Abstract
Changes in landscape structure have increased the edge amount between different environments, and its implications for biodiversity may vary depending on the level of analysis, ecological groups, and species life history. We investigated the effect of distance from the forest edge on species of solitary wasps and bees and their associated natural enemies, on a gradient from 200 m into the anthropogenic matrix to 200 m into the forest, in Atlantic Forest biome in Brazil. In general, species were positively affected by forest edge proximity, while those forest specialists were more negatively influenced. The natural enemy species were more dependent on the distribution of their hosts than on the edge effect directly. Caterpillar and spider hunters seem to be positively affected by the edge, while pollen collectors progressively decrease their richness and abundance from the matrix towards the interior of forest area, and cockroach hunters present an opposite response. We demonstrate the importance of forest conservation and reforestation programs, not only by some cavity-nesting wasps and bees being entirely dependent on forests to persist, but also because most species sampled here depend on being close to a forest edge (less than 100 m) to carry out their ecological holes. Therefore, understanding how different species respond to environmental and landscape changes and may depend on staying close to forests is essential for effective management and planning strategies for biodiversity conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tropical forests sustain the highest terrestrial biodiversity in the world (Laurance et al. 2012; Alroy 2017). However, they are highly threatened due to intense anthropic exploitation (Laurance et al. 2012; Kok et al. 2018). Habitat loss and fragmentation processes have increased the risk of extinction for many species worldwide, particularly forest-dependent species (Fahrig 2003; Souza et al. 2020).
One effect of intensive landscape modification is the formation of edges, which are interfaces between natural and anthropogenic environments (Ries et al. 2004). Approximately 20% of the world’s remaining forest cover is estimated to be within 100 m of an edge (Haddad et al. 2015), potentially subjecting many species to edge influences, particularly in smaller forest patches (Ries et al. 2004). The effect of edges on biodiversity arises from the existence of changes in biotic and abiotic conditions from the matrix to interior of forest patches (Villaseñor et al. 2014). The distance from the edges may influence both the direction and magnitude of the effects. Available evidence suggests that the most pronounced community changes occur within 100 m of the edge, but edge effects may extend to more than 1 km for insect and vertebrate species (Marsh et al. 2018; Willmer et al. 2022).
The presence of edges in landscapes may affect communities in three different ways: (1) abiotic effects (changes in environmental conditions due to proximity to the edge); (2) direct biological effects (changes in species abundance and distribution due to physiological tolerance of species near the edge); and (3) indirect biological effects (changes in interspecific interactions, such as herbivory, predation, competition, pollination, and parasitism) (Murcia 1995). These edge effects on communities may also vary depending on the life history traits of species, such as nesting strategy, dispersal capacity, body mass, and diet breadth (Caitano et al. 2020). Therefore, ecological responses to edge effects may be positive, negative, or neutral. Positive responses occur when the combination of two habitat types increases the availability of resources accessible to species. Negative responses occur when species avoid edges, which depends on the species’ sensitivity to the conditions of the surrounding anthropogenic matrix. Finally, neutral responses occur when species are able to use both types of environments because their realized niches are well distributed across the anthropogenic matrix, edge zone, and core habitat area (Ries et al. 2004; Haddad et al. 2015).
The edge effect on biodiversity can be considered an indirect consequence of the habitat fragmentation process, particularly prevalent in regions that have been heavily degraded by human actions, such as agriculture, changes in land use, and urban area expansion (Fahrig 2017). One example is the Atlantic Forest biodiversity hotspot in Brazil, which is one of the world’s richest biomes with many endemic species but is currently facing significant threats from anthropogenic actions (Myers et al. 2000). The forest cover in this region ranges from 16 to 28%, depending on the mapping method. Most fragments smaller than 50 ha are subject to several edge effects, such as increased temperature, reduced humidity, and greater exposure to human land use, mainly agriculture and urban areas (Ribeiro et al. 2009; Rezende et al. 2018; Haddad et al. 2015).
As a consequence of the landscape alterations in the Atlantic Forest biome, both ecological processes and the associated economy are affected, particularly with regard to communities and their associated ecosystem services, such as pollination and population regulation (Mitchell et al. 2015; Duarte et al. 2018). In this biome, pollination and biological population control services are mainly provided by insects, primarily hymenopteran species (Losey et al. 2006; Noriega et al. 2018).
Among Hymenoptera, solitary wasps and bees, as well as their associated natural enemies (parasitoids and kleptoparasites), exhibit a diverse array of species that may respond differently to the edge effect. By individually assessing each species, we can explore which of them are negatively, positively, or neutrally affected by edge effects. Moreover, an important aspect overlooked by previous studies with these species is how their responses to edge effects may vary according to functional groups. For example, Rocha-Filho et al. (2020) found that caterpillar-hunter species were more affected by a landscape resistance index, while spider-hunter species were unaffected by any of the landscape attributes. Thus, the responses of different functional groups may also vary in relation to edge distances, reflecting how groups with similar characteristics (e.g., trophic level or food resources) are affected by edges.
Considering the diversity and sensitivity of solitary wasps and bees and their associated natural enemies to environmental changes, this study aims to identify how these communities respond to edge effects. For this, we compared the communities along a gradient of distances across the anthropogenic matrix, including the edge zones and the interior of forest patches. We tested the following hypotheses: (1) habitat generalist species are positively affected by the edge (edge preference model) and (2) habitat specialist species are negatively affected by the edge, whereas matrix and forest specialist species are more abundant and have higher species richness in their preferred habitats (edge avoidance model). Our null hypothesis is that cavity-nesting hymenopterans are neutrally affected by the edge (Figure 1).
2 Material and methods
2.1 Study area
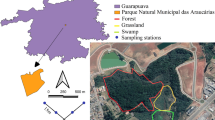
Our sampling was conducted in four forest patch areas and four surrounding anthropogenic matrices located within private properties in Vale do Jordão and Guabiroba, municipality of Guarapuava, Paraná state, northern Brazil (Figure 2). According to the Köppen classification, the climate in this region is humid mesothermal subtropical, characterized by abundant rainfall (annual average of 1961 mm) and the absence of a dry season (Kottek et al. 2006). The warmest months occur from September to April, with temperatures averaging 23 °C. The coldest months occur from May to August, with temperatures averaging 12.8 °C. During this period, frosts and temperatures below 0 °C are recorded (IAPAR 2014).
The main phytophysiognomy in the region is Araucaria Forest, which is surrounded by anthropogenic matrices consisting of grasslands used for cattle and sheep farming. Moreover, some properties in the area also have agricultural fields for corn, soybean, Eucalyptus, or Pinus. All of these properties are located in the rural area of the Guarapuava municipality (Figure 2, Table S1).
2.2 Sampling design
Sampling was conducted biweekly every month between August 2018 and August 2019. We used trap nests, a method widely used in studies aimed at sampling cavity-nesting wasps and bees and their associated natural enemies (Staab et al. 2018). This method consists of placing artificial cavities in the field to serve as nesting sites for wasps and bees. Trap nests can be built with several materials, and in this study, we used trap nests made of wood and bamboo. The wood trap nests were constructed according to Buschini et al. (2010). The bamboo trap nests ranged from 8 to 19 cm in length and from 0.4 to 1.6 cm in diameter. This broad range of cavity lengths and diameters enabled us to sample all cavity-nesting hymenopteran groups, given the substantial interspecific variation in body sizes within this group. Previous studies have also used similar-sized trap nests to those used here (MacIvor 2017; Staab et al. 2018). Both the wood and bamboo trap nests were longitudinally openable, allowing for visual inspection of their interiors (Figure S1).
In each forest patch and anthropogenic matrix area, we sampled six points, each located at a different distance from the edge. The distances from the edges were randomly chosen and ranged between 3 and 204 m from the edge on the matrix side and between 34 and 182 m from the edge on the forest side (Table S1). At each point, we installed a sampling station composed of 32 trap nests (16 made of wood and 16 made of bamboo), totaling 1536 nests available in the field. The wooden trap nests comprised four trap nests with 0.5, 0.7, 1.0, and 1.3 cm in diameter, and the bamboo trap nests had varied diameters. All nests were installed 1.5 m above the ground and covered to protect them from direct exposure to sunlight and rain (Figure S1).
The collection of occupied trap nests was done when the species had already finished building the nest, which was easily noticeable since most species that nest in pre-existing cavities close the nest entrance with some type of material, such as clay or some type of plant material. The collected nests were taken to the Bees and Wasps Biology and Ecology Lab (LABEVESP), at the Universidade Estadual do Centro Oeste, located in Guarapuava, Paraná State, Brazil, where they were placed inside PET bottles, closed with cotton, until the adults emerged, which were killed with ethyl acetate, dried, identified, and deposited in the laboratory collection.
2.3 Community composition and functional group levels
After species identification, we conducted the analysis at two levels of organization: species and community. At the species level, we analyzed each species separately. At the community level, we grouped the species into five functional groups as follows: (1) natural enemies, i.e., species that use their hosts as a trophic resource; (2) caterpillar hunters; (3) spider hunters; (4) cockroach hunters; and (5) pollen collectors.
At the species level, we used abundance and occurrence (0 = absence; 1 = presence) as response variables. At the community level, we used the abundance and richness of species of each functional group as response variables. We constructed separate models for each functional group. In both levels of analysis, we used distances to the edge as explanatory variables. Positive values were assigned to distances within the forests, and negative values were assigned to distances within the anthropogenic matrix. For the functional group of natural enemies, we also included host abundance and richness as explanatory variables. Moreover, for the host species and functional groups formed by them, we also conducted the analysis using the number of nest built as a proxy for abundance rather than the number of individuals that emerged from the nests.
2.4 Statistical analysis
For each pair of response and explanatory variables, we fitted two different models to evaluate the effect of different distances of sampling stations from the edge on the abundance and richness of cavity-nesting wasps and bees, as well as their associated natural enemies: (1) generalized additive models (GAMs) and (2) generalized additive mixed models (GAMMs), both to fit nonlinear response of richness and abundance of species and functional groups to edge distance, being the second used to we added the sample sites as random variables and and thus remove the effect of the different locations of collection points. The model’s response variables included the abundance and presence/absence of each species separately and the abundance and species richness for each functional group. For the presence/absence of species, we fitted a general linear model (GLM) with a binomial distribution (Zuur et al. 2009) (Table I).
To select the best type of models (GAM or GAMM models), we used the Akaike information criterion corrected for small sample sizes (AICc) (Burnham and Anderson 2004). The best models were those with the lowest AICc values (Table S2). For the GLM analysis, we used the “stats” package in R (R Core Team 2022). For the GAM and GAMM analyses, we used the “mgcv” package (Wood et al. 2011). All analyses were performed in R version 4.1.3 (R Core Team 2022).
3 Results
3.1 Community composition
We sampled 541 built nests, from which 1420 hosts and 254 natural enemies emerged. Among the hosts, most were wasps (N = 1395), while bees accounted for a smaller portion (N = 25). Wasps belonged to four families (Crabronidae, Pompilidae, Sphecidae, and Vespidae), nine genera, and sixteen species (Table S3). Bees belonged to two families (Apidae and Megachilidae), three genera, and four species (Table S3). In terms of diversity, Vespidae (S = 8) and Crabronidae (S = 4) families showed the highest richness among the wasp host species, whereas Megachilidae showed the highest richness among the bees. The natural enemies belonged to two orders: Diptera (N = 110) and Hymenoptera (N = 144). Dipterans were represented by two families (Bombyliidae and Sarcophagidae), three genera, and three species, whereas hymenopterans were represented by six families (Chalcididae, Chrysididae, Eulophidae, Ichneumonidae, Megachilidae, and Mutillidae), thirteen genera, and twenty-one species. The families Chrysididae and Ichneumonidae exhibited the highest species richness among the natural enemies (Table S4).
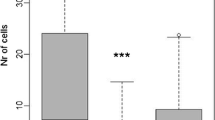
Using the number of individuals that emerged from nests, we found that the abundance of matrix and forest specialist species was higher than that of generalist species, while the richness of matrix specialist species was higher than that of forest specialist and generalist species (Figure 3A). Based on the data collected in this study, we categorized species as habitat specialists if they nested only in one habitat type (matrix or forest) and habitat generalists if they nested in both habitats. Similarly, when we analyzed the data using the number of nests built, matrix specialists had the highest abundance, followed by forest specialists and generalists, which was also observed for the richness data (Figure 3B).
Abundance and richness of cavity-nesting hymenopterans. A Abundance and richness calculated using the number of individuals that emerged from nests. B Abundance and richness of species calculated using number of nests (only for hosts). F, forest specialist species; M, matrix specialist species; MF, habitat generalist species. The letters above the boxplots indicate statistical differences observed using the t test.
3.2 Edge effect at the species level
At the species-level analysis, the impact of the edge varied for each species depending on whether the species was a specialist or generalist in nesting habitat choice and life history traits. For example, the most abundant forest specialist species (Trypoxylon agamemnon Richards 1934 and Ancistrocerus flavomarginatus Brèthies 1906) and matrix specialist species (Pachodynerus guadulpensis Saussure 1853 and Trypoxylon opacum Latreille 1796) did not show their highest abundance and presence/absence at the edge, but their occurrence was close to it (Figure 3). On the other hand, the habitat generalist Auplopus subaurarius Dreisbach (1963) showed a positive response towards inside the forest area, while Trypoxylon lactitarse Saussure, 1867 had its highest abundance at the edge between the matrix and the forest (Figure 4). When considering each natural enemy species, we observed that their response to the edge effect was linked to their hosts. Host-generalist natural enemies, such as Melittobia australica Girault (1912) and Chrysididae sp., showed a neutral response to the edge effect, with their abundance remaining stable from the matrix to the interior of forest area (Figures S4 and S5). However, host-specialist natural enemies, such as Xystromutilla bucki Suárez (1960) and Caenochrysis parvula (Fabricius 1804), exhibited a similar response to the edge as their hosts (Figures S4 and S6).
Species-level responses of cavity-nesting hymenopterans to forest edges. Blue dots and slopes were calculated using the number of individuals that emerged from the nests, while red dots and slopes were calculated using the number of nests. Positive values represent species found within forests, and negative values represent species found within anthropogenic matrices. Zero represents the edge. Only the most abundant species are shown.
3.3 Edge effect at the functional group level
At the functional group level, natural enemies exhibited their highest abundance and richness near the edge (matrix side), but their abundance also increased in higher distances inside forest area (Figure 5). Nevertheless, the response of natural enemies in terms of abundance and richness was better explained by the abundance and richness of their respective hosts than by the distance from the forest edge, and this relationship was positive (Table II, and Figure 5).
Functional group-level analysis of the natural enemies. The figure shows the responses of abundance and richness of natural enemies to forest edge distance, host abundance, and host richness. The AICc values are shown in Table II.
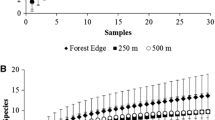
Caterpillar hunters exhibited a positive response to the edge in terms of both abundance and richness, regardless of whether we used the number of individuals that emerged from the nests or the number of built nests as a proxy for their abundance. Spider hunters showed a positive response to the edge only in abundance (both in the number of individuals that emerged and the number of nests), while their richness was unaffected (Figure 6). Cockroach hunters showed a negative relationship from the forest towards the matrix, while pollen collectors exhibited a negative relationship in the opposite direction, from the matrix towards the forest (Figure 7).
Functional group-level analysis of caterpillar and spider hunters. Blue dots and slopes were calculated using the number of individuals that emerged from the nests, while open red dots and slopes were calculated using the number of nests. Positive values indicate the presence within forests, and negative values indicate the presence within anthropogenic matrices. Zero represents the edge.
Functional group-level analysis of cockroach hunters and pollen collectors. Blue dots and slopes were calculated using the number of individuals that emerged from the nests, while open red dots and slopes were calculated using the number of nests. Positive values indicate the presence within forests, and negative values indicate the presence within anthropogenic matrices. Zero represents the edge.
4 Discussion
Our results demonstrate that the effects of the transition between the matrix and the forest on the communities of cavity-nesting wasps and bees depend heavily on the level at which analyses are conducted. All our hypotheses were confirmed, depending on the level of analysis (at the species or functional group levels) or the life history traits of each species (generalist and specialist in habitat use or trophic resource). Forest specialist species were more negatively affected by edge distance. On the other hand, natural enemy species were more dependent on the distribution of their hosts than on the edge effect directly. Furthermore, the response to the edge varied among functional groups, depending on the trophic resources they use. Caterpillar and spider hunters seem to be positively affected by the edge, mainly in their abundance, since the richness of the latter group was not influenced. An interesting observation was that pollen collectors progressively decreased their richness and abundance from the matrix towards the interior of forest area. Similarly, cockroach hunters were negatively affected when moving from the forest towards the matrix area.
An interesting point about our results and that requires caution is whether the edge should be classified as a distinct habitat type or just the cessation of one habitat and the beginning of another. Although we lack enough data to definitively classify the edge in our study area as a distinct habitat, information from the literature on cavity-nesting hymenopterans supports the idea that the ecotone between matrix and forest environments possesses unique characteristics (e.g., temperature, humidity, luminosity, and the presence of characteristic plant species) that differentiate the edge from other habitat types (Rocha-Filho et al. 2017; Stangler et al. 2015). Even so, the definition of edge as a distinct habitat may depend on the taxa analyzed, since as we show in our data, some species presents their higher abundance at the edge (e.g., caterpillar and spider hunters), and to them, this interface between matrix and forest probably is realized as a distinct environment.
This habitat perception by species may depend on some life history traits, such as dispersal capacity, niche breadth, and reproductive potential, which may be influenced in species distribution in fragmented landscapes (Ewers and Didham 2006; Öckinger et al. 2010). In our study, the dispersal capacity seems to be not very importance since solitary wasps and bees are able to foraging at least 1 km (Gathmann and Tscharntke 2002; Montagnana et al. 2021) and our edge distance gradient was 400 m from matrix to forest interior. So, other variables, such as niche breadth and reproductive potential, may be playing an important role in species habitat distribution of our study. Moreover, the individuals may also be having their behavior altered by interactions with other species, since they always are competing for food resources and nesting sites with other cavity nesting hymenopteran, and are being parasitized by natural enemies and had their nests predated by ants, which may influence the choice of nesting site (Corbara et al. 2009; Staab et al. 2018).
Our study presents a large gradient of edge distance from 200 m in the anthropogenic matrix to 200 m into the forest, and we obtained consistent results with other studies that had a minor gradient, that cavity-nesting wasps and bees, as well as their natural enemies, in general, exhibit higher richness and abundance in edge habitats than within forest fragments (Stangler et al. 2015; Rocha-Filho et al. 2017). The explanations for this pattern are commonly linked to the resources used by species. As edge areas are a union between different habitats, they are expected to have greater access to resources, including greater plant flowering, higher food availability, higher light availability, and greater understory cover (Stangler et al. 2015; Rocha-Filho et al. 2017). Moreover, higher environmental contrasts between the forest and matrix, as observed in our study, may increase species richness due to increased resource complementation between two different environments (Willmer et al. 2022). Nevertheless, this explanation may only apply to more tolerant species, which manage to survive edge conditions, as opposed to forest specialist species that avoid the edge because they are more sensitive to its effects (Matos et al. 2013, Vieira et al. 2015; Stangler et al. 2015).
At the species-level analysis, the availability of trophic resources may not be the only factor limiting species distribution. For instance, the habitat specialist wasps Trypoxylon agamemnon (which nests in forests) and Trypoxylon opacum Brèthes 1913 (which nests in open areas) are both spider predators (Moura et al. 2019). While spiders may be richer and more abundant at the edge (Prieto-Benítez and Méndez 2011; Rodrigues et al. 2014), these wasp species do not exhibit their highest abundance at the edge. Despite that, these species were also not positively affected by the higher distances inside their respective habitats (forest and matrix). Solitary wasps and bees have limited thermoregulatory capacity and may not survive in extreme temperatures and humidity (Loyola and Martins 2006; Stangler et al. 2015). Consequently, these species may avoid areas far from the edge, as they are unable to tolerate the high temperatures and low humidity of the matrix or the low temperatures and high humidity of the forest center (Stangler et al. 2015). In addition, by avoiding nest exactly in the edge, these species can benefit from the greatest amount of resources without having to compete with species from other environments, which are more common in forest edges (Youngentob et al. 2012).
On the other hand, habitat generalist wasp species, such as Trypoxylon lactitarse Saussure, 1867, may benefit from edge conditions. As a result, T. lactitarse may negatively influence other habitat specialist species at the edge, leading to a decrease in their occurrence and abundance. This effect can be observed for both forest-dependent species (e.g., T. agamemnon) and open-area species (e.g., T. opacum). This phenomenon could be attributed to competition between these species, but previous studies have reported that they do not overlap their trophic niche and do not use the same cavity diameter (Buschini and Wolff 2006; Buschini 2007; Buschini et al. 2010; Moura et al. 2019). Therefore, another plausible explanation may be the apparent competition process, which is a decrease in the abundance of one species due to the population increase of another species. These interactions occur due to the abundance increase of a third species at a higher trophic level, such as natural enemies (Holt 1977). Thus, the higher abundance of T. lactitarse at the edges may increase the density of natural enemies in these areas, leading to a decrease in the abundance of habitat specialist species. This process may explain the curve patterns observed in our results for generalist, specialist, and natural enemy species.
The response of species to edge effects may also depend on their life history traits. One example is A. subaurarius, an ectoparasitoid spider wasp that occurred in both forest and open areas but exhibited higher abundance further into the forest. This species provisions only one spider per brood cell, and many females use various wood materials in nest entrances, possibly to avoid hyperparasitoids (Buschini et al. 2007; Deus et al. 2023). Given that this species requires fewer prey items for its offspring than species that engage in mass provisioning and also rely on the forest for nest protection against natural enemies, it seems reasonable that we found its highest abundance towards the forest interior.
The abundance of bee species was notably low compared to wasp species, which makes it difficult to understand their response to the edge. Nonetheless, studies have shown that bees are positively affected by open areas and nearby forest patches (Gutiérrez-Chacón et al. 2018; Flores et al. 2018; Montagnana et al. 2021). For example, the distance of natural remnant edges in crop fields can positively affect bee abundance and richness, as these edges may be used by pollinators as nesting, foraging, and mating sites, thus increasing their diversity (Bailey et al. 2014). The low bee abundance may have been due to the trap nest methodology, which has been discussed that trap nesting rate may be higher in matrix areas due to the lack of natural cavities and lower in forest areas where natural cavities may be more abundant (Nether et al. 2019; Deus et al. 2022a, b). In our study, although we did not observe increased nesting within the forest, which would be more robust to indicate no influence of the methodology, most nesting occurred near the edge of the forest, where there could be more natural cavities compared to open areas of the matrix (Morato and Martins 2006). Anyway, this methodology has been widely used in various studies and has provided valuable insights into ecological processes (Staab et al. 2018), and in a previous study, comparing the occupancy of natural and artificial cavities by solitary wasps and bees found higher nesting rates in artificial nests (Westerfelt et al. 2015), indicating the effectiveness of this methodology for sampling cavity-nesting hymenopterans and their associated natural enemies.
In our study, the natural enemies were positively affected by the edge and also showed a positive response inside the forest area, which may only the reflect of their respective hosts at a species level analysis. These natural enemies’ species are entirely dependent on their hosts to complete their larval development (Sobek et al. 2009; Osorio-Canadas et al. 2018), and their responses to edge may vary depending on how they can use different host species. For example, the natural enemy M. australica Girault 1912, a gregarious species that parasitizes wasps and bees (Matthews et al. 2009), was found to be a generalist (parasitized several host species), occurring independently of edge distance, both in the matrix and forest directions. On the other hand, Caenochrysis nigropolita (Bischoff 1910) was positively affected by the forest edge, mainly because its specific hosts were more abundant in these areas. Therefore, the increased abundance of functional groups of natural enemies in the forest interior showed in our results may be attributed to the higher abundance of host species in these areas, such as A. subaurarius and T. agamemnon. Regardless, due to these great host dependence, natural enemy species may be more sensitive to environmental disturbances and may serve as bioindicators for assessing habitat quality (Staab et al. 2018).
The results from other functional groups also varied depending on the trophic resource used by species. The spider and caterpillar hunter were positively affected by edge while pollen collectors and cockroach hunters were negatively affected. The caterpillars preyed upon by wasps are typically herbivores, feeding on plant leaves, and they may benefit from the increased abundance of pioneer plants, especially herbaceous species at forest edges (Lôbo et al. 2011; Guimarães et al. 2014). In addition, spider species are also positively affected by the edge, as they show a mixture of species from the two adjacent environments (forest and matrix) (Rodrigues et al. 2014). As a result, trophic generalist species that are able to use more available food resources may benefit from the edge and exhibit higher abundance, which highly contributes to the positive edge effect when we analyze all species together, thus masking the effects of the edge on other species. Therefore, the general positive response of cavity nesting hymenopteran to the edge, shown here and in other studies, may merely reflect the disappearance of sensitive specialist species and the increase of species more tolerant to the edge effects in disturbed landscapes.
Our results highlight the importance of the analysis level and how it can modify the species response to edge effects. We demonstrate the importance of forest conservation and reforestation programs, not only by some cavity-nesting wasps and bees being entirely dependent on forests to persist, but also because most species sampled here depend on being close to a forest edge (less than 100 m) to carry out their ecological holes. Therefore, understanding how different species respond to environmental and landscape changes and may depend on staying close to forests is essential for effective management and planning strategies for biodiversity conservation.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary information files.
Code availability (software application or custom code)
Not applicable.
References
Alroy J (2017) Effects of habitat disturbance on tropical forest biodiversity. Proc Natl Acad Sci 114:6056–6061. https://doi.org/10.1073/pnas.1611855114
Bailey S, Requier F, Nusillard B, Roberts SP, Potts SG, Bouget C (2014) Distance from forest edge affects bee pollinators in oilseed rape fields. Ecol Evol 4:370–380. https://doi.org/10.1002/ece3.924
Bischoff H (1910) Die Chrysididen des Königlichen Zoologischen Museums zu Berlin. In Kommission bei R. Friedländer
Brèthies J (1906) Vespideos y Eumenídidos Sudamericanos. Anales del Museo Nacional de Buenos Aires, tomo XIII, p. 311–377
Brèthes J (1913) Himenópteros de la América Meridional. An. Mus. Nac. Hist. Nat. Buenos Aires, p. 24–35
Burnham KP, Anderson DR (2004) Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 33:261–304. https://doi.org/10.1177/0049124104268644
Buschini MLT (2007) Life-history and sex allocation in Trypoxylon (syn. Trypargilum) lactitarse (Hymenoptera; Crabronidae). J Zool Syst Evol Res 45:206–213. https://doi.org/10.1111/j.1439-0469.2006.00405.x
Buschini MLT, Wolff LL (2006) Notes on the biology of Trypoxylon (Trypargilum) opacum Brèthes (Hymenoptera; Crabronidae) in southern Brazil. Braz J Biol 66:907–917. https://doi.org/10.1590/S1519-69842006000500017
Buschini MLT, Luz V, Basilio S (2007) Comparative aspects of the biology of five Auplopus species (Hymenoptera; Pompilidae; Pepsinae) from Brazil. J Zool Syst Evol Res 45:329–335. https://doi.org/10.1111/j.1439-0469.2007.00407.x
Buschini MLT, Caldas TR, Borba NA, Brescovit AD (2010) Spiders used as prey by the hunting wasp Trypoxylon (Trypargilum) agamemnon Richards (Hymenoptera: Crabronidae). Zool Stud 49:169–175
Caitano B, Chaves TP, Dodonov P, Delabie JHC (2020) Edge effects on insects depend on life history traits: a global meta-analysis. J Insect Conserv 24:233–240. https://doi.org/10.1007/s10841-020-00227-1
Corbara B, Carpenter JM, Céréghino R, Leponce M, Gibernau M, Dejean A (2009) Diversity and nest site selection of social wasps along Guianese forest edges: assessing the influence of arboreal ants. CR Biol 332:470–479. https://doi.org/10.1016/j.crvi.2009.01.003
Deus JPA, Araujo G, Buschini MLT, Moura RR (2022a) Habitat types in the Atlantic Forest differently influence community structure and species interaction of cavity-nesting Hymenoptera and their natural enemies. J Insect Conserv 27:129–140. https://doi.org/10.1007/s10841-022-00440-0
Deus JP, Noga A, Brozoski F, Dias AM, Buschini ML (2022b) Trap-nesting biology of an ectoparasitoid spider wasp, Auplopus subaurarius (Hymenoptera: Pompilidae): the importance of wooded environments for niche generalist species. Braz J Biol 83:e269165. https://doi.org/10.1590/1519-6984.269165
Deus JPA, Noga A, Brozoski F, Dias AMP, Buschini MLT (2023) Trap-nesting biology of an ectoparasitoid spider wasp Auplopus subaurarius (Hymenoptera: Pompilidae): the importance of wooded environments for niche generalist species. Brazilian Journal of Biology 83. https://doi.org/10.1590/1519-6984.269165
Dreisbach RR (1963) New species of spider wasps, genus Auplopus, from the Americas South of the United States (Hymenoptera: Psammocharidae). Proceedings of the United States National Museum 114:137–211
Duarte GT, Santos PM, Cornelissen TG, Ribeiro MC, Paglia AP (2018) The effects of landscape patterns on ecosystem services: meta-analyses of landscape services. Landscape Ecol 33:1247–1257. https://doi.org/10.1007/s10980-018-0673-5
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81:117–142. https://doi.org/10.1017/S1464793105006949
Fabricius JC (1804) Systema Piezatorum secundum ordines, genera, species, adjectis synonymis, locis, observationibus, descriptionibus. Brunsvigae Reichard
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fahrig L (2017) Ecological responses to habitat fragmentation per se. Annu Rev Ecol Evol Syst 48:1–23. https://doi.org/10.1146/annurev-ecolsys-110316-022612
Flores L, Zanette LR, Araujo FS (2018) Effects of habitat simplification on assemblages of cavity nesting bees and wasps in a semiarid neotropical conservation area. Biodivers Conserv 27:311–328. https://doi.org/10.3097/LO.201976
Gathmann A, Tscharntke T (2002) Foraging ranges of solitary bees. J Anim Ecol 71:757–764. https://doi.org/10.1046/j.1365-2656.2002.00641.x
Girault AA (1912) A new Melittobia from Queensland, Australia. Jourbook: Psyche, Cambridge
Guimarães CDC, Viana JPR, Cornelissen T (2014) A meta-analysis of the effects of fragmentation on herbivorous insects. Environ Entomol 43:537–545. https://doi.org/10.1603/EN13190
Gutiérrez-Chacón C, Dormann CF, Klein AM (2018) Forest-edge associated bees benefit from the proportion of tropical forest regardless of its edge length. Biol Cons 220:149–160. https://doi.org/10.1016/j.biocon.2018.02.009
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A et al (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:e1500052. https://doi.org/10.1126/sciadv.1500052
Holt RD (1977) Predation, apparent competition, and the structure of prey communities. Theor Popul Biol 12:197–229. https://doi.org/10.1016/0040-5809(77)90042-9
IAPAR - Instituto de Desenvolvimento Rural do Paraná (2014) Historical and Current Meteorological Data, IAPAR
Kok MT, Alkemade R, Bakkenes M, Eerdt MV, Janse J et al (2018) Pathways for agriculture and forestry to contribute to terrestrial biodiversity conservation: a global scenario-study. Biol Cons 221:137–150. https://doi.org/10.1016/j.biocon.2018.03.003
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen-17 Geiger climate classification updated. Meteorol Z 15:259–263. https://doi.org/10.1127/0941-2948/2006/0130
Latreille PA (1796) Précis des caractères génériques des Insectes, disposés dans un ordre naturel. Paris et Brive
Laurance WF, Useche DC, Rendeiro J, Kalka M, Bradshaw CJ et al (2012) Averting biodiversity collapse in tropical forest protected areas. Nature 489:290–294. https://doi.org/10.1038/nature11318
Lôbo D, Leão T, Melo FPL, Santos AMM, Tabarelli M (2011) Forest fragmentation drives Atlantic Forest of northeastern Brazil to biotic homogenization. Divers Distrib 17:287–296. https://doi.org/10.1111/j.1472-4642.2010.00739.x
Losey JE, Vaughan M (2006) The economic value of ecological services provided by insects. Bioscience 56:311–323. https://doi.org/10.1641/0006-3568(2006)56[311:TEVOES]2.0.CO;2
Loyola RD, Martins RP (2006) Trap-nest occupation by solitary wasps and bees (Hymenoptera: Aculeata) in a forest urban remanent. Neotrop Entomol 35:41–48. https://doi.org/10.1590/S1519-566X2006000100006
MacIvor JS (2017) Cavity-nest boxes for solitary bees: a century of design and research. Apidologie 48:311–327. https://doi.org/10.1007/s13592-016-0477-z
Marsh CJ, Feitosa RM, Louzada J, Ewers RM (2018) Is β-diversity of Amazonian ant and dung beetles communities elevated at rainforest edges? J Biogeogr 45:1966–1979. https://doi.org/10.1111/jbi.13357
Matos MCB, Sousa-Souto L, Almeida RS, Teodoro AV (2013) Contrasting patterns of species richness and composition of solitary wasps and bees (Insecta: Hymenoptera) according to land-use. Biotropica 45:73–79. https://doi.org/10.1111/j.1744-7429.2012.00886.x
Matthews RW, González JM, Matthews JR, Deyrup LD (2009) Biology of the parasitoid Melittobia (Hymenoptera: Eulophidae). Annu Rev Entomol 54:251–266. https://doi.org/10.1146/annurev.ento.54.110807.090440
Mitchell MGE, Bennett EM, Gonzalez A (2015) Strong and nonlinear effects of fragmentation on ecosystem service provision at multiple scales. Environ Res Lett 10:094014. https://doi.org/10.1088/1748-9326/10/9/094014
Montagnana PC, Alves RSC, Garófalo CA, Ribeiro MC (2021) Landscape heterogeneity and forest cover shape cavity-nesting hymenopteran communities in a multi-scale perspective. Basic Appl Ecol 56:239–249. https://doi.org/10.1016/j.baae.2021.08.004
Morato EF, Martins RP (2006) An overview of proximate factors affecting the nesting behavior of solitary wasps and bees (Hymenoptera: Aculeata) in preexisting cavities in wood. Neotrop Entomol 35:285–298. https://doi.org/10.1590/S1519-566X2006000300001
Moura RR, Pitilin RB, Buschini MLT (2019) Specialization in prey capture drives coexistence among sympatric spider-hunting wasps. Ecol Entomol 44:639–647. https://doi.org/10.1111/een.12743
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62. https://doi.org/10.1016/S0169-5347(00)88977-6
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Nether MC, Dudek J, Buschini MLT (2019) Trophic interaction and diversity of cavity-nesting bees and wasps (Hymenoptera: Aculeata) in Atlantic Forest fragments and in adjacent matrices. Apidologie 50:104–115. https://doi.org/10.1007/s13592-018-0623-x
Noriega JA, Hortal J, Azcárate FM, Berg MP, Bonada N et al (2018) Research trends in ecosystem services provided by insects. Basic Appl Ecol 26:8–23. https://doi.org/10.1016/j.baae.2017.09.006
Öckinger E, Schweiger O, Crist TO, Debinski DM, Krauss J, Kuussaari M, Petersen JD, Pöyry J, Settele J, Summerville KS, Bommarco R (2010) Life-history traits predict species responses to habitat area and isolation: a cross-continental synthesis. Ecol Lett 13:969–979. https://doi.org/10.1111/j.1461-0248.2010.01487.x
Osorio-Canadas S, Arnan X, Bassols E, Vicens N, Bosch J (2018) Seasonal dynamics in a cavity-nesting bee-wasp community: shifts in composition, functional diversity and host-parasitoid network structure. PLoS ONE 13:e0205854. https://doi.org/10.1371/journal.pone.0205854
Prieto-Benítez S, Méndez M (2011) Effects of land management on the abundance and richness of spiders (Araneae): a meta-analysis. Biol Cons 144:683–691. https://doi.org/10.1016/j.biocon.2010.11.024
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rezende CL, Scarano FR, Assad ED, Joly CA, Metzger JP, Strassburg BBN, Tabarelli M, Fonseca GA, Mittermeier RA (2018) From hotspot to hopespot: an opportunity for the Brazilian Atlantic Forest. Perspect Ecol Conserv 16:208–214. https://doi.org/10.1016/j.pecon.2018.10.002
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Cons 142:1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021
Richards OW (1934) The American species of the genus Trypoxylon. Transactions of the Royal Entomological Society of London 82:73–362
Ries L, Fletcher RJ, Battin J, Sisk TD (2004) Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu Rev Ecol Evol Syst 35:491–522
Rocha-Filho LC, Rabelo LS, Augusto SC, Garófalo CA (2017) Cavity-nesting bees and wasps (Hymenoptera: Aculeata) in a semi-deciduous Atlantic forest fragment immersed in a matrix of agricultural land. J Insect Conserv 21:727–736. https://doi.org/10.1007/s10841-017-0016-x
Rocha-Filho LC, Montagnana PC, Boscolo D, Garófalo CA (2020) Green patches among a grey patchwork: the importance of preserving natural habitats to harbour cavity-nesting bees and wasps (Hymenoptera) and their natural enemies in urban areas. Biodivers Conserv 29:2487–2514. https://doi.org/10.1007/s10531-020-01985-9
Rodrigues ENL, Mendonça MDS, Costa-Schmidt LE (2014) Spider diversity responds strongly to edge effects but weakly to vegetation structure in riparian forests of Southern Brazil. Arthropod Plant Interact 8:123–133. https://doi.org/10.1007/s11829-014-9294-3
Saussure H (1853) Description de quelques crustacés nouveaux de la côte occidentale du Mexique. Revue et Magasin de Zoologie Pure et Appliquée 5:354–368
Sobek S, Tscharntke T, Scherber C, Schiele S, Steffan-Dewenter I (2009) Canopy vs. understory: does tree diversity affect bee and wasp communities and their natural enemies across forest strata? For Ecol Manage 258:609–615. https://doi.org/10.1016/j.foreco.2009.04.026
Souza TB, França FM, Barlow J, Dodonov P, Santos JS, Faria D, Baumgarten JE (2020) The relative influence of different landscape attributes on dung beetle communities in the Brazilian Atlantic Forest. Ecol Ind 117:106534. https://doi.org/10.1016/j.ecolind.2020.106534
Staab M, Pufal G, Tscharntke T, Klein AM (2018) Trap nests for bees and wasps to analyse trophic interactions in changing environments—a systematic overview and user guide. Methods Ecol Evol 9:2226–2239. https://doi.org/10.1111/2041-210X.13070
Stangler ES, Hanson PE, Steffan-Dewenter I (2015) Interactive effects of habitat fragmentation and microclimate on trap-nesting Hymenoptera and their trophic interactions in small secondary rainforest remnants. Biodivers Conserv 24:563–577. https://doi.org/10.1007/s10531-014-0836-x
Suárez FJ (1960) Datos sobre mutílidos neotropicales I. Nuevas especies de Sphaerophthalminae (Hymenoptera) 36:451–480
Vieira CR, Pitts J, Colli GR (2015) Microhabitat changes induced by edge effects impact velvet ant (Hymenoptera: Mutillidae) communities in southeastern Amazonia, Brazil. J Insect Conserv 19:849–861. https://doi.org/10.1007/s10841-015-9803-4
Villaseñor NR, Driscoll DA, MaH E, Gibbons P, Lindenmayer DB (2014) Urbanization impacts on mammals across urban-forest edges and a predictive model of edge effects. PLoS ONE 9:e97036. https://doi.org/10.1371/journal.pone.0097036
Westerfelt P, Widenfalk O, Lindelöw Å, Gustafsson L, Weslien J (2015) Nesting of solitary wasps and bees in natural and artificial holes in dead wood in young boreal forest stands. Insect Conserv Divers 8:493–504. https://doi.org/10.1111/icad.12128
Willmer JNG, Püttker T, Prevedello JA (2022) Global impacts of edge effects on species richness. Biol Cons 272:109654. https://doi.org/10.1016/j.biocon.2022.109654
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodol 73:3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x
Youngentob KN, Yoon HJ, Coggan N, Lindenmayer DB (2012) Edge effects influence competition dynamics: a case study of four sympatric arboreal marsupials. Biol Cons 155:68–76. https://doi.org/10.1016/j.biocon.2012.05.015
Zuur A, Ieno E, Walker N, Saveliev A, Smith G (2009) Ebooks Corporation. Mixed Effects Models and Extensions in Ecology with R. Stat Biol Health. Springer, New York. https://doi.org/10.1007/978-0-387-87458-6
Acknowledgements
We thank the excellent team of experts who identified the species: M.Sc. Bolívar Rafael Garcete Barrett (Federal University of Paraná), Dr. Gabriel Augusto Rodrigues de Melo (Federal University of Paraná), Dr. Jorge M. González (California State University, Fresno), Dra. Angélica Maria Penteado Dias (Federal University of São Carlos), Dra. Cátia Mello Patiu (Federal University of Rio de Janeiro), and Dr. Marcelo Teixeira Tavares (Federal University of Espírito Santo). We are grateful to the owners of the farms in the municipality of Guarapuava where collections were conducted.
Funding
The study was financially supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Contributions
CNQ, JPAD collected the data and GAA made the preparation to analysis. MCR,PCM and JPAD made the analysis. GAA, MLTB and JPAD wrote the manuscript. MLTB was supervisor. All authors revised the text.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: James Nieh
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alves, G.A., de Deus, J.P.A., Montagnana, P.C. et al. Edge effects on the cavity-nesting hymenopteran communities and their natural enemies within fragmented landscapes. Apidologie 55, 24 (2024). https://doi.org/10.1007/s13592-024-01061-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-024-01061-z