Abstract
Sophora japonica has been shown many beneficial pharmacological activities, including the neuroprotective effects. Flavonoids, isoflavonoids, triterpenes, alkaloids are bioactive compounds identified presence in Sophora japonica. The present study aimed to evaluate the neuroprotective effects of ethanolic extract of Sophora japonica flower buds on scopolamine (SCP)-induced cognitive deficits in mice. The modulatory effect of Sophora japonica on memory impairment was investigated using Y-maze and the Morris water maze tasks. Acetylcholine (ACh) levels and acetylcholinesterase enzyme (AChE) activity were measured in brain tissue to investigate the cholinergic effect of Sophora japonica. Pro-inflammatory tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and anti-inflammatory interleukin 10 (IL-10) were also evaluated in mice brain tissue to investigate the anti-inflammatory effect of Sophora japonica. Scopolamine induced the cognitive deficits in Y-maze and Morris water maze test along with reducing ACh level and increasing AChE activity and inflammation in brain tissue. Treatment with ethanolic extract of Sophora japonica flower buds reduced the SCP-induced memory impairment in both behavioral tests along with reducing inflammation and AChE activity, and increasing ACh level in brain tissue. Our data demonstrated that ethanolic extract of Sophora japonica flower buds enhanced cognitive deficits in mice induced by scopolamine, and it is a promising source for the treatment of Alzheimer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease and leads to cognitive deficits in the older people. AD has characteristics with the presence of Aβ plaques and neurofibrillary tangles formation, over oxidative stress status and excess of inflammatory processes (Bonda et al. 2010; Hardy 2006; Heppner et al. 2015). It also showed a decrease of cholinergic transmission, behavior disorders and progressive memory loss. The AD’s pathogenesis remains still not completely understood until now. To explain the AD’s pathogenesis, there are various hypothesis that have been proposed. One of them is the cholinergic hypothesis. The cholinergic hypothesis states that the AD’s pathogenesis is associated with the cholinergic deficits (Davies 1999). The improving of cholinergic function may decrease the process of AD. Enhancing the acetylcholine (ACh) level into the neuronal synapses has been used for increasing cholinergic transmission. Some acetylcholinesterase (AChE) inhibitor such as physostigmine, tacrine, have been used for the treatment of Alzheimer’s symptoms (Mukherjee et al. 2007). However, these drugs have been shown several adverse reactions including diarrhea, nausea, abdominal pain, vomiting (Birks 2006). Therefore, there is a need to find out others inhibitors of AChE with stronger effects, more selective and less adverse reaction for the treatment of Alzheimer. Many researchers have tried to find the AChE inhibitors from medicinal plants with the aim of enhancing the inhibitory effect, selectivity and without adverse reaction for the treatment of AD (Bui and Nguyen 2017).
Medicinal plants have been used in traditional medicine to treat cognitive impairment and memory loss. Sophora japonica (Fabaceae) has been used in traditional medicine for treatment of many disorders such as hypertension, arteriosclerosis, hematemesis, intestinal hemorrhage, leukorrhea, metrorrhagia, conjunctivitis, pyoderma and dizziness (He et al. 2016). The main compounds of Sophora japonica are flavonoids, isoflavonoids, triterpenes and alkaloids. Kaempferol, isorhamnetin, quercetin, genistein and rutin are some flavonoids which have been isolated from Sophora japonica (Abdallah et al. 2014; Sun et al. 2007). Previous study showed that rutin is the most abundant compound of Sophora japonica (Chua 2013). These compounds provided for Sophora japonica various pharmacological activities, including anti-inflammatory, antioxidant, antitumor, antibacterial, antiviral, hemostatic, and anti-atherosclerotic effects (Chua 2013; Kim et al. 2003; Kimura and Yamada 1984; Krishna et al. 2012; Wang et al. 2006). However, there are very few reports about the neuroprotective effect of Sophora japonica plant (Chen and Hsieh 2010). In the present study, we investigated the neuroprotective effects of ethanolic extract of Sophora japonica flower buds to alleviate cognitive deficits induced by scopolamine in mice, by using the Y-maze and Morris water maze tests in mice and evaluating the AChE activity, ACh level and pro-inflammatory TNF-α, IL-1β, and IL-10 in mice brain tissue.
Material and methods
Reagents
5,5′-dithio-bis-(2-nitro) benzoic acid (DTNB) (Himedia, India), scopolamine hydrobromide (Sigma, Singapore), acetylthiocholine iodide (ATCI) (Sigma, Singapore). Solvent ethanol was analytical grade.
Plant material
The Sophora japonica flower buds were collected in Hoa Binh, Vietnam during 2015 and authenticated by Department of Pharmacognosy and Traditional Pharmacy, School of Medicine and Pharmacy, Vietnam National University, Hanoi, Vietnam (SMP-VNU). A voucher specimen has been deposited in the SMP-VNU. Dried samples (3 kg) were extracted with 96% ethanol (10 L) by ultrasonic at 40 °C for three hours for three times. The extracts were filtered, combined and evaporated under low pressure to afford the EtOH extract (354.8 g) (yield: 11.83%).
Animals and experimental design
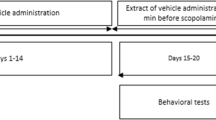
Eight-week-old male C57BL/6 J mice were used in our study. Animals were housed in enriched environmental conditions in groups of 5 animals per polycarbonate cage in a colony room under a 12 h light/dark cycle (12:00 AM – 12:00 PM) under controlled temperature (25 ± 3 °C) and humidity. All animals were maintained accordingly to a protocol approved by the Ethical Committee of the Vietnam National University, Hanoi and following the international rules for animal research. Animals were received water ad libitum as a vehicle and standard diet administration. After adaptation for five days, the mice were randomly divided into four groups of ten mice each, as follows: The SCP group was treated with scopolamine (1 mg/kg,i.p.) without any treatment. Ex-100 group received orally Sophora japonica extract (100 mg/kg) and was treated with scopolamine (1 mg/kg, i.p.). Ex-200 group received orally Sophora japonica extract (200 mg/kg) and was treated with scopolamine (1 mg/kg, i.p.). The normal control (NC) group was treated with saline. For Y-maze test, the treatment was as follows: thirty minutes after oral administration of Sophora japonica extract, each mouse was injected intraperitoneally of 1 mg/kg of scopolamine (Yahaya et al. 2013). For Morris water maze test, the treatment was as follows: mice were given daily extract for fourteen days. At the 15th day, after 60 min of administration extract, mice was injected intraperitoneally scopolamine for four consecutive days (Tung et al. 2017).
Y-maze test
Spontaneous alternation is a behavioral test which shows the capacity of spatial learning and memory. Spatial working memory performance was assessed by recording spontaneous alternation behavior in a Y-maze as previously described (Ma et al. 2007). The Y-maze is a three–arm (assigned as A, B and C) horizontal maze (40 cm long and 5 cm wide with walls 10 cm high) in which the three arms are symmetrically separated at 120o. Twenty minutes after administration of scopolamine, each mouse was initially placed within one arm (A). The sequence and number of all arm entries were recorded for each mouse over a 8 min period. Alternation was determined from successive entries into the three arms on overlapping triplet sets in which three different arms are entered. An actual alternation was defined as entries into all three arms consecutively (i.e. ABC, CAB or BCA but not BAB). The maze arms were cleaned with 70% ethanol between tasks to remove residual odours. An entry was defined as placing all four paws within the boundaries of the arm. The percentage of alternation was calculated using the following formula
Morris water maze test
Morris water maze test has been widely used for study the learning and memory deficits in Alzheimer’s disease model in mice (Bromley-Brits et al. 2011). Morris water maze task was performed 30 min after SCP injection to evaluated memory-related behaviors. Morris water maze is a large circular tank (110 cm in diameter, 35 cm in height), filled to a depth of 20 cm with water at a temperature of 25 ± 1 °C. Water was made opaque by adding powdered milk to prevent the mice from seeing the platform. The platform (10 cm × 10 cm) was made of clear acrylic and was hidden 2 cm below the water surface in a fixed location. The training of mice was during four consecutive days, in each day the mice were given four trials during which they were allowed to find the submerged platform. The starting position was randomized over the four days and was the same for all mice. If the mouse successfully found the platform within 90 s, it was allowed to rest on the platform for 10 s. The time from the mouse being placed in the water to finding the platform was recorded as the escape latency in each trial. If the mouse failed to find the platform within 90 s, its escape latency was recorded as 90 s, and it was physically placed on the platform for 10 s. On the fifth day, mice were subjected to a probe trial session in which the platform was removed from the pool for examining the retention of spatial reference memory. The mice were allowed to swim freely for 90 s. The number of platform crossings and swimming time in the ‘target quadrant’, where the platform had previously been placed, was recorded as indices of spatial memory.
Collection and processing of brain samples
The brain samples were collected and homogenized according to the method described in previous studies, with minor modifications. At the end of the MWM task, the mice were sacrificed by decapitation. Immediately after decapitation, the brains were carefully removed from the skull and were washed twice with cold normal saline solution. The tissue of brain was homogenized in 9 volumes of ice-cold tissue lysis buffer containing 150 mM sodium chloride, 1.0% NP-40, 50 mM Tris, pH 8.0 and 1 mM PMSF (phenylmethanesulfonylfluoride) with protease inhibitors (Sigma, Singapore). Homogenates were centrifuged at 1000×g for 10 min at 4 °C. The supernatant was used for the estimation of AChE activity and ACh level. Protein concentration was determined by Bradford’s method (Bradford 1976).
AChE activity determination
The AChE activity in the brain homogenates was estimated using commercial Acetylcholinesterase Activity Assay Kit according to the manufacturer’s protocols. AChE hydrolyze the neurotransmitter acetylcholine (ACh) to acetate and choline. AChE activity determination was evaluated by Ellman’s method (Ellman et al. 1961). This method based on thiocholine, which is produced by AChE, reacts with 5,5′-dithio-bis-(2-nitro) benzoic acid (DTNB) to form a colorimetric (at 412 nm) product, proportional to the AChE activity present. AChE activity was expressed as U/mg protein.
ACh level determination
The level of ACh in the brain homogenates were estimated using commercial Acetylcholine Assay Kit according to the manufacturer’s protocols. The method consisted in ACh is hydrolyzed by AChE to choline which is oxidized by choline oxidase to betaine and H2O2 (Gilberstadt and Russell 1984). The resulting H2O2 reacts with a specific dye reagent to form a pink colored product. The color intensity at 570 nm is directly proportional to the ACh concentration in the sample. The ACh level in the brain tissues was normalized and expressed as μg/mg protein.
Pro-inflammatory cytokines determination
The level of proinflammatory cytokine IL1β, TNFα and IL10 in the brain homogenates were determined with commercially available Enzyme-Linked ImmunoSorbent Assay (ELISA) kits according to the manufacturers’s instructions. Determination of IL-1β, TNF-α, and IL-10 were performed using a sandwich ELISA method. Briefly, 96-well plates were coated overnight at 4 °C with 100 μl of monoclonal antibody against IL-1β (2,0 μg/ml) or TNF-α (1,0 μg/ml) or IL-10 (2,0 μg/ml) in PBS1x buffer (pH 7.2). The plate was then washed four times with wash buffer (PBS1x + 0.05% Tween-20), blotted dry, and then incubated with blocking solution (PBS1x + 1% bovine serum albumin) for 1 h. The plate was then washed and 100 μl of each homogenate sample or standard was added. Then the plate was incubated at room temperature for 2 h, followed by washing, and added 100 μl of detection antibody IL-1β (0,5 μg/ml) or TNF-α (0,25 μg/ml) or IL-10 (0,5 μg/ml). The antibody was incubated at room temperature for 2 h. Following additional washing, 100 μl of avidin-HRP conjugated (1:2000) was added to each well, followed by a 30 min incubation. After thorough washing, plate development was performed using ABTS (2,2′-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) liquid substrate solution. Then the plate was incubated at room temperature for color development and the color was monitored using a microplate reader at 405 nm with wavelength correction set at 650 nm. The standard curve for the ELISA was established by using murine standard IL-1β or TNF-α or IL-10 diluted in PBS 1× buffer. All standard curves obtained an r2 value between 0.98 and 1. Results were normalized to total protein content in the brain samples. Data are reported as μg cytokine per milligram protein.
Statistical analysis
All data are shown as the mean ± standard error of the mean (SEM) using SigmaPlot 10 (Systat Software Inc., US). The data were checked the normality before choosing the statistical test to use. Multiple comparisons were made by one-way analysis of variance (ANOVA) followed by Dunn’s test. For escape latency test in the Morris water maze, data were analyzed by two ways analysis of variance (ANOVA) followed by Dunn’s test. Statistical significance was set at p < 0.05.
Results
Effect of Sophora japonica extract on scopolamine-induced cognitive deficits in the Y maze task
We have found that the percentage of spontaneous alternation in SCP group significantly lower than NC group. Extract of Sophora japonica at dose 100 mg/kg tends to increase the spontaneous alternation and at a dose of 200 mg/kg significantly increased the spontaneous alternation as compared with SCP group (p < 0.05) (Fig. 1a). We did not find any difference significant between the numbers of arm entries in all mice groups, demonstrating that the general locomotor activity was not affected by the extract (Fig. 1b).
Effect of Sophora japonica extract on scopolamine-induced cognitive deficits in the Morris water maze task
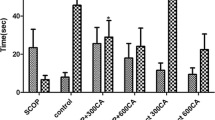
Our data showed that the escape latency decreased gradually during the acquisition training; this means all mice were able to locate the platform (Fig. 2a). The mice in SCP group showed a significantly slowed the learning speed as compared with those in NC group. The mice were treated with Sophora japonica extract significantly decreasing the escape latency on the fourth days as compared to SCP group (p < 0.05) (Fig. 2a).
Effect of Sophora japonica extract on Escape latency of acquisition trial over 4 days (a); swimming time in target quadrant of probe trial (b) and crossing numbers over the platform position (c) in scopolamine-induced cognitive impairment mice in Morris water maze test. Each bar with vertical line represents the mean of 10 animals ± SEM. *p < 0.05 vs. NC group. #p < 0.05 vs. SCP group
The swimming time in the target quadrant and the crossings number have been used to evaluate retention of spatial memory. The swimming time spent in the target quadrant significantly decreased in the SCP group as compared with NC group (Fig. 2b and 2c). Interestingly, the extract of Sophora japonica at dose 100 mg/kg tends to increase the swimming time in the target quadrant and the crossings number. Moreover, at dose 200 mg/kg mice exhibited a significantly increasing these values as compared with mice in SCP group (Fig. 2b and 2c). These data indicate that extract markedly improved the cognitive deficits in mice induced by scopolamine.
Effect of Sophora japonica extract on scopolamine -induced AChE activity and ACh level in brain tissue
Figure 3a showed the effect of Sophora japonica extract on AChE activity in brain tissue. The scopolamine significantly increased the AChE activity in SCP group as compared with NC group (p < 0.05). Treatment with different doses of extract significantly reduced the AChE activity as compared with those in SCP group.
Figure 3b showed that the ACh level in the brain was significantly lower in the SCP group than those in the NC group. The mice were treated with extract at a dose of 100 mg/kg tended to increase the ACh level compared with those in the SCP group. Interestingly, treated mice with a dose of 200 mg/kg exhibited significantly increasing the ACh level as compared with those in SCP group.
Effect of Sophora japonica extract on scopolamine -induced proinflammatory cytokines in brain tissue
We found that IL-1β and TNF-α level significantly increased in SCP group compared with control group as shown in Fig. 4a and 4b. Interestingly, treatment with extraction of Sophora japonica at dose of 100 mg/kg showed a tendency to decrease and at dose of 200 mg/kg significantly decreased those proinflammatory cytokines in mice (Fig. 4a and 4b). Moreover, we found the level of IL-10 decreased in SCP group as compared with the control group. Treatment with different doses of extract significantly increased this cytokine as compared with those in SCP group (Fig. 4c).
Discussion
AD is a neurodegenerative disease characterized by an accumulation of Aβ plaques and neurofibrillary tangles. Cholinergic transmission deficits, increasing the oxidative stress and inflammation process in the brain have been considered main causation and development of AD (Bonda et al. 2010; Davies 1999; Heppner et al. 2015). Medicinal plants have been used in traditional medicine for treating neurodegenerative diseases. Among them, Sophora japonica was traditionally used for calm and improving the memory. In the present study, we examined the effects of Sophora japonica extract on scopolamine-induced cognitive deficits by using the Y-maze task, and the Morris water maze task in mice. Scopolamine, a nonselective muscarinic antagonist, has been widely used to induce the cognitive deficits (Stone et al. 1988).
The ACh level plays an important role in improving the cognitive functions. AD patients have shown a markedly decreased the cholinergic functions and lowed the ACh level in brain (Muir 1997). Therefore, behavior disorders and progressive memory loss are main clinical manifestations in early stage of AD (Mega et al. 1996). Y-maze test is widely used to investigate the drug’s effect on spatial short term memory. The spontaneous alternation behavior is an indicator of spatial memory (Lalonde 2002). We have shown that SCP significantly reduced the spontaneous alteration behavior, whereas Sophora japonica extract reversed SCP-induced reduction in spontaneous alternation behavior. This data suggested that Sophora japonica extract might improve the short-term memory. The total arm entries in all mice group were not significantly different shown that there was not alteration in general locomotor activity of animals.
The Morris water maze test is widely used to evaluate the effects of drugs on spatial learning and memory (Bromley-Brits et al. 2011). Escape latency, number of platform crossings and swimming time spent in the target quadrant were used to investigate cognitive deficits and spatial retention. Our results showed that SCP continuously increased the escape latency, illustrating that cognitive deficits were induced by SCP. This result is agreed with a number of platform crossings and swimming time spent in the target quadrant decreasing in SCP group as compared with NC group. Interestingly, the treatment with Sophora japonica extract not only increased the escape latency, but also enhanced the number of platform crossings and swimming time spent in the target quadrant. Results of the behavioral study demonstrated that Sophora japonica extract may protect mice from SCP-induced learning and memory impairment.
The neurotransmitter cholinergic plays an important role vital role in the regulation of cognitive function (Giacobini 2003). The memory impairment is closely associated with deficits of neurotransmitters. The neurotransmitter ACh, which is hydrolyzed by AChE after its release, is important for learning and memory functions. Strategy to inhibits the AChE has been used for the treatment of AD (Massoud and Léger 2011). SCP has been widely used to induce the cognitive deficits and memory loss by inhibiting cholinergic signaling (Liu et al. 2017). Previous study showed that SCP markedly augmented the AChE activity and reduced ACh level (Liu et al. 2017). In our study, we used SCP to evaluate the effects of Sophora japonica extract on the neurotransmitter cholinergic system. Our study demonstrated that the treatment with Sophora japonica extract significantly inhibited AChE activity and increased ACh level, therefore improving cognitive function in mice.
Many studies have shown that systemic inflammation is associated with AD and stimulates the cognitive deficits (Lee et al. 2010). The neuroinflammatory processes play a critical role in AD’s pathogenesis. In details, proinflammatory cytokines play an important role in the development of AD’s pathogenesis (Swardfager et al. 2010). IL-1β level has been showed elevation in brain tissue from patients who suffered to brain injury or stroke and has implicated in AD’s pathogenesis (Shaftel et al. 2008). TNF-α is a central mediator of amyloid β protein (Aβ) action and can be targeted for AD drug development (Medeiros et al. 2007). TNF-α has been showed increasing in serum from patients with AD compared to healthy person (Fillit et al. 1991). On the other site, IL-10 is a potent anti-inflammatory cytokine that mediates the inflammatory process. IL-10 can regulate Aβ-induced production of the inflammatory cytokines, therefore it may alleviate the risk of AD (Agostinho et al. 2010; Azizi and Mirshafiey 2012). In order to determine if inflammation increases in mice brain and the effect of Sophora japonica extract, we have evaluated the levels of proinflammatory cytokines in whole brain homogenates. In our study, treatment with Sophora japonica extract significantly reduced IL-1β, TNF-α levels and enhanced IL-10 level in the brains of SCP group mice. Our findings suggest that Sophora japonica extract may maintain the balance between pro-inflammatory and anti-inflammatory mediators and may improve memory function in AD.
Our results showed the Sophora japonica extract produced effects in both the cholinergic and inflammatory pathway. In details, the Sophora japonica extract increased the ACh level, reduced the AChE activity and at the same time decreased the IL-1β and TNF-α levels and enhanced the IL-10 level. Our data provide evidence to prove the link between the cholinergic pathway and the inflammatory pathway since the extract acts in both pathways. This data may be explained by using cholinergic anti-inflammatory pathway hypothesis. Many studies have shown that ACh may suppress the cytokine release through a cholinergic anti-inflammatory pathway (Rosas-Ballina and Tracey 2009). Indeed, the cholinergic anti-inflammatory pathway has been well explained link between parasympathetic and innate immune system (Pohanka 2014; Rosas-Ballina and Tracey 2009). The central nervous system reduces inflammation mediated by macrophages or any other immune cells having α7 nicotinic acetylcholine receptor (nAChR). Ach, an agonist of α7 nAChR, was released from the vagus nerve termination and then opens the central channel allowing an influx of Ca2+ into cells (Pavlov et al. 2009; Pohanka 2012). Increasing levels of Ca2+ trigger the mitogen-activated protein kinases (MAPK) and nuclear factor κ B (NF κB), induce the suppression of cytokine production including TNFα, IL-6 (Sun et al. 2013). Our findings suggested that the mechanism of anti-inflammatory effects in mice brain tissue of Sophora japonica extract may be related to suppress the cytokine production by the activation of cholinergic pathway.
Conclusion
Sophora japonica extract exhibits neuroprotective effects, by improving cholinergic neurotransmission and reducing neuroinflammation. Further studies are needed to investigate the detailed mechanism of the Sophora japonica extract on neuroprotective effect.
References
Abdallah HM, Al-Abd AM, Asaad GF, Abdel-Naim AB, El-halawany AM (2014) Isolation of Antiosteoporotic compounds from seeds of Sophora Japonica. PLoS One 9:e98559. https://doi.org/10.1371/journal.pone.0098559
Agostinho P, Cunha RA, Oliveira C (2010) Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer's disease. Curr Pharm Des 16:2766–2778
Azizi G, Mirshafiey A (2012) The potential role of proinflammatory and antiinflammatory cytokines in Alzheimer disease pathogenesis. Immunopharmacol Immunotoxicol 34:881–895
Birks JS (2006) Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database of Systematic Reviews 2006, Issue 1. https://doi.org/10.1002/14651858.CD005593
Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA (2010) Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology 59:290–294
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bromley-Brits K, Deng Y, Song W (2011) Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp 53:2920. https://doi.org/10.3791/2920
Bui TT, Nguyen TH (2017) Natural product for the treatment of Alzheimer’s disease. Journal of basic and clinical physiology and pharmacology retrieved 5 Sep. 2017, from https://doi.org/10.1515/jbcpp-2016-0147
Chen H-N, Hsieh C-L (2010) Effects of Sophora Japonica flowers (Huaihua) on cerebral infarction. Chin Med 5:1
Chua LS (2013) A review on plant-based rutin extraction methods and its pharmacological activities. J Ethnopharmacol 150:805–817
Davies P (1999) Challenging the cholinergic hypothesis in Alzheimer disease. JAMA 281:1433–1434
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fillit H, Ding W, Buee L, Kalman J, Altstiel L, Lawlor B, Wolf-Klein G (1991) Elevated circulating tumor necrosis factor levels in Alzheimer's disease. Neurosci Lett 129:318–320
Giacobini E (2003) Cholinergic function and Alzheimer's disease. Int J Geriatr Psychiatry 18:S1–S5
Gilberstadt ML, Russell JA (1984) Determination of picomole quantities of acetylcholine and choline in physiologic salt solutions. Anal Biochem 138:78–85. https://doi.org/10.1016/0003-2697(84)90772-3
Hardy J (2006) Alzheimer's Disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis 9:151–153
He X et al (2016) Local and traditional uses, phytochemistry, and pharmacology of Sophora Japonica L.: a review. J Ethnopharmacol 187:160–182
Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16:358–372
Kim BH, Chung EY, Min B-K, Lee SH, Kim M-K, Min KR, Kim Y (2003) Anti-inflammatory action of legume isoflavonoid sophoricoside through inhibition on cyclooxygenase-2 activity. Planta Med 69:474–476
Kimura M, Yamada H (1984) Interaction in the antibacterial activity of flavonoids from Sophora Japonica L. to Propionibacterium. Yakugaku zasshi: J Pharm Soc Jpn 104:340
Krishna PM, KNV R, Banji D (2012) A review on phytochemical, ethnomedical and pharmacological studies on genus Sophora, Fabaceae. Rev Bras 22:1145–1154
Lalonde R (2002) The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26:91–104
Lee Y-J, Han SB, Nam S-Y, Oh K-W, Hong JT (2010) Inflammation and Alzheimer’s disease. Arch Pharm Res 33:1539–1556
Liu W, Rabinovich A, Nash Y, Frenkel D, Wang Y, Youdim MBH, Weinreb O (2017) Anti-inflammatory and protective effects of MT-031, a novel multitarget MAO-A and AChE/BuChE inhibitor in scopolamine mouse model and inflammatory cells. Neuropharmacology, 113, Part A:445–456. https://doi.org/10.1016/j.neuropharm.2016.10.028
Ma M, Chen Y, He J, Zeng T, Wang J (2007) Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience 147:1059–1065
Massoud F, Léger GC (2011) Pharmacological treatment of Alzheimer disease. Can J Psychiatr 56:579–588
Medeiros R et al (2007) Connecting TNF-α signaling pathways to iNOS expression in a mouse model of Alzheimer's disease: relevance for the behavioral and synaptic deficits induced by amyloid β protein. J Neurosci 27:5394–5404
Mega MS, Cummings JL, Fiorello T, Gornbein J (1996) The spectrum of behavioral changes in Alzheimer's disease. Neurology 46:130–135
Muir JL (1997) Acetylcholine, aging, and Alzheimer's disease. Pharmacol Biochem Behav 56:687–696
Mukherjee PK, Kumar V, Mal M, Houghton PJ (2007) Acetylcholinesterase inhibitors from plants. Phytomedicine 14:289–300
Pavlov VA et al (2009) Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun 23:41–45
Pohanka M (2012) Alpha7 Nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int J Mol Sci 13:2219–2238
Pohanka M (2014) Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Int J Mol Sci 15:9809–9825
Rosas-Ballina M, Tracey K (2009) Cholinergic control of inflammation. J Intern Med 265:663–679
Shaftel SS, Griffin WST, O'Banion MK (2008) The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation 5:1
Stone WS, Croul CE, Gold PE (1988) Attenuation of scopolamine-induced amnesia in mice. Psychopharmacology 96:417–420
Sun A, Sun Q, Liu R (2007) Preparative isolation and purification of flavone compounds from Sophora Japonica L. by high-speed counter-current chromatography combined with macroporous resin column separation. J Sep Sci 30:1013–1018
Sun P et al (2013) Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS One 8:e69424
Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N (2010) A meta-analysis of cytokines in Alzheimer's disease. Biol Psychiatry 68:930–941
Tung BT, Hai NT, Thu DK (2017) Antioxidant and acetylcholinesterase inhibitory activities in vitro of different fraction of Huperzia Squarrosa (Forst.) Trevis extract and attenuation of scopolamine-induced cognitive impairment in mice. J Ethnopharmacol 198:24–32
Wang Z, Sun J, Wang D, Xie Y, Wang S, Zhao W (2006) Pharmacological studies of the large-scaled purified genistein from Huaijiao (Sophora Japonica–Leguminosae) on anti-osteoporosis. Phytomedicine 13:718–723
Yahaya TA, Adeola SO, Emma UU (2013) Neuro-protective effect of Carvedilol, an adrenergic antagonist against scopolamine-induced cognitive impairment in mice. J Appl Pharm Sci (8 Suppl 1) 3:S32–S36
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statements
The experimental protocols were approved by the Ethical Committee of School of Medicine and Pharmacy, Vietnam National University Ha Noi, Vietnam.
Conflict of Interest
The authors have declared that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Bui, T.T., Nguyen, H.T. Ethanolic extract of Sophora japonica flower buds alleviates cognitive deficits induced by scopolamine in mice. Orient Pharm Exp Med 17, 337–344 (2017). https://doi.org/10.1007/s13596-017-0286-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-017-0286-6