Abstract
Almond is a high-market value crop that benefits from honeybee pollination services, even for self-compatible varieties. Besides, it has been recently shown that the offering of food scented with floral mimic odors specific to different crops biased honeybee foraging preferences towards sunflowers, pear, or apple trees. Herein, we analyzed the floral volatiles of two almond self-compatible varieties to propose potential mimic odors. The mixture which bees discriminated the least from the natural floral scent in olfactory conditioning assays was chosen as almond mimic (AM). In the field, colonies fed AM-scented sucrose solution increased their foraging activity and amounts of stored pollen compared with colonies fed unscented food. Our results support the conditioning of honeybees to a floral mimic odor as a potential tool to bias their foraging preferences in almond, even applicable to self-compatible varieties. Future studies should address its effect on yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The expansion of areas cultivated with pollinator-dependent crops in recent years increases the global demand for pollination services (Aizen et al. 2019). Although the honeybee Apis mellifera L. is not the only managed bee, it is the most economically valuable pollinator in agriculture (Kevan 2018). Almond (Prunus dulcis (Mill.) D.A.Webb) is among the crops that most rely on honeybee services (Klein et al. 2007; Macfarlane 2018). In 2020, approximately 11 million colonies were necessary to pollinate an area of 2.2 million ha worldwide (FAOSTAT 2022).
By the time almond trees bloom, honeybee colonies have a reduced population in late winter when temperature restricts foraging activity to a few hours per day (Macfarlane 2018). Associated with the stress of transporting the hives to the plantation, exposure to pesticides within the agricultural setting increases the risk of infection by pathogens (Ahn et al. 2012). In addition, the transport of hives to a novel environment causes a delay on the onset of foraging. Altogether, these challenges lead us to consider new strategies for honeybee management in agricultural settings. To initiate a prompt foraging activity in the target crop, the conditioning of honeybee colonies to an odor that mimics the crop floral scent is a procedure that promotes a more efficient pollination (Farina et al. 2020, 2022). Based on mechanisms that enable social learning, this strategy allows conditioning honeybees by giving them early access to olfactory information (the floral scent) that predicts the oncoming food sources. Given the eusociality of honeybees, the distribution of the scented food inside the nest via mouth-to-mouth trophallactic contacts provides colony-mates with information that is learned and biases colony-foraging decisions (Farina et al. 2005; Arenas et al. 2007, 2008; Balbuena et al. 2012). Recently, it has been shown that honeybees can generalize a specific mimic odor with the natural floral scent of sunflower, pear, or apple crops (Farina et al. 2020, 2022). In these studies, the offering of food scented with each mimic odor led to higher foraging activity, increased the density of bees on the crops, and translated into higher yields.
As almond production is highly dependent on honeybee pollination services, breeders have been interested in the development of self-fertile varieties to reduce the number of honeybee colonies rented (Champetier et al. 2019). However, the extent to which the self-compatible varieties depend on bees under field conditions is not clear. In fact, it has been recently reported that honeybee visits to some self-fertile varieties improve crop yield and nut quality (Sáez et al. 2020, 2022). Considering the previous positive results in other Scrops (Farina et al. 2020, 2022), it is relevant to develop a specific mimic odor for almond flowers and test whether conditioning honeybees can increase foraging activity on an almond self-compatible variety.
Encompassing different experimental assays, we aimed to develop an almond mimic (AM) odor that honeybees could generalize to the natural floral scent. Then, we evaluated whether feeding colonies sucrose solution scented with the AM biased their foraging preferences towards almond trees in the field, as a potential tool to be considered as part of a pollination strategy, even applicable to self-compatible varieties.
2 Materials and methods
2.1 Study sites, honeybee colonies, and chemicals
Almond (Prunus dulcis, cv. Guara, Viu, two self-compatible varieties) floral volatiles were collected in a commercial orchard, “Finca Angel del Viento” near San Martín, province of Mendoza, Argentina (33° 07′ 11″ S, 68° 50′ 31″ W), during the blooming season of 2013. The identification of volatile compounds was carried out at the Chemical Ecology Laboratory of Facultad de Química (Universidad de la República, Montevideo, Uruguay). Chemicals used to prepare the different synthetic mixtures were obtained from Sigma-Aldrich, Steinheim, Germany. Almond mimic (AM) was composed of benzaldehyde (product # B1334), R-(+)-limonene (product # 183,164), and ( ±)-linalool (product # L2602); AMI was composed by benzaldehyde, R-(+)-limonene, ( ±)-linalool, and nonanal (product # 30,803); AMII was composed by benzaldehyde, ( ±)-linalool, (R)-(+)-limonene, and 2-octanone (product # O4709). For details of their proportions in the mixtures, see Patent PCT/IB2018/055549 (Farina et al. 2018). Jasmine mimic (JM) was a commercial extract obtained from Firmenich S.A.I.C. y F., Argentina, a scent chosen based on its presumed dissimilarity with the almond floral scent.
Conditioning experiments were performed with naïve bee foragers captured at the entrance of Langstroth hives located at least 5 km away from an almond orchard during August 2014. Almond flowers (cv. Viu) whose volatiles were used as stimulus during conditioning were collected in the commercial orchard, “Finca Angel del Viento.”
Field experiments were performed in a plantation for nut production (7,000,000 m2), “Finca Santa Mónica,” located near Lavalle, province of Mendoza, Argentina, (32° 35′ 37″ S; 68° 37′ 20″ W), during the almond blooming season of 2015 (August–September). The plantation contained several almond varieties (mixed planting design), among which the self-compatible cultivar Guara (planted in year 2008 at a density of 330 trees per hectare) was the only one in bloom during the experimental period, subjected to the same agricultural management (i.e., irrigation, fertilization, and pruning). No other competitive flora was found in bloom within the plantation during the experimental period (mid-winter in Argentina).
Commercial Langstroth single-story deep-frame hives (15,000 worker strength) were introduced in the field to provide pollination services at a stocking density of approximately 4 hives per hectare. A total of 125 experimental beehives were selected and inspected before conducting the study, presenting similar sizes (on average 7 frames covered by bees). Honeybee colonies were placed in 15 groups of 7–10 distributed along the borders of the field, providing pollination services for approximately 5100 Guara trees (see Supplementary Table S1). The minimum distance between the groups of hives receiving the different treatments was 600 m, in order to ensure that bees visiting almond flowers would more likely belong to the hives nearby receiving one of the two treatments.
2.2 Collection and identification of almond floral volatiles
To collect volatile compounds from almond flowers, an almond tree branch of each cultivar (i.e., Guara and Viu) was selected having 20–30 fresh flowers in the following stages: “bud” and “one-day-old flower.” The branches were enclosed in polyester bags (30 × 45 cm) gently sealed around the stems with plastic seals, for a period of 4 h (between 1000 and 1400 h, when large numbers of bee foragers were flying), and the volatiles were sampled with a SPME (solid phase microextraction) fiber (PDMS, 100 μm, Supelco®, product # 57300U) placed inside the bag. SPME fibers were then carried out to the laboratory for analysis. Volatiles were desorbed at 250 ℃ for 1 min in the injection port of a Shimadzu QP–2010 GC–MS device, with an AT–5 column (30 m, 0.25 mm i.d., 0.25 µm film thickness). The injection port was operated in the splitless mode with helium as carrier gas with a constant column flow of 1.0 mL/min. The initial oven temperature was 40 °C, held for 1 min, ramped at 5 °C/min to 200 °C, and ramped at 15 °C/min to 300 °C, then held for 1 min. The mass spectrometer was operated in the electron ionization mode at 70 eV, in the scan mode (m/z 40 to 500) with an ion source temperature of 200 °C. Compounds were identified on the basis of their mass spectra and retention indices in comparison with those of NIST17 database (Adams 2007; El-Sayed 2022). Experimental ion fragmentation patterns of the floral volatile compounds are available upon request.
2.3 Similarity of mimic odors to the natural almond floral scent
Once we had identified the main volatiles that make up the natural floral scent of the almond tree, we set out to develop a mixture of three or four compounds that would mimic the natural blend so that the bees would not be able to discriminate between them. Since insects do not need to assess all the single odorant components to recognize a complex bouquet (Riffell et al. 2009a) and due to in-hive learning procedure by using simple mixtures of representative odors which promoted bees’ generalization towards the floral blend of the target crop biasing their foraging preferences (Farina et al. 2020, 2022), we evaluated three candidate mixtures in olfactory conditioning assays to test how they were discriminated against the natural almond floral scent.
In the laboratory, bees’ learning abilities can be quantified using the Pavlovian conditioning of the proboscis extension reflex (PER), by pairing an originally neutral odor (conditioned stimulus, CS) with a sucrose reward (unconditioned stimulus, US) that elicits the proboscis extension as response (Takeda 1961; Bitterman et al. 1983).
To assay the PER, a device that delivered a continuous airflow (50 mL/s) was used for the application of the odorant. Four microliters of a synthetic odorant mixture impregnated on 30 × 3 mm filter paper inside a syringe was delivered through a secondary airstream (6.25 mL/s) to the head of the bee. A fan extracted the released odors to avoid contamination (Fernández et al. 2009). Each learning trial lasted 39 s. Before odor presentation, bees rested for 15 s in the airflow for familiarization as well as for testing the bees’ response towards the mechanical stimulus. For the training procedure of the classical conditioning, we presented the CS for 6 s. Reinforcement (1.8 M sucrose solution) was presented on the proboscis (mouthparts) and occurred for 3 s, 3 s after the onset of the CS. Only those bees that showed the unconditioned response (the reflexive extension of the proboscis after applying a 1.8 M sucrose solution to the antennae) and did not respond to the mechanical airflow stimulus were used. Also, at the end of the procedure, the unconditioned response was verified and bees that did not present it were discarded.
Experimental bees were randomly captured at the hive entrance. Then, bees were anesthetized at −4 °C for 1 min and individually harnessed in metal tubes. Harnessing restrained bees’ body movement but allowed them to freely move the antennae and mouthparts (Frings 1944). Afterwards, bees were kept in darkness in an incubator at 30 °C and 60% relative humidity, for 1 h, prior to the conditioning experiments.
2.3.1 Differential olfactory conditioning
To assess the bee’s ability to discriminate between two odors, naïve foragers can be trained in a differential PER conditioning (Bitterman et al. 1983), in which two odors, one rewarded (CS+) and the other unrewarded (CS−), are presented. In this study, three synthetic mixtures that mimic the almond floral odor (almond mimic, AM; almond mimic I, AMI; and almond mimic II, AMII) and a control synthetic mixture (jasmine mimic, JM) were presented as CS+ and the natural almond floral scent as CS−, swept from a 100 g sample of flowers (cv. Viu) contained in a Büchner flask by means of a continuous airflow. Bees were conditioned by four rewarded and four non-rewarded learning trials (inter-trial interval of 10 min) in a pseudo-random order (+ − − + + − − +). After training, bees’ responses were evaluated against the same odors, without reward, presented in a randomized order (test). The ability of bees to discriminate between the CS+ and CS− was analyzed by means of a discrimination index (DI) which allowed us to compare between the responses of bees trained with each synthetic blend. The DI was calculated for each bee as the difference between the response to the CS+ minus the response to the CS− during the testing period. The DI could take values of 0, if the bee responded equally to both odors, or 1, if it only responded to the CS+. Only one bee responded to the CS− and was discarded from the analysis.
2.3.2 Absolute olfactory conditioning
To study if bees could generalize the mimic formulation to the natural almond floral scent, bees were subjected to a conditioning procedure in which they learned to associate either JM or AM (CS) with the US. After four training trials (inter-trial interval of 10 min), bees stayed harnessed for 15 min and were then subjected to the presentation of two novel odors (test), (1) JM (when the CS was AM) or AM (when CS was JM) and (2) the natural almond floral scent, both without reinforcement. The presentation order of the odors during the tests was balanced, and a time gap of 15 min was used between each presentation.
In summary, absolute and differential conditionings were used to evaluate how honeybees perceive the stimuli (synthetic mixture or natural floral scent), and both provide useful information for investigating how bees learn, generalize, and discriminate odorant mixtures from floral bouquets (Farina et al. 2020, 2022).
2.4 Feeding protocol in field experiments
Based on the results obtained in both types of olfactory conditioning assays, we selected the almond mimic formulation to be tested in field experiments with honeybee colonies pollinating an almond orchard. We used the offering of scented food within the hive as a standardized procedure to establish long-term olfactory memory in honeybees (Arenas et al. 2007, 2008; Balbuena et al. 2012; Farina et al. 2020, 2022). Scented food was obtained by diluting 50 µL of AM per liter of sucrose solution (1.8 M). Colonies were fed in situ in a single event, by pouring either 500 mL of AM-scented sucrose solution (SS + AM, N = 62) or 500 mL of unscented 1.8 M sucrose solution (SS, N = 63) as control, over the top of the central frames of the hives. We did not consider a group of untreated hives as an additional control since it is a common practice of beekeepers to feed their hives sugar syrup during this time of the year when nectar is in short supply or unavailable. The researchers involved in the recording of the data (see sections below) remained blind with respect to the treatments to avoid any observer bias.
2.5 Honeybee foraging–related activity
To evaluate the effect of the circulation of AM-scented food on the colony-foraging activity, we recorded the number of incoming bees (incoming rate) at the entrance of the hive. We counted the arrivals in 20 colonies fed SS + AM and in 21 colonies fed SS for 1 min every day (from 10:00 to 14:00 h), once before treating the colonies and three times after (on days 1, 3, and 5 post-stimulation) (Supplementary Table S1). We performed this measurement each day at the same time frame in both treatments, to control for the influence of the time of day.
To evaluate whether the offering of AM-scented food biased honeybee foraging activity towards almond flowers, we measured the density of foragers visiting almond trees in the surroundings of treated hives (N = 20 trees randomly chosen within 40 m of SS + AM-treated colonies; N = 20 trees within 40 m of SS-treated colonies; Supplementary Table S1). Although we could not identify the hive each bee belonged to, the probability that the selected trees were visited by bees from the other treated hives (more distant) was lower, given the abundance of flowers, the lack of alternative flora nearby, and because the number of foraging bees decreases as the distance from the hives increases (Noetzel 1968; Johannsmeier et al. 1997; Johannsmeier and Mostert 2001).
Each tree was labeled and then daily monitored for 3 days (on days 2, 4, and 6 post-stimulation) during honeybee foraging hours (from 1100 to 1700 h). To avoid any bias due to differences in luminosity, ten trees in each treatment were monitored on their north side and the remaining ten on their south side. Standing on a fixed point, the observers surveyed all visible flowers following a clockwise direction, encompassing the whole tree surface (in no more than 1 min), and obtained the total number of foragers per tree. To consider the flower density over the course of the behavioral study, we estimated the blooming percentage as the number of open flowers (hence accessible for the bees) in relation to the total number of flowers in an almond tree. This procedure was repeated on each one of the trees selected for recording the density of bees.
2.6 Honeybee colony–stored pollen
To analyze the effect of feeding colonies AM-scented food on nest pollen reserves, we estimated the amount of stored pollen in each hive the day before the administration of the treatments (initial area) and 8 days later (final area). To this end, we sequentially removed the frames of each hive and measured the height and width of the area occupied by cells containing pollen on both sides of each frame with a measuring tape (N = 6 SS + AM-treated colonies; N = 6 SS-treated colonies; Supplementary Table S1). In case of widely separated cells, we grouped them together (Scheiner et al. 2013). The total pollen area (in cm2) of a hive was obtained by adding the areas of all sides of the frames. Finally, for each hive, we calculated the initial and final areas, measured on each date.
2.7 Statistical analysis
All statistical tests were performed with R v4.2.0 (R Core Team 2022), using the glmmTMB package (Brooks et al. 2017). Models in general were simplified as follows: significance of the different terms was tested starting from the higher-order term model using likelihood ratio tests (LRT) to compare between models (Chambers 1992). Non-significant terms (p > 0.05) were removed (see Supplementary Table S2). We considered the use of generalized linear and generalized linear mixed-effect models (GLM and GLMM) because they allow analyzing response variables whose errors are not normally distributed, avoiding the transformation of the response variable or the adoption of non-parametric methods (Crawley 2013) (Supplementary Tables S3, S5). Post hoc comparisons using contrast matrixes were performed with the emmeans function (Lenth 2022) (Supplementary Tables S4, S6, S7).
Differences in the DI obtained in the differential olfactory conditioning were assessed by means of a GLM following a binomial (Bernoulli) error distribution, in which we considered the conditioned stimuli (a four-level factor: AM+, JM+, AMI+, AMII+) as fixed effect.
To analyze differences in olfactory memory retrieval and in generalization to the almond flower odor, we compared the responses to the CS obtained in the fourth (last) trial with the responses obtained during the testing trials for each bee by means of a GLMM following a binomial (Bernoulli) error distribution. We considered the presented odor (a three-level factor: either JM+, AM, and almond flower or AM+, JM, and almond flower) as fixed effect and the bee as random effect.
To test for differences among incoming rates and the density of bees on almond flowers between treatments, we proposed two GLMMs and included the interaction between time (days, three-level factor) and treatment (a two-level factor). In the former, we considered a negative binomial error distribution to account for the overdispersion of the data and included the incoming rate before treatment (day 0) as covariate and the hive as random factor. In the latter, we considered a Poisson error distribution and included tree as random factor and blooming percentage as an offset, to correct the number of events for an estimate of population size.
To test for differences in the amount of stored pollen, we proposed two linear models, considering treatment (a two-level factor) as fixed factor. It is worth to mention that there were no pollen reserves before the treatment.
3 Results
3.1 Identification of floral almond volatiles
The analysis of the headspace from almond flowers (Prunus dulcis, cv. Guara and Viu) revealed more than 20 volatile compounds (Figure S1, Table I). These included eight monoterpenoids, one sesquiterpene, four aliphatic aldehydes, three aliphatic ketones, four aromatics, two alcohols, and one linear hydrocarbon. Identification was based on mass spectra fragmentation patterns and retention indices (Table I). Natural almond floral scent was dominated by limonene as the major compound in both cultivars (44–46%, peak #9). In addition, linalool, benzaldehyde, 2-octanone, p-cymene, 2-nonanone, and nonanal were the main floral volatiles identified in both cultivars, with varying relative abundances (peaks #11, 4, 6, 8, 10, and 12, respectively; Table I, Figure S1). These seven compounds, commonly found in floral blends, were selected as the representative volatile components of almond flowers scent.
3.2 Similarity of mimic odors to the natural almond floral scent under the PER paradigm
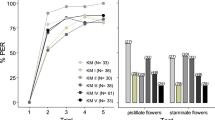
Following the identification of the main volatiles that make up the floral scent of the almond tree, we developed three synthetic mixtures of three and four compounds each and evaluated if bees were able to distinguish between them and the natural almond floral scent. Bees trained with the AM as rewarded conditioned stimulus (CS+) showed high levels of response (higher than 50%) towards both the CS+ and the natural almond floral scent (here, as CS−) (Fig. 1a). In contrast, bees trained either with the unrelated novel odor JM (Fig. 1b) or with AMI and AMII as CS+ (Figs. 1c and d, respectively) showed high levels of response towards the learned odors (CS+) but not towards the CS− (the natural almond floral scent).
Discrimination between mimic odors and the natural floral scent. Bees’ ability to discriminate was evaluated after differential olfactory conditioning. The almond flower scent was used as unrewarded stimulus (CS−), and the following synthetic blends were used as rewarded stimuli (CS+): a almond mimic (AM), b jasmine mimic (JM), c AMI, and d AMII. In all cases, the left panel contains the percentage of bees that extended their proboscis (% PER, observed data) during four conditioning training trials. Statistical analysis was performed on a discrimination index (DI, e), based on the bee’s ability to discriminate between CS+ and CS− in the final presentation of the odors (test). The DI of conditioning assays with AM as CS+ was lower than the DI in which the CS+ was JM, indicating that bees discriminated the least between AM and the unrewarded floral natural scent (GLM predicted data, p > 0.05; Supplementary Tables S2, S3). Numbers between brackets indicate sample size. Different letters indicate significant differences
Comparisons of the DIs revealed significant differences between the conditioned stimuli (Fig. 1e; model: DI ~ conditioned stimuli, likelihood ratio test (LRT) = 10.31, p = 0.016, Supplementary Tables S2, S3). The DIs obtained in AM conditioning resulted statistically different from those obtained in bees trained with JM (pairwise contrast: t.ratio = −3.04, p = 0.0147; Supplementary Table S4), indicating that the bees differentiated the fragrance of jasmine more than that of AM from the natural almond floral scent. In turn, the DIs obtained for the AMI and AMII showed no significant differences neither with AM nor the JM (p > 0.05; Supplementary Table S4).
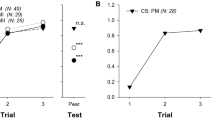
We also conducted absolute conditionings to evaluate whether the conditioned responses to JM or AM could be generalized between them and to the natural almond floral scent. Bees could be successfully conditioned to JM along the 4 learning trials (Fig. 2a; model: response ~ odor + 1|bee, LRT = 34.9, p < 0.001; Supplementary Tables S2, S3). However, bees did not generalize JM to AM or the natural almond floral scent presented as novel odors during testing (pairwise contrast JM vs AM: t.ratio = 3.48, p < 0.01, and JM vs almond flower: t.ratio = 4.05, p < 0.001, Supplementary Table S4). On the other hand, bees successfully conditioned to AM (Fig. 2b; model: response ~ odor + 1|bee, LRT = 25.4, p < 0.001; Supplementary Tables S2, S3) generalized the learned response towards the novel natural almond floral scent (pairwise contrast AM vs almond flower: t.ratio = 0.95, p > 0.05, Supplementary Table S4), but not to JM (pairwise contrast AM vs JM: t.ratio = 3.65, p < 0.01, Supplementary Table S4), showing that bees perceived AM and the natural almond floral scent as more similar.
Generalization of memories from synthetic mimic odors to almond floral natural odor. Bees’ ability to generalize was tested towards the single presentation of the almond natural odor after a synthetic mimic odor was used as conditioned stimulus (CS) during an absolute classical conditioning of the proboscis extension reflex (PER). The odors used as rewarded stimuli (CS) were a jasmine mimic (JM) and b Almond mimic (AM). In both cases, the left panel contains the percentage of bees that extended their proboscis (% PER, observed data) during four conditioning training trials, while the right panel contains the response in the final presentation of two odors (test). Letters indicate significant differences between responses to the CS in trial 4 and the two tested odors (GLMM predicted data, p < 0.001; Supplementary Tables S2, S3). Numbers between brackets indicate sample size
Considering the results obtained in laboratory assays, we tested the effect of AM in field experiments with honeybee colonies pollinating an almond orchard.
3.3 Honeybee foraging–related activity
Honeybee colonies settled inside the almond orchard increased their general activity over the course of time after the offering of AM-scented food, as the analysis detected a significant interaction between treatment and time for the rate of incoming bees (Fig. 3a; model: incoming bees per minute ~ treatment × time + incoming bees day 0 + (1|hive); LRT = 6.49, p < 0.05; Supplementary Tables S2, S5). The incoming bee rates were significantly higher in colonies fed SS + AM than in those fed unscented sucrose solution (SS) in days 3 and 5 post-stimulation (pairwise contrasts: day 3, SS vs SS + AM: t.ratio = −0.79, p < 0.01; day 5, SS vs SS + AM: t.ratio = −3.89, p < 0.001; Supplementary Tables S6).
Effect of almond mimic-scented food on bees foraging–related activity. Colonies were stimulated with unscented sucrose solution (white) or almond mimic-scented sucrose solution (gray). a The number of incoming bees per minute was monitored up to 5 days post-stimulation (SS, N = 21; SS + AM, N = 20). b The density of bees foraging on almond flowers was quantified in trees within 40 m of the treated beehives up to 6 days post-stimulation (SS, N = 20 trees; SS + AM, N = 20 trees). Boxplots (observed data) show the median and interquartile range (IQR), with whiskers showing the maximum value within 1.5 IQR, and individual points mark values outside this range. Asterisks indicate significant differences between treatments (**p < 0.01, ***p < 0.001, GLMM predicted data; Supplementary Tables S2, S5, S6, S7)
To evaluate whether the offering of AM-scented solution biased honeybee foragers towards the target crop, we assessed the density of bees foraging on almond flowers in trees within 40 m of the SS + AM-treated colonies or the control colonies, up to 6 days after treatment. We found a significant interaction between treatment and time (Fig. 3b; model: bees per tree ~ treatment × time + (1|tree) + offset (log (blooming)); LRT = 14.05, p < 0.001; Supplementary Table S2, S5). While statistically significant differences were found only in day 4 (t.ratio = 3.25, p < 0.01; Supplementary Table S7), the overall mean density of foragers on almond trees near colonies fed SS + AM doubled those near control colonies (mean ± SE: 6.9 ± 0.6 bees/tree and 3.4 ± 0.3 bees/tree, respectively).
3.4 Honeybee colony–stored pollen
Although none of the experimental hives had any pollen reserves when inspected before treatment, in 8 days, SS + AM-fed colonies collected and stored significantly higher amounts of pollen (more than twice) than SS-fed colonies (Fig. 4; model: final pollen area ~ treatment; F = 15.84, p < 0.01; Supplementary Tables S2, S5).
Effect of the almond formulation on the amount of stored pollen in honeybee colonies. Colonies were stimulated with unscented sucrose solution (SS) or almond mimic-scented sucrose solution (SS + AM). The total pollen area of beehives was assessed the day before applying the treatments and 8 days afterwards. Boxplots (observed data) show the median and interquartile range (IQR), with whiskers showing the maximum value within 1.5 IQR, and individual points mark values outside this range. The number of hives is indicated in brackets. The asterisks indicate significant differences (**p < 0.01, GLM predicted data; Supplementary Tables S2, S5)
4 Discussion
In this study, we evaluated the effect of conditioning honeybees to a floral mimic odor on the performance of colonies pollinating a self-compatible almond variety. Based on almond floral volatiles, we developed a synthetic blend (almond mimic, AM) which harnessed honeybees perceived as similar to almond flower scent under laboratory conditions. Later, in the field, the offering of AM-scented food to honeybee colonies promoted the establishment of specific olfactory memories which ultimately translated into higher foraging activity and higher amounts of stored pollen.
To our knowledge, reports about almond floral volatiles are scarce, with a few studies focusing on the volatiles emitted by the fruit which could be considered as part of integrated pest management (Beck et al. 2012; Nawade et al. 2019). In the present study, we conducted the identification of the main compounds present in the floral blend of two self-compatible almond varieties (Prunus dulcis, cv. Guara and Viu). The natural floral scents of both cultivar varieties were dominated by limonene as the major compound. Among other abundant components, we found linalool, benzaldehyde, 2-octanone, p-cymene, 2-nonanone, and nonanal, with varying relative abundances. Most of them are commonly found in the inflorescences of Rosaceae plant species; and four of them are present in the volatiles of other members of the genus Prunus (Knudsen et al. 2006; El-Sayed et al. 2018). Still, very few studies investigated volatiles in flowers of this genus from the perspective of plant-pollinator interactions (El-Sayed et al. 2018).
Honeybees rely on floral volatiles to recognize rewarding flowers, and they can discriminate mixtures with varying proportions of their components (Ditzen et al. 2003; Deisig et al. 2006Giurfa et al. 2012). Interestingly, bees can ignore information about the ratio of the components of mixtures if they lead to a reward, showing similar response levels to all mixtures of the same odorants (Wright et al. 2008). Thus, based on the ability of bees to learn that perceptually distinct olfactory stimuli can lead to common outcomes (i.e., generalization, Shepard 1987), we developed a simple synthetic mixture AM (composed by only a few of the almond floral volatiles identified herein) that bees could generalize to the natural almond floral scent. We showed that there is generalization between the mimic and the natural floral odor (although different) when the former is associated to a reward. Even though the generalization (and/or discrimination) between the AM and the natural mixture was not symmetric (i.e., the response was different when the natural scent was rewarded), our results show that the generalization of the rewarded mimic odor towards the natural blend is well suited for the purpose of improving bee management for pollination. From this perspective, it is also relevant that the floral mimic developed, composed of only three of the more than 20 volatiles identified in the present study, was enough to elicit bee response levels like those towards the complete natural blend. These results are in accordance with the previous studies which propose that the insect olfactory system has evolved to process complex floral olfactory stimuli just by a small subset of odors (Riffell et al. 2009a, b; Reinhard et al. 2010; Twidle et al. 2015; Mas et al. 2018).
In the field, feeding honeybee colonies with AM-scented food resulted in higher activity levels at the hive entrance as well as on almond trees. Bias of the forager workforce towards the target crop might be due to the in-hive learning of olfactory information about the availability of resources in the environment (Farina et al. 2005; Arenas et al. 2007, 2008; Balbuena et al. 2012). In fact, previous studies show that bees conditioned to an odor under the PER paradigm can use learned information in subsequent orientation tasks to search for food outside the nest (honeybees, Chaffiol et al. 2005; bumblebees, Nery et al. 2020). In addition, the increase in the colony activity that we observed here was coupled with a higher amount of pollen stored during the almond blooming period, suggesting that the floral mimic odor learned with sucrose solution as reward can be functional for guiding bees to almond flowers which offer pollen as well as nectar. Consistently, honeybee foragers have been shown to actively collect almond pollen (Estravis-Barcala et al. 2021), which is hoarded in the nest and is then used to feed larvae (Brodschneider and Crailsheim 2010). This would be highly relevant considering the challenging environment faced by the hives that pollinate almond trees, as it will result in a better and faster development of the colony in early spring. Even if the administration of scented sucrose solution is expected to mainly activate nectar foragers, recent evidence suggest that this group of bees show a higher probability of transitioning to pollen collection when they encounter nectars of decreasing quality (Arenas and Kohlmaier 2019). In this sense, the lower quality of the nectar offered by almond flowers (estimated nectar sugar concentration, 16.7 ± 1.1% w/w, N = 46, unpublished data) might induce bees to switch to pollen collection.
Overall, the results obtained herein support the conditioning of honeybees to a floral mimic odor as a tool to bias their foraging preferences in an almond self-compatible variety. As Farina et al. (2020, 2022) demonstrated in other crops, the use of in-hive learning with mimic odors increased honeybee foraging activity on the target crop, which translated into higher yields. While the effect of this procedure on almond yield should be further investigated, a pilot trial showed a 1.6-fold increase in terms of nuts per tree in the surroundings of treated colonies (N = 7 trees per treatment, unpublished data).
Moreover, breeding of self-compatible cultivars has become one of the main objectives of this high-value industry (Martínez-Gómez et al. 2007) in an attempt to decrease the dependence on managed pollinators and, thus, to reduce the number of rented hives (Champetier et al. 2019). However, the use of managed honeybees improved yield in a presumed pollinator-independent almond variety (Sáez et al. 2020). In this line, the conditioning of honeybees to a floral mimic odor could be considered as an alternative tool to optimize pollination services, even applicable to self-compatible high-value crops.
Data availability
The datasets generated for this study are available upon request to the corresponding author.
Code availability
Not applicable.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation, Carol Stream
Ahn K, Xie X, Riddle J, Pettis J, Huang ZY (2012) Effects of long distance transportation on honey bee physiology. Psyche vol 2012 Article ID 193029 https://doi.org/10.1155/2012/193029
Aizen MA, Aguiar S, Biesmeijer JC, Garibaldi LA, Inouye DW, Jung C, Martins DJ, Medel R, Morales CL, Ngo H, Pauw A, Paxton RJ, Sáez A, Seymour CL (2019) Global agricultural productivity is threatened by increasing pollinator dependence without a parallel increase in crop diversification. Glob Chang Biol 25(10):3516–3527. https://doi.org/10.1111/gcb.14736
Arenas A, Fernández VM, Farina WM (2007) Floral odor learning within the hive affects honeybees’ foraging decisions. Naturwissenschaften 94:218–222. https://doi.org/10.1007/s00114-006-0176-0
Arenas A, Fernández VM, Farina WM (2008) Floral scents experienced within the colony affect long-term foraging preferences in honeybees. Apidologie 39:714–722. https://doi.org/10.1051/apido:2008053
Arenas A, Kohlmaier MG (2019) Nectar source profitability influences individual foraging preferences for pollen and pollen-foraging activity of honeybee colonies. Behav Ecol Sociobiol 73:1–10. https://doi.org/10.1007/s00265-019-2644-5
Balbuena MS, Arenas A, Farina WM (2012) Floral scents learned inside the honeybee hive have a long-lasting effect on recruitment. Anim Behav 84:77–83. https://doi.org/10.1016/j.anbehav.2012.04.008
Beck JJ, Higbee BS, Light DM, Gee WS, Merrill GB, Hayashi JM (2012) Hull split and damaged almond volatiles attract male and female navel orangeworm moths. J Agric Food Chem 60(33):8090–8096. https://doi.org/10.1021/jf302658v
Bitterman ME, Menzel R, Fietz A, Schäfer S (1983) Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97(2):107–119. https://doi.org/10.1037/0735-7036.97.2.107
Brodschneider R, Crailsheim K (2010) Nutrition and health in honey bees. Apidologie 41:278–294. https://doi.org/10.1051/apido/2010012
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9(2):378–400
Chaffiol A, Laloi D, Pham-Delègue MH (2005) Prior classical olfactory conditioning improves odour-cued flight orientation of honey bees in a wind tunnel. J Exp Biol 208(19):3731–3737. https://doi.org/10.1242/jeb.01796
Chambers JM (1992) Linear models In: Chambers JM, Hastie TJ (eds) Statistical Models in S. Wadsworth & Brooks/Cole, Belmont
Champetier A, Lee H, Sumner DA (2019) Are the almond and beekeeping industries gaining independence? Choices 34(4):1–8
Crawley MJ (2013) The R Book. JohnWiley & Sons Ltd., Hoboken
Deisig N, Giurfa M, Lachnit H, Sandoz JC (2006) Neural representation of olfactory mixtures in the honeybee antennal lobe. Eur J Neurosci 24(4):1161–1174. https://doi.org/10.1111/j.1460-9568.2006.04959.x
Ditzen M, Evers JF, Galizia CG (2003) Odor similarity does not influence the time needed for odor processing. Chem Sens 28(9):781–789. https://doi.org/10.1093/chemse/bjg070
El-Sayed AM (2022) The Pherobase: database of pheromones and semiochemicals. Retrieved from https://www.pherobase.com
El-Sayed AM, Sporle A, Colhoun K, Furlong J, White R, Suckling D (2018) Scents in orchards: floral volatiles of four stone fruit crops and their attractiveness to pollinators. Chemoecology 28(2):39–49. https://doi.org/10.1007/s00049-018-0254-8
Estravis-Barcala MC, Sáez A, Graziani MM, Negri P, Viel M, Farina WM (2021) Evaluating honey bee foraging behaviour and their impact on pollination success in a mixed almond orchard. Apidologie 52(4):860–872. https://doi.org/10.1007/s13592-021-00872-8
FAOSTAT (2022) Food and Agriculture Organization Corporate statistical database. Retrieved from http://www.fao.org/faostat/en
Farina WM, Grüter C, Díaz PC (2005) Social learning of floral odours inside the honeybee hive. Proc R Soc B: Biol Sci 272(1575):1923–1928. https://doi.org/10.1098/rspb.2005.3172
Farina WM, Estravis-Barcala C, Palottini, F (2018) Formulation for promoting targeted pollination of almond tree crops in honey bees. PCT: 25/7/2018. PCT/IB2018/055549
Farina WM, Arenas A, Díaz PC, Susic Martin C, Estravis-Barcala MC (2020) Learning of a mimic odor within beehives improves pollination service efficiency in a commercial crop. Curr Biol 30(21):4284–4290. https://doi.org/10.1016/j.cub.2020.08.018
Farina WM, Arenas A, Díaz PC, Susic Martin C, Corriale MJ (2022) In-hive learning of specific mimic odours as a tool to enhance honey bee foraging and pollination activities in pear and apple crops. Sci Rep 12(1):20510. https://doi.org/10.1038/s41598-022-22985-5
Fernández VM, Arenas A, Farina WM (2009) Volatile exposure within the honeybee hive and its effect on olfactory discrimination. J Comp Physiol A 195(8):759–768. https://doi.org/10.1007/s00359-009-0453-4
Frings H (1944) The loci of olfactory end-organs in the honey-bee, Apis mellifera Linn. J Exp Zool 97:123–124. https://doi.org/10.1002/jez.1400970203
Giurfa M, Sandoz JC (2012) Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn Mem 19(2):54–66. https://doi.org/10.1101/lm.024711.111
Johannsmeier MF, Mostert JN (2001) Crop pollination. In: Johannsmeier MF (ed) Beekeeping in South Africa, 3rd edn.. Agricultural Research Council of South Africa, Pretoria, pp 235–250
Johannsmeier MF, Swart DJ, Morudu TM (1997) Honeybees in an avocado orchard: forager distribution, influence on fruit set and colony development. South African Avocado Growers’ Association Yearbook, 20:39–41. https://www.avocadosource.com/Journals/SAAGA/SAAGA_TOC.htm Accessed 25 April 2023
Kevan PG (2018) Conserving pollinators for agriculture, forestry and nature. In: Roubik DW (ed) Pollination of cultivated plants: A compendium for practitioners, vol 1. Food and Agriculture Organization of the United Nations, Rome, pp 29–33
Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc B: Biol Sci 274(1608):303–313. https://doi.org/10.1098/rspb.2006.3721
Knudsen JT, Eriksson R, Gershenzon J, Ståhl B (2006) Diversity and distribution of floral scent. Bot Rev 72(1):1–120. https://doi.org/10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2
Lenth R (2022) Emmeans: EstimatedMarginalMeans, aka Least-Squares Means. R package version 1.7.4–1. https://CRAN.R-project.org/package=emmeans
Macfarlane RP (2018) Applied pollination in temperate and subtropical areas. In: Roubik DW (ed) Pollination of cultivated plants: A compendium for practitioners, Volume 1 Food and Agriculture Organization of the United Nations, Rome, pp 137–181
Martínez-Gómez P et al (2007) Almond. In: Kole C (ed) Genome Mapping and Molecular Breeding in Plants, vol 4. Fruits and nuts. Springer, Berlin, pp 229–242
Mas F, Harper A, Horner R, Welsh T, Jaksons P, Suckling DM (2018) The importance of key floral bioactive compounds to honey bees for the detection and attraction of hybrid vegetable crops and increased seed yield. J Sci Food Agricult 98(12):4445–4453. https://doi.org/10.1002/jsfa.8967
Nawade, B, Yahyaa M, Reuveny H, Shaltiel-Harpaz L, Eisenbach O, Faigenboim A, Bar-Yaakov I, Holland D, Ibdah M (2019) Profiling of volatile terpenes from almond (Prunus dulcis) young fruits and characterization of seven terpene synthase genes. Plant Sci 287:110187. https://doi.org/10.1016/j.plantsci.2019.110187
Nery D, Palottini F, Farina WM (2020) Classical olfactory conditioning promotes long term memory and improves odor-cued flight orientation in the South American native bumblebee Bombus pauloensis. Curr Zool 67(5):561–563. https://doi.org/10.1093/cz/zoaa073
Noetzel DM (1968) Insect pollination results on sunflower. Department of Entomology, North Dakota State University, Fargo USA [Mimeograph], pp 108–112
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reinhard J, Sinclair M, Srinivasan MV, Claudianos C (2010) Honeybees learn odour mixtures via a selection of key odorants. PloS One 5(2):e9110. https://doi.org/10.1371/journal.pone.0009110
Riffell JA, Lei H, Christensen TA, Hildebrand JG (2009a) Characterization and coding of behaviorally significant odor mixtures. Curr Biol 19:335–340. https://doi.org/10.1016/j.cub.2009.01.041
Riffell JA, Lei H, Hildebrand JG (2009b) Neural correlates of behavior in the moth Manduca sexta in response to complex odors. Proc Natl Acad Sci USA 106(46):19219–19226. https://doi.org/10.1073/pnas.0910592106
Sáez A, Aizen MA, Medici S, Viel M, Villalobos E, Negri P (2020) Bees increase crop yield in an alleged pollinator-independent almond variety. Sci Rep 10(1):1–7. https://doi.org/10.1038/s41598-020-59995-0
Sáez A, Aguilar R, Ashworth L, Gleiser G, Morales CL, Traveset A, Aizen MA (2022) Managed honeybees decrease pollination limitation in self-compatible but not in self-incompatible crops. Proc R Soc B: Biol Sci 289:20220086. https://doi.org/10.1098/rspb.2022.0086
Scheiner R, Abramson CI, Brodschneider R, Crailsheim K, Farina WM, Fuchs S, Grünewald B, Hahshold S, Karrer M, Koeniger G, Koeniger N, Menzel R, Mujagic S, Radspieler G, Schmickl T, Schneider C, Siegel AJ, Szopek M, Thenius R (2013) Standard methods for behavioural studies of Apis mellifera. J Apic Res 52(4):1–58. https://doi.org/10.3896/IBRA.1.52.4.04
Shepard RN (1987) Toward a universal law of generalization for psychological science. Science 237(4820):1317–1323. https://doi.org/10.1126/science.3629243
Takeda K (1961) Classical conditioned response in the honey bee. J Insect Physiol 6(3):168–179. https://doi.org/10.1016/0022-1910(61)90060-9
Twidle AM, Mas F, Harper AR, Horner RM, Welsh TJ, Suckling DM (2015) Kiwifruit flower odor perception and recognition by honey bees. Apis Mellifera J Agricult Food Chem 63(23):5597–6560. https://doi.org/10.1021/acs.jafc.5b01165
Wright GA, Kottcamp SM, Thomson MGA (2008) Generalization mediates sensitivity to complex odor features in the honeybee. PLoS One 3(2):e1704. https://doi.org/10.1371/journal.pone.0001704
Acknowledgements
The authors are grateful to G.P. Ramirez for her support during the measuring period. We also thank José Gobbi (Almenci S.A.) and Martín Fernández (Aceitera General Deheza S.A.) for the access to the orchards.
Funding
This project was supported by the National Scientific and Technical Research Council of Argentina (CONICET), the National Agency for Scientific and Technological Promotion (ANPCYT), and the University of Buenos Aires.
Author information
Authors and Affiliations
Contributions
Walter M. Farina was responsible for the study conceptualization and funding acquisition. Walter M. Farina, Florencia Palottini, M. Cecilia Estravis-Barcala, and Andrés Arenas contributed to the data collection. Florencia Palottini, M. Cecilia Estravis-Barcala, M. Sol Balbuena, and Andrés González performed the data analysis. Walter M. Farina, Florencia Palottini, and M. Cecilia Estravis-Barcala wrote the first complete draft. All the authors contributed to the writing of the manuscript, and all the authors read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study did not require any ethics approval.
Consent to participate
This manuscript had no human participants.
Consent for publication
Data contributors were all contacted for, and received, consent to use their data prior to the submission of this manuscript.
Conflict of interest
National Scientific and Technical Research Council of Argentina (CONICET) and the University of Buenos Aires has filed the patent application (PCT/IB2018/055549) on the commercial use of the almond formulation to improve honeybee pollination efficiency, in which Walter M. Farina, Florencia Palottini, and M. Cecilia Estravis-Barcala are coinventors. Walter M. Farina is coinventor and shareholder of ToBEE S.A., the licensee of this technology. Andrés Arenas, M. Sol Balbuena, and Andrés González declare no competing interests.
Additional information
Handling editor: James Nieh
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farina, W.M., Palottini, F., Estravis-Barcala, M.C. et al. Conditioning honeybees to a specific mimic odor increases foraging activity on a self-compatible almond variety. Apidologie 54, 40 (2023). https://doi.org/10.1007/s13592-023-01019-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-023-01019-7