Abstract
Cataract is one of the primitive secondary complications of diabetes mellitus and contributes to 50% blindness worldwide. Aldose reductase (AR) is the first and rate limiting enzyme in the intracellular polyol pathway responsible for the development of cataract. Thus, there is an imperative need for modest, non-surgical way in order to treat cataract. In Ayurveda, Punica granatum (Pomegranate) is considered as “a pharmacy unto itself”. Pomegranate leaves are traditionally used for their anti-diabetic activity. In this study, we investigated AR inhibitory activity and anti-cataract potential of Pomegranate leaves in glucose - induced cataract in goat lens. AR inhibitory activity was evaluated in vitro in goat lenses. In glucose induced cataract model, goat lenses were incubated in artificial aqueous humor containing 55 mM glucose along with methanolic extract of Punica granatum leaves (MPGL) or quercetin for 72 h. After 72 h, photographic evaluation of the lens was carried out and the lens antioxidant parameters were determined. Different concentrations of MPGL were found to inhibit lens AR activity with an IC50 value of 83.55 ± 3.92 μg/ml. Quercetin was taken as positive control which exhibited 56.461% AR inhibition at a concentration of 500 μg/ml. Cataractous lenses exhibited marked opacity and decrease in the levels of reduced glutathione and superoxide dismutase. Treatment with MPGL and quercetin prevented the opacity and restored back the antioxidant activity. Together, Punica granatum leaves ameliorated glucose-induced cataractogenesis via AR inhibition, reduction in oxidative stress and improvement in antioxidant defense system.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cataract is visual impairment caused due to significant opacity of the eye lens (Kumar et al. 2011) and is one of the principal causes of blindness worldwide (Kurmi et al. 2014). In Ayurveda, cataract is known as linganasha (Dhiman et al. 2010). It is a multifactorial disease which occurs primly due to accumulation of large protein aggregates in the lens. Under normal conditions lens transparency is maintained due to the lens Na+- K+- ATPase activity. In cataractous conditions, deterioration of this activity leads to accumulation of Na+ and loss of K+ which is further accompanied by hydration and swelling of the lens fibers eventually causing cataract (Kumar et al. 2011). Moreover, formation of advanced glycation end products further aggravates the development of cataract as glycation of sugars and their binding with amino groups on proteins leads to disruption of biological properties of proteins (Kurmi et al. 2014). High level of glucose is one of the foremost reasons leading to the development of cataract (Kumar et al. 2011) making it one of the earliest secondary diabetic complications (Stefek 2011).
Aldose reductase (AR) is a member of the aldo-keto reductase super family. It is the initial and rate-limiting enzyme (Saraswat et al. 2008) in the intracellular polyol pathway and hence, responsible for the development of this diabetic complication (Bhadada et al. 2016). The polyol pathway typically exhibits a negligible course of glucose usage and elucidates <3% of glucose utilization. On the other hand, the activity of polyol pathway escalates 10 folds in the presence of high glucose and can show up to 30% of entire glucose utilization (Saraswat et al. 2008). In a diabetic state, AR raises the polyol pathway activity (Kumar et al. 2014) by reducing excess D-glucose into D-sorbitol with complementary conversion of NADPH into NADP+. Thus, aberrant activation of polyol pathway in the face of diabetes advances to an aggregation of polyol (sorbitol) in lens fibers causing influx of water followed by generation of osmotic as well as oxidative stress (Saraswat et al. 2008) which eventually leads to sugar cataracts (Kumar et al. 2014).
Aldose reductase has a pivotal role not only in the development of cataract in the lens but also in the inception of diabetes induced complications such as diabetic nephropathy, diabetic retinopathy and diabetic neuropathy (Bhadada et al. 2016). Progression of cataract is followed by a decrease in the activities of antioxidant enzymes such as reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) which are protective in nature (Kurmi et al. 2014) along with an increase in the level of lipid peroxidation products (LPO) (Shabeer et al. 2011).
Considerable documentations have affirmed that cataract advancement can be impeded or blocked by exploiting plants which have high total flavonoid content and have shown extensive hypoglycemic and in vivo AR inhibitory activity (Kumar et al. 2014; Goodarzi et al. 2006; Lim et al. 2001). In recent times, plants such as Houttuynia cordata Thunb (Kumar et al. 2014), Cinnamomum cassia (Lee 2002), Zingiber officinale (Kumar et al. 2011), Asparagus Racemosus (Shah et al. 2013), Nigella sativa (Shabeer et al. 2011), Achyranthes aspera Linn. (Umamaheshwari et al. 2012), Tamarindus indica Linn (Merugu et al. 2012) have been noted for their AR inhibitory activity as well as their anti-cataract potential.
Punica granatum (Pomegranate) is a species of its genus and is native to Iran (Middha et al. 2013). In Ayurveda, it is treated as “a pharmacy unto itself”. Since ancient times Punica granatum has been traditionally used in diverse countries. In Turkey, the ashes of the fruit peel were used to counteract skin infection; in Iran Punica granatum flowers have been employed as an astringent, hemostatic and antibacterial; in Chinese medicine these flowers have been exploited for the treatment of injuries from falls and grey hair (Garachh et al. 2012). Furthermore, Punica granatum flower extract has demonstrated anti-diabetic action by activation of peroxisome proliferator-activated receptor (PPAR) – gamma (Huang et al. 2005). In India Punica granatum root, bark, flowers, fruit and leaves are used in traditional ayurvedic therapy and medicinal applications (Ganguly 2017).
Extensive class of phytoconstituents reported in Punica granatum leaves are ellagitannins (punicalin and punicafolin) (Garachh et al. 2012); flavonols such as luteolin and apigenin (Sharma and Maity 2010); sterols, saponins, flavanoids, tannins, flavones and glycosides (Sreekumar et al. 2014) whereas the major compounds stated to be present are brevifolin, brevifolin carboxylic acid, ethyl brevifolincarboxylate, corilagin, ellagic acid, gallic acid, punicalagin, punicafolin, punicalin, apigenin, luteolin (Wang et al. 2010), strictinin and tercatain (Sharma and Maity 2010). Pharmacological activity of this part of the plant includes antibacterial (Sharma and Maity 2010), antihyperlipidemic (Miguel et al. 2010), antioxidant (Amjad and Shafighi 2012), anti-obesity (Adnyana et al. 2014), hypolipidemic, antidiabetic (Salwe et al. 2015), anti-inflammatory and analgesic activity (Bagri et al. 2010).
It is probable that drugs with anti-cataract potential have antioxidant action (Woolard et al. 1990). Based on preceding studies, it is ascertained that Punica granatum leaves exert anti-diabetic effects by augmenting the levels of antioxidant enzymes thus indicating its antioxidant potential (Salwe et al. 2015; Das and Barman 2012). In addition to this, Pomegranate extracts and their active components have been demonstrated to possess great medical potential for the treatment of type 2 diabetes and its pathological concerns (Banihani et al. 2013). Previous studies have exhibited AR inhibitory activity and antioxidant capacity of Punica granatum seed (Karasu et al. 2012) and peel (Hui et al. 2008). Furthermore, studies have implied that polyphenols and vitamins which have antioxidant properties might be effective in countering damage caused by cataract (Sharma and Maity 2010).
However there is no scientific data available establishing the AR inhibitory activity and anti-cataract potential of Pomegranate leaves. Therefore, present study was undertaken to evaluate the protective effects of Punica granatum leaves in inhibiting in vitro cataractogenesis in experimental isolated goat eye lens model by studying its AR inhibitory activity.
Material and methods
Plant material
Punica granatum leaves were collected from Nashik, Maharashtra, India in the month of January, 2014. The plant was identified and authenticated by Dr. Ganesh Iyer, Botany Department, Ruia College, Mumbai, Maharashtra, India. A voucher specimen (No. 2014/01) of the plant has been deposited in the Department of Pharmacology, Institute of Chemical Technology, Mumbai, Maharashtra, India.
Preparation of extract
Punica granatum leaves were washed with water, shade dried and made into a homogenous coarse powder. Dried powdered material (100 g) was initially defatted using 1 L petroleum ether by soxhletion followed by extraction with 1 L methanol until the whole powdered material was completely exhausted. The resulting extract was concentrated under reduced pressure to obtain a dark crude residue (yield: 50.707% w/w) and stored in an airtight container for subsequent use.
Preliminary phytochemical screening
Preliminary phytochemical screening of methanolic extract of Punica granatum leaves (MPGL) was carried out following the standard procedure of (Wagner and Bladt 1996). MPGL was screened for the presence of steroids, glycosides, flavonoids and tannins. Total phenolic compounds in the extract were estimated by Folin-Ciocalteu method using gallic acid as standard and expressed as mg/g of gallic acid equivalents (GAE). Total flavonoid content of the extract was measured as mg/g of rutin equivalents using aluminium chloride colorimetric method (Mannan et al. 2013).
Isolation of aldose reductase
Isolation of crude AR enzyme was undertaken in accordance to the method published by Lee (2002) with slight modifications (Lee 2002). Fresh goat eyes were collected from slaughter house at Govandi, Mumbai. Non-cataractous transparent lenses were isolated by extracapsular extraction and 10% w/v homogenate was prepared in 0.1 M phosphate buffer (pH 7.0). The homogenate was centrifuged at 5000 xg for 30 min at 4 °C to obtain the supernatant. After centrifugation, the resulting supernatant was collected and retained as an enzyme preparation.
Assay of aldose reductase activity
AR activity was assayed as per the method described by Dongare V. et al. (Dongare et al. 2012). Aldose reductase reduces excess D-glucose into D-sorbitol with concomitant conversion of NADPH into NADP+. Formation of NADP is recorded at 340 nm for 3 min (Kumar et al. 2014). MPGL was dissolved in phosphate buffered saline (PBS). A sample cuvette containing freshly prepared lens homogenate, varying concentrations of MPGL (10–1000 μg/ml), 0.1 M phosphate buffer (pH 7.0), 0.03 mM NADPH was read against a reference cuvette containing all components except the substrate. The enzymatic reaction was initiated after the addition of 0.04 mM D-xylose (substrate). The decrease in absorbance was measured at 340 nm for 3 min. Percent inhibition of AR activity was calculated as follows with reference to the activity of normal lens as 100%.
Collection of eye balls
Fresh goat eye balls were collected from slaughter house of Govandi, Mumbai. These eye balls were immediately transported to laboratory at 0–4 °C.
Preparation of lens culture
The lenses were removed by extracapsular extraction and incubated in artificial aqueous humor (pH 7.8) at room temperature for four to six hours. The artificial aqueous humor consisted of NaCl – 140 mM, KCl – 5 mM, MgCl2–2 mM, NaHCO3–0.5 mM, NaH2PO4.2H2O – 0.5 mM, CaCl2–0.4 mM and glucose – 5.5 mM. Penicillin 32 mg% and streptomycin 250 mg% were added to the incubated media to prevent bacterial contamination (Kumar et al. 2011).
Drug concentration and groups
Damaged lenses were discarded and intact lenses were divided into following groups (n = 4 in each group):
-
Group I:
Normal control [Glucose content 5.5 mM]
-
Group II:
Negative control [Glucose content 55 mM]
-
Group III:
Positive control [Glucose content 55 mM + Quercetin (500 μg/ml)]
-
Group IV:
Glucose content 55 mM + MPGL (250 μg/ml)
-
Group V:
Glucose content 55 mM + MPGL (500 μg/ml)
-
Group VI:
Glucose content 55 mM + MPGL (1000 μg/ml)
Induction of in vitro cataract
Glucose in a concentration of 55 mM was used to induce cataract. The goat lenses were incubated with glucose 5.5 mM, 55 mM, different concentrations of MPGL and quercetin respectively at 37 °C, pH 7.8 for 72 h.
Photographic evaluation and homogenate preparation
After 72 h of incubation, the lenses were removed from respective media and washed with normal buffer. The lenses which were washed were then kept on graph papers with posterior surface touching the graph paper and the number of squares clearly visible through the lens were counted to measure the opacity of the lens (Dongare et al. 2012). Then they were homogenized in Tris buffer (0.23 M; pH 7.8) containing 0.25 × 10−3 M ethylenediaminetetraacetic acid to obtain a 10% w/v homogenate. The homogenate was further centrifuged at 10000 g at 4 °C for 15 min and the supernatant was separated (Kumar et al. 2011). The supernatant was used for the analysis of biochemical parameters such as reduced glutathione, superoxide dismutase and lipid peroxidation.
Biochemical estimation
5–5-dithiobis (2-nitrobenzoic acid) (DTNB) reagent was used to estimate reduced glutathione (GSH) level in lens homogenate. 10 μl of lens homogenate was incubated with 200 μl of DTNB reagent in a 96 well plate for 15 min at room temperature. The absorbance was measured at 412 nm using micro plate reader (epoch, synergy). The amount of GSH in the sample was calculated in microgram per ml from a standard curve obtained and represented in GSH per total protein.
Evaluation of lens homogenate for superoxide dismutase (SOD) activity was carried out following the method published by Nishi et al. (Nishi and Kumar 2013). The assay mixture consisted of 180 μl of phosphate buffer (pH 7.4), 10 μl of pyrogallol (2.6 mM in 10 mM HCl) and 10 μl of lens homogenate in a total volume of 200 μl. SOD activity was measured at 325 nm in microplate reader (epoch, synergy) and expressed as Units per 100 g protein. One Unit of enzyme represents the enzyme activity that inhibits auto-oxidation of pyrogallol by 50%.
Formation of Thiobarbituric acid reactive substances (TBARS) as a product of Lipid peroxidation was estimated in the lens. 0.2 ml of 10% lens homogenate (0.1 M phosphate buffer, pH 7.4) was treated with 0.2 ml of sodium dodecyl sulfate (SDS), 1.5 ml acetic acid (20%, pH 3.5) and 1.5 ml of 0.8% TBA. The above reaction mixture was made up to 4 ml with distilled water, placed in boiling water bath for 60 min and cooled under tap water. 4 ml of butanol:pyridine (15:1 v/v) was added to above mixture, mixed vigorously and centrifuged at room temperature for 10 min. 200 μl of clear supernatant was collected and measured against reference blank at 532 nm in a 96 microplate reader (epoch, synergy). The TBARS content was calculated and expressed as nmol malondialdehyde (MDA) formed per 100 g protein (Halliwell and Chirico 1993).
Results
Preliminary phytochemical screening
The preliminary phytochemical screening indicated the presence of phytoconstituents such as steroids, glycosides, saponins, flavonoids, tannins, carbohydrates. The total phenolic content was found to be 328.75 ± 0.625 mg of gallic acid/g of extract whereas the total flavonoid content was found to be 126.667 ± 6.5 mg of rutin/g of extract respectively.
Aldose reductase inhibitory activity
Different concentrations of MPGL were found to inhibit lens AR to various extent with IC50 value of 83.55 ± 3.92 μg/ml (Fig. 1). It is evident from the figure that MPGL at the concentration of 1000 μg/ml possessed maximum AR inhibitory activity. At a concentration of 1000 μg/ml, MPGL showed AR inhibition of 94.218%. Quercetin, which is well known for its AR inhibition, was used as a positive control which showed 56.461% AR inhibition at a concentration of 500 μg/ml.
The effect of MPGL and quercetin on aldose reductase activity. Different concentrations of MPGL were incubated with 0.1 M phosphate buffer (pH 7.0), 0.03 mM NADPH 0.04 mM D-xylose. The decrease in absorbance was measured at 340 nm for 3 min. Values are expressed as Mean ± SEM (n = 3). SEM, standard error of mean. MPGL: methanolic extract of Punica granatum leaves
Photographic evaluation
Incubation of lenses with 55 mM glucose showed opacification starting post 24 h at the periphery which progressively increased towards the centre with complete opacification at the end of 72 h as compared to lenses incubated in 5.5 mM glucose. Lenses incubated in 5.5 mM glucose were transparent post 72 h as evident by the number of squares clearly visible through the lenses (Table 1). Treatment with MPGL significantly reduced the onset and extent of cataract formation. Lenses incubated with 55 mM glucose along with quercetin (500 μg/ml) showcased more transparency as compared to the cataractous lenses in the negative control group (Fig. 2).
The effect of MPGL and quercetin on opacity of lens. (a) Lens incubated in glucose 5.5 mM (Normal Control); (b) Lens incubated in glucose 55 mM (Negative Control); (c) Lens incubated in glucose (55 mM) and MPGL 250 μg/ml; (d) Lens incubated in glucose (55 mM) and MPGL 500 μg/ml; (e) Lens incubated in glucose (55 mM) and MPGL 1000 μg/ml; (f) Lens incubated in glucose (55 mM) and Quercetin 500 μg/ml (Positive Control). MPGL: methanolic extract of Punica granatum leaves
Effect of MPGL on lens antioxidants
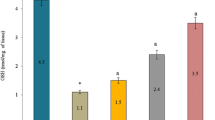
Incubation with glucose 55 mM for 72 h produced a significant decrease in the levels of reduced glutathione and superoxide dismutase and increase in the malondialdehyde (MDA) levels in the lenses respectively as compared to the normal control group. Incubation with different concentrations of MPGL simultaneously with glucose 55 mM restored the levels of both reduced glutathione and superoxide dismutase and decreased the MDA levels in lenses. Quercetin, maintained as standard, also significantly increased the reduced glutathione and superoxide dismutase activity and decreased MDA levels (Fig. 3).
The effect of MPGL and quercetin on lens antioxidants. (a) Reduced glutathione in lens homogenate of normal control, negative control, MPGL treated groups and positive control (Quercetin) (b) Superoxide dismutase in lens homogenate of normal control, negative control, MPGL treated groups and positive control (Quercetin) (c) MDA levels in lens homogenate of normal control, negative control, MPGL treated groups and positive control (Quercetin). MDA: Malondialdehyde. MPGL: methanolic extract of Punica granatum leaves. *** Treatment group was compared with negative control, p < 0.001; ### Negative control group was compared with normal control group, p < 0.001, using one-way ANOVA with Dunnett’s test. Values are expressed as mean ± SEM (n = 4). SEM, standard error of the mean. ANOVA: Analysis of variance
Discussion
Diabetes has been the foremost origin of blindness. Diabetic retinopathy and cataracts are well known causes of blindness in diabetics. Opacification of lens in cataract has been known to be responsible for the genesis of around 50% blindness and visual impairment worldwide (Kumar et al. 2014).
In diabetic patients, cataracts advance at an early age, generally constraining surgical intervention (Skarbez et al. 2010). Activation of polyol pathway due to increased activity of AR, a lens enzyme has been implicated in the formation of cataract (Kumar et al. 2011). Moreover, this enzyme has been involved in the pathogenesis of diabetic complications such as nephropathy, neuropathy, retinopathy including cataractogenesis (Brownlee 2005). Growing evidence indicates that cataract advancement can be slowed down by using natural therapies, especially plants with high total flavonoid content and AR inhibitory activity (Kumar et al. 2014).
Literature has shown herbal drugs such as Ocimum sanctum, Emblica officinalis, Ginkgo biloba and green tea to impede the inception and progression of cataract advancement due to their antioxidant potential (Gupta et al. 2010). In spite of the availability of clinically tested AR inhibitors such as tolrestat, epalrestat and sorbinil, there is an increased concern lately to identify natural sources for their curative properties, chiefly because of the application of plants in traditional medicine, diet and also since majority of the plants are devoid of adverse effects (Akileshwari et al. 2012).
Punica granatum L. shrub, the leaves of which are rich in polyphenolic compounds including tannins and flavonoids (Al-Muammar and Khan 2012) has aroused great interest due to its anti-diabetic and antioxidant potential which could be considered as a lead to further study the effect of this part of the plant on cataractogenesis (Patel et al. 2014). Taking this into account, in our present study we have investigated the anti-cataract effect of Punica granatum considering the inhibition of AR enzyme in the polyol pathway.
Quercitin was used as a positive control in our study as it is a potent AR inhibitor for sorbitol accumulation in polyol pathway and particularly is considered as therapeutic promise for treatment of diabetic retinopathy and cataract (Goodarzi et al. 2006). MPGL exhibited in-vitro AR inhibition in dose-dependent manner and IC50 was found to be 83.55 ± 3.92 μg/ml. The results of our study suggested the potent AR inhibitory activity of MPGL which may be attributed to the presence of high flavonoid contents. Since Punica granatum demonstrated satisfactory inhibitory activity against AR, it was concluded that it could be possibly used to treat diabetic cataract in the initial phase.
Incubation of goat lens in media containing high glucose (55 mM) showcased progression of opacity which might be due to aggregation of Na+ and loss of K+ accompanied with hydration and swelling of lens fibers accelerating cataract formation (Kumar et al. 2011). This was quite evident from the drastic reduction in the number of squares observed through the lenses. It is extensively established that oxidative stress is a major aspect in the development of cataract induced by glucose (Kumar et al. 2011; Akileshwari et al. 2012). The negative control group in which goat lens were incubated in 55 mM glucose, demonstrated a significant decrease in the levels of antioxidant enzymes – reduced glutathione and superoxide dismutase and increased levels of MDA which is a significant indicator of free radical – induced lipid peroxidation (Al-Muammar and Khan 2012), thus implicating oxidative stress in the cells. When goat lens were incubated in high glucose content aqueous humor at 37 °C for 72 h in presence of MPGL, the extract prevented the formation of cataract in a dose dependent manner.
GSH acts as the major antioxidant in the lens and treatment with MPGL was found to restore significant level of GSH as compared to the negative control group (Gupta et al. 2010). SOD, a chain-breaking antioxidant converts superoxide to H2O2 (Shah et al. 2013). Altered activity of antioxidant enzyme SOD suggested an increased oxidative stress in diabetic cataract lens. However, MPGL prevented alterations in SOD. A significant reduction in MDA levels by MPGL distinctly demonstrated the lens protection against lipid peroxidation.
The present study revealed that Punica granatum decreased the oxidative stress and positively balanced the level of antioxidant enzyme levels such as GSH and SOD. These effects might be attributed to the presence of high amount of flavonoids in the extract since flavonoids are known as multifunctional agents and literature has reported their antioxidant, AR inhibitory and advanced glycation end-products inhibitory properties (Stefek 2011; Cheng et al. 2013).
Thus, MPGL prevented cataract formation due to inhibition of AR enzyme. This also suggested that MPGL could be promising therapeutics in diabetes induced retinopathy.
Conclusions
These findings indicated that MPGL exhibited significant inhibitory effect on AR enzyme which may be attributed to the presence of high phenolic and flavonoid content. Further, the extract also showcased its potential as a promising source in the treatment of cataract. Our data suggested that the extract might be helpful in delaying the development of cataract due to the presence of varied constituents in it. This beneficial effect may be due to its significant inhibition of AR enzyme in combination with its anti-oxidant activities. These results suggest that Punica granatum could potentially provide a new natural treatment for diabetic complications and might hold a promise to treat diabetic cataract.
References
Adnyana IK, Sukandar EY, Yuniarto A, Finna S (2014) Anti-obesity effect of the pomegranate leaves ethanol extract (Punica granatum L.) in high-fat diet induced mice. Int J Pharm Pharm Sci 6(4):626–631
Akileshwari C, Muthenna P, Nastasijevic B, Joksic G, Petrash JM, Reddy GB (2012) Inhibition of aldose reductase by Gentian lutea extracts. Exp Diabetes Res 2012:1–8
Al-Muammar MN, Khan F (2012) Obesity: the preventive role of the pomegranate (Punica granatum). Nutrition 28:595–604
Amjad L, Shafighi M (2012) Antioxidant activity of leaf different extracts in Punica granatum. Int J Biol Med Res 3(3):2065–2067
Bagri P, Ali M, Aeri V, Sultana S, Bhowmik M (2010) Evaluation of anti-inflammatory and analgesic activity of Punica granatum Linn. Int J Drug Develop Res 2(4):698–702
Banihani S, Swedan S, Alquraan Z (2013) Pomegranate and type 2 diabetes. Nutr Res 33(5):341–348
Bhadada SV, Vyas VK, Goyal RK (2016) Protective effect of Tephrosia purpurea in diabetic cataract through aldose reductase inhibitory activity. Biomed Pharmacother 83:221–228
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54(6):1615–1625
Cheng D, Liang B, Li Y (2013) Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. Biomed Res Int 2013:1–7
Das S, Barman S (2012) Antidiabetic and antihyperlipidemic effects of ethanolic extract of leaves of Punica granatum in alloxan-induced non-insulin dependent diabetes mellitus albino rats. Indian J Pharmacol 44(2):219–224
Dhiman KS, Dhiman K, Puri S, Ahuja D (2010) A comprehensive review of cataract (Kaphaja Linganasha) and its surgical treatment in Ayurvedic literature. Ayu 31(1):93–100
Dongare V, Kulkarni C, Kondawar M, Magdum C, Haldavnekar V, Arvindekar A (2012) Inhibition of aldose reductase and anti cataract action of trans-anethole isolated from Foeniculum vulgare Mill. Fruits. Food Chem 132:385–390
Ganguly S (2017) Medicinal utility of Pomegranate fruit in regular human diet: a brief review. Int J Forestry and Horticulture 3(1):17–18
Garachh D, Patel A, Chakraborty M, Kamath JV (2012) Phytochemical and pharmacological profile of Punica granatum: an overview. Int Res J Pharm 3(2):65–68
Goodarzi MT, Zal F, Malakooti M, Safari MR, Sadeghian S (2006) Inhibitory activity of flavonoids on the lens aldose reductase of healthy and diabetic rats. Acta Medica Iranica 44(1):41–45
Gupta SK, Kalaiselvan V, Srivastava S, Saxena R, Agrawal SS (2010) Trigonella foenum-graecum (Fenugreek) protects against selenite-induced oxidative stress in experimental cataractogenesis. Biol Trace Elem Res 136:258–268
Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement and significance. Am J Clin Nutr 57:715S–724S
Huang TH, Peng G, Kota BP, Li GQ, Yamahara J, Roufoqalis BD, Li Y (2005) Anti-diabetic action of Punica granatum flower extract: activation of PPAR-gamma and identification of an active component. Toxicol Appl Pharmacol 207(2):160–169
Hui S, Dong-ying J, Kai Y (2008) Inhibitory effect of polyphenol extract from pomegranate peel on aldose reductase activity. Nat Prod Res Develop 20(3):508–510
Karasu C, Cumaoglu A, Gurpinar AR, Kartal M, Kovacikova L, Milackova I et al (2012) Aldose reductase inhibitory activity and antioxidant capacity of pomegranate extracts. Interdiscip Toxicol 5(1):15–20
Kumar M, Singh T, Ali J, Tyagi LK (2011) In vitro anticataract activity of Zingiber officinale on goat lenses. Int J Pharm Biol Archives 2(5):1430–1433
Kumar M, Laloo D, Prasad SK, Hemalatha S (2014) Aldose reductase inhibitory potential of different fractions of Houttuynia cordata Thunb. J Acute Disease 3(1):64–68
Kurmi R, Ganeshpurkar A, Bansal D, Agnihotri A, Dubey N (2014) Ethanol extract of Moringa oliefera prevents in vitro glucose induced cataract on isolated goat eye lens. Indian J Ophthalmol 62(2):154–157
Lee H (2002) Inhibitory activity of Cinnamomum cassia bark-derived component against rat lens aldose reductase. J Pharm Pharmaceut Sci 5(3):226–230
Lim SS, Jung SH, Ji J, Shin KH, Keum SR (2001) Synthesis of flavonoids and their effects on aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. J Pharm Pharmacol 53(5):653–658
Mannan H, Ghazaleh M, Mohammad RA, Hossein K, Naficeh S, Reza OM et al (2013) Total phenolics, flavonoids, tannin content and antioxidant power of some Iranian pomegranate flower cultivars (Punica granatum L.) Am J Plant Sci 4:1815–1820
Merugu S, Veeresh B, Rekulapally D, Swetha T (2012) Study of in vitro anticataract activity of Tamarindus indica Linn. on isolated goat lenses. Int J Pharm 2(4):758–763
Middha SK, Usha T, Pande V (2013) A review on antihyperglycemic and antihepatoprotective activity of eco-friendly Punica granatum peel waste. Evid Based Complement Alternat Med 2013:1–10
Miguel MG, Neves MA, Antunes MD (2010) Pomegranate (Punica granatum L.): A medicinal plant with myriad biological properties – A short review. J Med Plants Res 4(25):2836–2847
Nishi AA, Kumar P (2013) Protective effect of chlorogenic acid against diabetic nephropathy in high fat diet/ streptozotocin induced type-2 diabetic rats. Int J Pharm Pharm Sci 5:489–495
Patel AN, Bandwane DD, Mhetre NK (2014) Pomegranate (Punica granatum Linn.) leaves attenuate disturbed glucose homeostasis and hyperglycemia mediated hyperlipidemia and oxidative stress in streptozotocin induced diabetic rats. Eur J Integr Med 6:307–321
Salwe KJ, Sachdev DO, Bahurupi Y, Kumarappan M (2015) Evaluation of antidiabetic, hypolipidemic, antioxidant activity of hydroalcoholic extract of leaves and fruit peel of Punica granatum in male Wistar albino rats. J Nat Sci Biol Med 6(1):56–62
Saraswat M, Muthenna P, Suryanarayana P, Petrash JM, Reddy GB (2008) Dietary sources of aldose reductase inhibitors: prospects for alleviating diabetic complications. Asia Pac J Clin Nutr 17(4):558–565
Shabeer AN, Niyas AI, Ashar Waheed MP, Abdul Jafar AH (2011) Anticataract activity of ethanolic extract of Nigella sativa on glucose induced cataract in goat eye lens. Int J Appl Biol Pharm Biol 2(4):274–279
Shah NK, Patel PK, Vyas BA, Joshi SV (2013) Evaluation of anti-cataract activity of Asparagus racemosus root extract using in vitro model of goat lens. Int J Pharm Res Schol 2(3):19–26
Sharma J, Maity A (2010) Pomegranate phytochemicals: nutraceutical and therapeutic values. Fruit Veg Cereal Sci Biotech 4(2):56–76
Skarbez K, Priestly Y, Hoepf M, Koevary SB (2010) Comprehensive review of the effects of diabetes on ocular health. Expert Rev Ophthalmol 5(4):557–577
Sreekumar S, Sithul H, Muraleedharan P, Azeez JM, Sreeharshan S (2014) Pomegranate Fruit as a rich source of biologically active compounds. Biomed Res Int 2014:1–12
Stefek M (2011) Natural flavonoids as potential multifunctional agents in prevention of diabetic cataract. Interdiscip Toxicol 4(2):69–77
Umamaheshwari M, Dhinesh S, Sivashanmugam T, Subhadradevi V, Puliyath J, Madeswaran A (2012) Anticataract and antioxidant activities of Achyranthes aspera Linn. against glucose-induced cataractogenesis using goat lenses. J Nat Prod Plant Resour 2(1):153–161
Wagner H, Bladt S (1996) Plant drug analysis. A thin layer chromatography Atlas, 2nd edn. Springer, Berlin/New York
Wang R, Ding Y, Liu R, Xiang L, Du L (2010) Pomegranate: constituents, bioactivities and pharmacokinetics. Fruit Veg Cereal Sci Biotech 4(2):77–87
Woolard AC, Wolff SP, Bascal ZA (1990) Antioxidant characteristics of some potential anticataract agents. Studies of aspirin, paracetamol and bendazac provide support for an oxidative component of cataract. Free Radic Biol Med 9(4):299–305
Acknowledgements
This study has been supported by a grant from INSPIRE Program, Department of Science & Technology, New Delhi (No. DST/INSPIRE Fellowship/2013/1152).
The authors would like to acknowledge Dr. Ganesh Iyer for carrying out authentication of the plant and Mr. Sanjay Devare for arranging and providing plant material for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by a grant from INSPIRE Program, Department of Science & Technology, New Delhi (No. DST/INSPIRE Fellowship/2013/1152).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Statements
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(DOCX 70 kb)
Rights and permissions
About this article
Cite this article
Mestry, S.N., Juvekar, A.R. Aldose reductase inhibitory potential and anti-cataract activity of Punica granatum Linn. leaves against glucose-induced cataractogenesis in goat eye lens. Orient Pharm Exp Med 17, 277–284 (2017). https://doi.org/10.1007/s13596-017-0274-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-017-0274-x