (p < 0.05). Also, group 3 (STZ/FR10) and group 2 (STZ control) have significant higher levels of creatinine compared to NC (p < 0.05), HbA1c compared to NC and Group 7 (Gli) (p < 0.05) and ALT compared to NC and group 2 (STZ control) (p < 0.05). However, AST levels was significantly lower compared to NC (p < 0.05) in the same group (STZ/FR10 control). Glucose levels were significantly lower in groups 4 (T. iboga 50 mg/kg), 6 (T. iboga 200 mg/kg) and 8 (FR10, no STZ) (p < 0.05). Creatinine levels decreased significantly in groups treated with T. iboga (50 to 200 mg/kg), group 7 (Gli) and group 8 (FR10, no STZ) compared to group 3 (STZ/FR10 control) and group 2 (STZ control) (p < 0.05). HbA1c levels were significantly increased in group 3 (STZ/FR10 control) compared to NC and group 7 (Gli) (p < 0.05) and in group 8 (FR10, no STZ) compared to NC and Group 2 (STZ control) (p < 0.05). Whereas, AST levels were significantly lower in groups treated with T. iboga (50 to 200 mg/kg) and group 7 (Gli) compared to NC, group 2 (STZ control) and group 3 (STZ/FR10 control) (p < 0.05).
As shown on Table 9, T. iboga treatment in T2D rats for a period of 4 weeks did not influence lipid profile, except for the group 6 (T. iboga 200 mg/kg) which exhibited significant increase in HDL-c levels compared to group 3 (STZ/FR10 control) (p < 0.05). However, triglycerides levels were significantly increased in group 3 (STZ/FR10 control) compared to NC (p < 0.05).
Discussion
The subacute toxicity study revealed non-significant differences in the consumption of food and water at graded doses of aqueous extract of T. iboga groups, in both female and male rats and in control group. This indicate that the animals exhibit a healthy growth over 28 days. Rats in all experimental groups gained body weight throughout this study, except for female rats in the group that received 200 mg/kg of T. iboga (Table 4). Thus, it can be inferred that growth prevention did not happen during the journey of repeated daily administration of T. iboga aqueous extract root barks. Likewise, alteration in organ weights is a toxicity indication in animals which is easily confirmed by toxicity tests (Adewale et al. 2016). In this study, there was no major change in the organ weights of all experimental groups (Table 7). Since blood is the major means of transport for a large number of nutrients and foreign elements within the body, blood constituents including white blood counts, blood red cells, haemoglobin and platelets are first in contact with concentrations of possible toxic compounds from ingested plant extract (Adewale et al. 2016). T. iboga aqueous extract of root barks did not induce any alteration to the blood cells of healthy Wistar rats since haemoglobin levels in all groups were not altered after the plant administration (Table 6). Furthermore, aspartate and alanine transaminases (AST and ALT) which are strong criterions of liver harm, alkaline phosphatase (ALP), that indicates bile ducts obstruction or liver and gallbladder inflammation, urea and creatinine which are known to be good indicators of the kidneys function (Adewale et al. 2016) were measured (Tables 6, 8) and indicated a non-toxicity of the plant extract to the liver. Moreover, creatinine levels exhibited (Table 8) may be an evidence that the extract did not inhibit the liver and the kidney capacities to excrete this plant metabolites and may potentiate/stimulate these organs functions. Also, T. iboga may improve insulin resistance as creatinine levels were significantly decreased at selected doses (50, 100 and 200 mg/kg). Thus, these outcomes suggest that T. iboga aqueous extract does not have negative effects on liver and kidney, but rather seems to have a protective effect on these organs.
Our study revealed an in vivo hypoglycaemic effect of T. iboga aqueous extract (50 to 200 mg/kg p.o.) on healthy rats after 3 h. However, T. iboga aqueous extract did not show a dose-dependent hypoglycaemic effect; the hypoglycaemic effect was delayed and was shown after 3 h only, which may indicate an intracellular action of T. iboga aqueous extract as previously shown in isolated pancreatic ß-cells by Souza et al. 2011, where, T. iboga aqueous extract (1 µg/ml) potentiates insulin release from isolated pancreatic islets at stimulatory glucose concentrations. The insulin secretion potentiation by T. iboga at 1 µg/ml was shown to follow a similar pattern of sulfonylureas such as glibenclamide, which induces intracellular Ca2+ influx by closing \({\text{K}}^{ + }_{\text{- ATP}}\) channels in pancreatic cells and triggering insulin release (Souza et al. 2011). However, it has been demonstrated that T. iboga active principles including ibogaine, noribogaine and its alkaloids related congeners may induce intracellular Ca2+ rise through an interaction with σ2 receptors (Bowen 2001; Souza et al. 2011; Ahn et al. 2015). Nevertheless, previous studies have shown that σ2 receptors ligands may induce sustainable and latent rise of Ca2+ involved in processes leading to toxicity and cell death (Bowen 2001).
Furthermore, we explored the anti-diabetic effect of T. iboga aqueous extract (50 to 200 mg/kg p.o.) in a non-genetic fructose-10/STZ rat model of T2D (STZ/FR10, Wilson and Islam 2012). Significant polydipsia was occurring in diabetic control groups in our investigation. The intake of fluid was significantly higher while the body weight gain was significantly lower in T2D (group 3) in comparison to control groups, which might be related to diabetic conditions severity, in addition to an excessive energy expenditure via urinary glucose elimination in our study (Fig. 4). The experimental T2D (group 3) did not show actual weight loss as observed in animal models and T1D patients (King 2012; Eddouks et al. 2012) whereas exhibited reduced weight gain comparing to the control groups. Furthermore, it has been reported in previous work that animals treated with STZ/FR10 exhibited less body weight gain compared to NC and STZ groups, in agreement with our results (Wilson and Islam 2012).
In the present study, animals treated with fructose alone, STZ alone and FR10/STZ T2D rats treated with glibenclamide gained significantly more weight than negative control (NC) animals (p < 0.0001), while animals in groups treated with STZ/FR10 and T. iboga at 50, 100 and 200 mg/kg gained less weight than NC group (Fig. 3). These outcomes are in agreement with effects already described in this type 2 diabetic model and the negative impact of excessive fructose consumption within the body, including fat accumulation, weight gain and circulating levels of triglycerides (Köseler et al. 2018, Table 9). A significant excessive fluid intake (p < 0.0001) was noticed in groups treated with STZ, FR10 and STZ/FR10 compared to NC group (Fig. 4) but not doses of T. iboga or Glibenclamide. Thus, T. iboga aqueous extract at 50 to 200 mg/kg may exert some activity that prevent diabetic features such as polydipsia and an avoidance of body weight gain, which is a usual side effect of type 2 anti-diabetic drugs such as glibenclamide seen also in our study (Skliros et al. 2016, Fig. 3b). T. iboga may prevent excess of energy loss via urinary glucose excretion, commonly seen in diabetic condition (Wilson and Islam 2012).
The OGTT performed at 2 weeks showed that in groups treated with T. iboga at 50 and 100 mg/kg, the peak of glucose level was reached within 60 min after glucose load and decreased slowly after the 1 h period and was brought to levels close to those of NC group (Fig. 6a, b). These results suggest a rapid metabolism of glucose that may be potentiated by T. iboga at these doses (50 and 100 mg/kg) compared to T2D STZ/FR10 rats. Moreover, the OGTT performed after 4 weeks’ period treatment of T. iboga showed a more rapid peak of glucose level (after 30 min) and a sharp fall of glucose levels from 30 min to decrease slowly after 60 min and reached levels close to those of NC group. The significant hypoglycaemic response of T. iboga aqueous extract was clearly shown at 50 mg/kg while, at higher doses (100 and 200 mg/kg), less hypoglycaemic responses were evidenced. The area under the curves (AUC) at the OGTT test at 2 weeks of T. iboga treatment showed significant glucose intolerance in T2D, in group treated with T. iboga at 100 mg/kg and also group treated with Glibenclamide, which is in contrast to the small but significant effect seen in this fructose/STZ-induced T2D rat model. Indeed, in groups treated with T. iboga at 100 mg/kg, blood glucose levels were significantly elevated after 120 min test compared to those of NC group and groups treated with T. iboga at 50 and 200 mg/kg (Fig. 6c, d). These reduced hypoglycaemic responses at higher doses of the plant extract has already been observed with numerous indigenous plants (Singh et al. 2007; Jaiswal et al. 2009) and suggest a dual effect of the plant extract.
Similarly, ITT performed at 2 weeks showed a marked insulin resistance during the first 30 min of the test, in groups treated with T. iboga at 50 and 100 mg/kg, while, in other experimental groups glucose levels patterns showed moderate sign of insulin resistance (Fig. 7c, d). However, when ITT was performed at 4 weeks’ period of T. iboga treatment, insulin resistance was increased in groups treated with STZ/FR10, in line with previous work and is a characteristic of T2D insulin resistance (Wilson and Islam 2012), and in T. iboga at 100 mg/kg group, while significant insulin resistance improvement was noticed in groups treated with T. iboga at 50 mg/kg and 200 mg/kg superior to the anti-diabetic drug glibenclamide (Fig. 7c, d). These results support the hypoglycaemic effect of T. iboga at 50 and 200 mg/kg. Moreover, in our study, plasma insulin could not be measured and HOMA-ß and HOMA-IR could not be calculated which would emphasis pancreatic ß-cells function and the state of insulin resistance in all treated groups.
Biochemical analyses revealed liver and testis weight alterations (Table 7), which reflect the negative effect of FR10 treatment on liver and hepatic functions, already reported (Wilson and Islam 2012; Köseler et al. 2018) and suggest possible deleterious influence on sexual organs of high fructose diets. Plasma glucose was also significantly lower in FR10 group compared to STZ/FR10 group (p < 0.05), which support the well-known STZ consequences on glucose levels supplemented by fructose effects (Wilson and Islam 2012). Furthermore, our results demonstrated kidney damage in STZ alone and STZ/FR10 groups evidenced by elevated plasma creatinine levels, which is in agreement with previous data (Mawa et al. 2019; Al Hroob et al. 2018; Wilson and Islam 2012). Additionally, reduced plasma AST, ALT and ALP levels indicated that T. iboga aqueous extract at 50, 100 and 200 mg/kg may ameliorate diabetes induced liver damage in a similar way of glibenclamide (Table 8, Chukwunonso et al. 2016) and may exert a protective effect of liver and kidney organs by preventing deleterious change in transaminases and creatinine, as well as urea levels. These results are in line with previous data which demonstrated increased levels of AST, ALT and ALP parameters following STZ, STZ/FR10 or FR10 treatments (Köseler et al. 2018; Saeed et al. 2008) as seen in our results (Table 8). Diets high in fructose can cause hyperlipidaemia and have been linked to insulin resistance (Huang et al. 1987). The significantly higher circulating plasma triglyceride levels observed in the FR10/STZ and not STZ and NC groups (Table 9) in line with Wilson and Islam (2012) is a risk factor for insulin resistance in rats. Four weeks of daily dosing of T. iboga aqueous extract (all doses) and Glibenclamide significantly reversed the elevated triglyceride plasma levels which may contribute to the control of insulin sensitivity and non-fasting glucose tolerance.
Our study, in line with others, has demonstrated that 10%-fructose-fed-40 mg/kg b.w.-STZ- injected rats develop a stable diabetic type 2 condition over 6 weeks experimental period. One of the key components of type 2 diabetes therapy is the discovery of novel pharmaceuticals to improve blood glucose control, without negative side effects.
This is the first study to report that the T. iboga aqueous extract (50 and 200 mg/kg once a day over 4 weeks) has hypoglycaemic effects in healthy rats and anti-hyperglycaemic effects in fructose-fed STZ type 2 diabetic rats. The plant aqueous extract at these selected doses does not influence body and major organs weight or parameters reflecting no side effects after 28 days administration.
Further investigations are now needed to evaluate appropriate safe doses of active constituents within the T. iboga aqueous extract in controlled long term clinical trials to validate the clinical use of T. iboga aqueous extract for the management of diabetes in Gabon. Moreover, investigations are crucial to unveil the anti-diabetic mechanism of action of T. iboga active constituents.
References
Adewale OB, Onasanya A, Anadozie SO, Abu MF, Akintan IA, Ogbole CJ, Olayide II, Afolabi OB, Jaiyesimi KF, Ajiboye BO, Fadaka AO (2016) Evaluation of acute and subacute toxicity of aqueous extract of Crassocephalum rubens leaves in rats. J Ethnopharmacol 188:153–158
Ahmed M (2015) Acute toxicity (lethal dose 50 calculation) of herbal drug Somina in rats and mice. Pharmacol Pharm 6:185–189
Ahn C, An B-S, Jeung E-B (2015) Streptozotocin induces endoplasmic reticulum stress and apoptosis via disruption of calcium homeostasis in mouse pancreas. Mol Cell Endocrinol 412:302–308
Al Hroob AM, Abukhalil MH, Alghonmeen RD, Mahmoud AM (2018) Ginger alleviates hyperglycaemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed Pharmacother 106:381–389
Bading-Taika B, Bouckandou M, Souza A, Bourobou Bourobou HP, MacKenzie LS, Lione L (2018a) An overview of anti-diabetic plants used in Gabon: pharmacology and toxicology. J Ethnopharmacol 216:203–228
Bading-Taika B, Akinyeke T, Alcazar Magana A, Choi J, Ouanesisouk M, Samson Torres ER, Lione LA, Maier CS, Bobe G, Raber J, Miranda CL, Stevens JF (2018b) Phytochemical characterisation of Tabernanthe iboga root bark and its effects on dysfunctional metabolism and cognitive performance in high-fat-fed C57BL/6J mice. J Food Bioact 3:11–123
Bowen WD (2001) Chapter 9: Sigma receptors and iboga alkaloids. The Alkaloids 56:173–191
Chukwunonso BO, Chinwuba Okoye T, Okpashi VE, Igwe CI, Alumanah EO (2016) Comparative study of the antioxidant effects of metformin, glibenclamide, and repaglinide in alloxan-induced diabetic rats. J Diabetes Res 2016:1–5
De la Garza-Rodea AS, Knaän-Schanzer S, Den Hartigh JD, Verhaegen APL, Van Bekkum DW (2010) Anomer-Eliquilibrated Streptozotocin Solution for the induction of experimental diabetes in mice (Mus musculus). J Am Assoc Lab Anim Sci 49(1):40–44
Domingueti CP, Dusse LM, Carvalho M, de Souza LP, Gomes BK, Fernandes AP (2016) Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complicat 30(4):738–745
Eddouks M, Chattopadhyay D, Zeggwagh N (2012) Animal models as tools to investigate antidiabetic and anti-inflammatory plants. Evid Based Complement Altern Med 142087:1–14
González-Castejón M, Rodriguez-Casado A (2011) Dietary phytochemichals and their potential effects on obesity. Pharmacol Res 64:438–455
Guarino MP, Dantos AI, Mota-Carmo M, Costa PF (2013) Effects of anaesthesia on insulin sensitivity and metabolic parameters in Wistar rats. In Vivo 27:127–132
Hodge A, Sterner B (2005) Toxicity classes. In: Canadian Center for Occupational Health and Safety. http://www.ccohs.ca/oshanswers/chemicals/id50.htm. Accessed 2018
Hosseini A, Shorofi SA, Davoodi A, Azadbakht M (2018) Starting dose calculation for medicinal plants in animal studies; recommendation of a simple and reliable method. Res J Pharmacogn 5(2):1–7
Huang IS, Ho H, Hoffman BB, Reaven GM (1987) Fructose induced insulin resistance and hypertension in rats. Hypertension 10:512–516
Jaiswal D, Rai PK, Kumar A, Mehta S, Watal G (2009) Effect of Moringa oleifera Lam. Leaves aqueous extract therapy on hyperglycemic rats. J Ethnopharmacol 123:392–396
King AJF (2012) The use of animal models in diabetes research. Br J Pharmacol 166:877–894
Köseler E, Kiziltan G, Türker PF, Saka M, Ok MA, Baccanh D, Aydos TR, Bayraktar N, Özdemir H (2018) The effects of glucose and fructose on body weight and some biochemical parameters in rats. Prog Nutr 20(1):46–51.1
Kubilienė A, Marksienė R, Kazlauskas S, Sadauskienė I, Ražukas A, Ivanov L (2008) Acute toxicity of ibogaine and noribogaine. Medicina (Kaunas) 44(12):984–988
Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54(4):275–287
Mawa J, Rahman M, Hashem MA, Hosen M (2019) Leea macrophylla root extract upregulates the mRNA expression for antioxidative enzymes and repairs the necrosis of pancreatic ß-cell and kidney tissues in fructose-fed Type 2 diabetic rats. Biomed Pharmacother 110:74–84
Motala A, Ramaiya K (2010) Diabetes: the hidden pandemic and its impact on Sub-Saharan Africa. Diabetes Leadership Forum, Africa, Johannesburg, 30 September and 1 October, 2010
Sadeghi A, Beigy M, Alizadeh S, Mazloom H, Vakili S, Ahmadi S, Meshkani R (2017) Synergistic effects of ad libitum low-dose fructose drinking and low-dose streptozotocin treatment in Wistar rats: a mild model of type 2 diabetes. Acta Med Iran 55(5):304–340
Saeed MK, Deng Y, Dai R (2008) Attenuation of biochemical parameters in streptozotocin-induced diabetic rats by oral administration of extracts and fractions of Cephalotaxus sinensis. J Clin Biochem Nutr 42:21–28
Singh SK, Kesari AN, Rai PK, Watal G (2007) Assessment of glycemic potential of Musa paradisiaca stem juice. Indian J Clin Biochem 22(2):48–52
Skliros NP, Vlachopoulos C, Tousoulis D (2016) Treatment of diabetes: crossing to the other side. Hellenic J Cardiol 57:304–310
Souza A, Mbatchi B, Herchuelz A (2011) Induction of insulin secretion by an aqueous extract of Tabernanthe iboga (Apocynaceae) in rat pancreatic islets of Langerhans. J Ethnopharmacol 133:1015–1020
Tripathi BK, Srivastava AK (2006) Diabetes mellitus: complications and therapeutics. Med Sci Monit 12(7):RA130–RA147
Wilson DR, Islam M (2012) Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep 64:129–139
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We are grateful for the support of Dr Adelaide Nieguitsila, Miss Hortense Massalina Souza and Edelweiss Medical Center Port-Gentil/Gabon in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All study protocols were approved by the National Committee of Ethics in Scientific Research of Gabon (0013/2019/ MESRSTT/USTM/VR).
Conflict of interest
Bayissi Bading-Taika has no conflict of interest. Alain Souza has no conflict of interest. Henri-Paul Bourobou Bourobou has no conflict of interest. Lisa A. Lione has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bading-Taïka, B., Souza, A., Bourobou Bourobou, HP. et al. Hypoglycaemic and anti-hyperglycaemic activity of Tabernanthe iboga aqueous extract in fructose-fed streptozotocin type 2 diabetic rats. ADV TRADIT MED (ADTM) 21, 281–295 (2021). https://doi.org/10.1007/s13596-020-00484-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-020-00484-0