Abstract
Grooming behavior confers resistance to honey bees against Varroa destructor, being of interest to social immunity studies and breeding programs. The objective of this study was to characterize at the individual level the grooming behavior of mite-resistant (R) and susceptible (S) A. mellifera stocks from Argentina. Assays were performed in experimental arenas by applying two treatments to nurse bees: (1) placing a V. destructor mite on the bee’s thorax and (2) touching the bee with a paintbrush. Grooming reactions were recorded on bees from both stocks at the ages of 6, 10, and 14 days after emergence. R bees exhibited lower time of first response against the mite, performed more cleaning attempts, and used all their legs with a higher probability compared to S bees. The same pattern was evident when younger and older bees from the R stock were compared. The results demonstrate that bee age and genetic origin are critical factors of grooming behavior in honey bees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Grooming behavior has been described in the honey bee Apis mellifera Linnaeus (Hymenoptera: Apidae) as a social trait involving the physical removal of parasitic mites, such as Varroa destructor Anderson and Trueman (Acari: Varroidae), from the body of adult bees. Through this behavior, the parasitized bees can dislodge themselves and injure mites using their legs and mandibles (autogrooming) or receiving help from other bees (allogrooming) (Boecking and Spivak 1999). Such behavior is considered an important trait in the defense against V. destructor in Apis cerana Fabricius, the original host species of the mite (Büchler et al. 1992; Peng et al. 1987; reviewed by Pritchard 2016). Even though the importance of grooming behavior as a mechanism conferring mite resistance to A. mellifera colonies has been a controversial issue, a growing body of evidence suggests that this trait can increase mite mortality and hence modulate its population growth in colonies, specifically in Africanized honey bee populations (Morse et al. 1991; Moosbeckhofer 1992; Ruttner and Hänel 1992; Boecking and Ritter 1993; Moretto et al. 1993; Bienefeld et al. 1999; Arechavaleta-Velasco and Guzmán-Novoa 2001; Guzmán-Novoa et al. 2012; Invernizzi et al. 2016; Nganso et al. 2017) as well as stocks of European origin (Dadoun et al. 2020; Russo et al. 2020).
The field assessment of grooming behavior towards V. destructor is challenging, due to the difficulty of directly observing and quantifying its expression. Indirect methods of assessing this behavior are based on counting the number of fallen and mutilated mites on screened bottom boards of honey bee hives (Ruttner and Hänel 1992). Nevertheless, a proportion of injured mites may be the product of hygienic behavior (the cleaning of cells with infested brood) (Rosenkranz et al. 1997) or predation (Bienefeld et al. 1999; Szabo and Walker 1995; Boecking and Spivak 1999). Direct observation of grooming performed by individual bees under controlled conditions, though time-consuming, can substantially reduce the unreliable nature of indirect methods and complement the field characterization of honey bee stocks of interest. Direct methods of assessing the behavior include the visualization of worker bees grooming their bodies for a period of time when artificially infested with varroa mites (Peng et al. 1987; Fries et al. 1996). Grooming behavior has been assessed under controlled conditions in small groups of bees infested by V. destructor (Andino and Hunt 2011; Arechavaleta-Velasco and Guzmán-Novoa 2001; Currie and Tahmasbi 2008; Invernizzi et al. 2016) or by observing the behavior of individual bees in a Petri dish after placing a mite on its body (Aumeier 2001; Guzmán-Novoa et al. 2012).
Results obtained by Guzmán-Novoa et al. (2012) indicated that bee genotypes with lower mite infestation levels and higher proportions of injured mites in their colonies removed a higher percentage of mites in individual grooming tests. Specifically, these authors found that Africanized, Russian, and Canadian honey bee genotypes with low mite population growth removed significantly more mites from their bodies than European honey bees at the individual level. This finding highlights the importance of performing integral evaluations of honey bee mite-resistance stocks to explore the intensity and effectiveness of grooming at both the individual and colony levels.
Laboratory assays have made it possible to characterize the grooming reactions displayed by V. destructor-infested bees (Aumeier 2001; Invernizzi et al. 2016). In a pioneer study, Aumeier (2001) reported five reaction behaviors: cleaning intensity, shaking, biting, rolling, and attempting to fly. However, grooming behavior seems to be highly variable, and marked differences have been found in the proportion of bees that display these behaviors and can shed the mite between different genotypes and studies (Aumeier 2001; Guzmán-Novoa et al. 2012; Invernizzi et al. 2016). In addition, age is an important factor in the division of labor in honey bees, and age polyethism was described for immunity traits as hygienic behavior in A. mellifera (e.g., Panasiuk et al. 2010; Scannapieco et al. 2016). Even though a recent study (Dadoun et al. 2020) detected a lower percentage of grooming towards V. destructor in 21-day-old bees than in younger bees, little is known about the age factor in grooming behavior elicited by honey bees against the mite.
A naturally mite-surviving honey bee stock from northeast Argentina that expresses high grooming behavior at the colony level was recently characterized under field conditions by our research group (Russo et al. 2020). However, the detailed description of this behavior at the individual and group levels and its association with grooming phenotype in the field remains to be explored in this selected honey bee stock. In the present study, we evaluated self-grooming behavior intensity and the latency time to initiate the response toward V. destructor in resistant and susceptible A. mellifera stocks under laboratory conditions. In addition, we analyzed whether grooming variables exhibit differences between bee ages in these populations. We tested the hypotheses that (i) bees from resistant colonies display more intense grooming reactions toward the mite than bees from susceptible colonies, and (ii) grooming intensity decreases as the age of the bees increases, regardless of their genetic origin.
2 Materials and methods
This study was conducted during December–March (2017–2018) (summer in the southern hemisphere) at Instituto de Genética of the Instituto Nacional de Tecnología Agropecuaria (INTA), Buenos Aires, Argentina.
2.1 Honey bee stocks
Apis mellifera colonies from two stocks previously characterized by Russo et al. (2020) were used as follows: stock 1, a V. destructor-resistant honey bee population that exhibited high levels of grooming behavior at the colony level (hereafter named R stock), and stock 2, a susceptible honey bee population that showed low levels of grooming behavior at the colony level (hereafter named S stock). Three colonies from each stock (R and S) exhibiting contrasting grooming phenotypes (percentage of damaged mites registered in screened bottom boards; Russo et al. 2020) were used for the experiments. The mean percentages of damaged mites were 44.21 in the studied R colonies and 5.55% in S colonies.
Two frames containing capped brood (i.e., pupae with dark eyes and cuticle) were selected from each colony (Figure 1). Twenty-four hours prior to the bee’s emergence, the frames were individually placed in a screened cage and stored in an incubator at 33 ± 0.5 °C, with 75 ± 2% relative humidity (RH) (Figure 1). The emerging bees of each colony were individually marked with a color on the thorax using a queen bee marker (Figure 1). Different colors were used to identify the age of the bee and its stock origin (R and S). For each stock, cohorts of one hundred color-marked worker bees of the same age were obtained and returned to the corresponding colony. At the age of 6, 10, and 14 days, the color-marked worker bees were sampled and transported to the laboratory to conduct behavioral assays (Figure 1). The selection of ages was based on previous studies on the age range in which bees can potentially perform grooming behavior (Moore et al. 1995; Dadoun et al. 2020).
2.2 V. destructor source
Mites were collected from donor colonies with high V. destructor phoretic loads (> 5%), using the icing sugar method (Dietemann et al. 2013). Phoretic mites were shaken onto a damp paper towel and rinsed with a drop of distilled water. Mites that could not clutch the paintbrush bristles were considered unhealthy and discarded. Healthy mites were transferred to Petri dishes with moist pieces of paper towel, placed in a laboratory room kept at 33 ± 0.5 °C, and used as soon as possible to artificially infest the color-marked bees. Only active vital mites that were able to attach to the host immediately were used for the bioassays. Mites that were not used within 2 h of collection time were discarded.
2.3 Bioassays
To register grooming behavior, observation frames were used according to Arechavaleta-Velasco et al. (2012) with modifications. Briefly, a wooden frame was placed over a broodless Langstroth standard wooden framework (42 × 20 cm) containing honey at the top. Two acetate rings (9 cm diameter), used as paired-experimental arenas, were embedded in the wax (Figure 1). Two acetate lids facilitated the introduction of the color-marked worker bees. Outside the rings, non-marked bees from the same colony were introduced into the observation frame to create a more natural environment (Figure 1).
Two treatments were performed for each bee age and colony: (i) infesting bees with a V. destructor mite by placing it on the dorsal part of the thorax using a small paintbrush (mite infestation treatment) and (ii) touching bees once, at the beginning of the observation period, with a small paintbrush on the dorsal part of the thorax (control treatment).
A color-marked bee was placed in the experimental arena jointly with ten nurse bees (not color-marked) from the same colony and allowed to acclimate for 3 min before we started the observations (Figure 1). A stopwatch was started immediately upon the simultaneous application of the treatments in the paired experimental arenas (Figure 1). Color-marked bees were observed for up to 3 min by two previously trained observers to avoid potential bias in the records. The grooming behavior was recorded according to previous assays (Aumeier 2001; Arechavaleta-Velasco et al. 2012) including the following variables: the time of first grooming response, the number of legs involved in this first attempt, the total number of grooming attempts during the observation time, and the cleanliness intensity. Cleanliness was categorized as a dichotomous variable according to how vigorous or weak the body and leg movements of the color-marked bee were (Aumeier 2001). A fifth variable, “Anxiety,” was recorded and categorized as “no anxiety” (being quiet) or “anxiety” (walking quickly with nervous movements and on occasions, nudging other bees) (Bąk and Wilde 2015). During the observation time, we also determined whether the color-marked bee displayed rolling movements and whether it effectively removed the mite during the observation time (Aumeier 2001). About 15 to 20 color-marked bees were individually evaluated per stock (R, S: three colonies each), age (6, 10, and 14 days old), and treatment (mite infestation and control), reaching a total of 584 bees analyzed. Visual observations were made under red light, in a laboratory room with controlled temperature (33 ± 0.5 °C).
2.4 Statistical analysis
Differences in the grooming variables were independently analyzed with a generalized linear mixed model (GLMM) considering age, stock, and treatment as fixed factors and evaluation day, colony, and paired bees as random factors. The response variables “time of first grooming response” and “number of attempts” were adjusted to Gamma and Poisson distribution, respectively. The variable “number of legs used during the first grooming attempt” was adjusted to a binomial distribution and logit link function (number of legs used vs. number of legs not used). The rest of the variables (anxiety level, cleanliness intensity, and rolling behavior) were adjusted to a Bernoulli distribution. In these cases, the models were tested using the Hosmer–Lemeshow test for goodness of fit. To obtain the most appropriate structure of variance, the Akaike information criterion was used. To compare the response variables among factors (ages, stocks, and treatments), post hoc analysis was performed using Fisher’s least significant difference (LSD) test (α = 0.05).
To evaluate differences in grooming effectiveness between stocks, the frequencies of bees that successfully groomed off the mite from their bodies during the observation period were compared by Fisher’s exact test. All analyses were performed using the glmer function in R package “lme4” (Bates et al. 2015; R Core Team 2017).
Principal component analysis (PCA) was used to describe the relationship among the grooming variables and compare samples from mite-infested S and R bees at the multidimensional space. Only statistically significant variables from GLMM analysis were considered. This analysis was conducted using InfoStat 2016 (Di Rienzo and Montiglio 2016).
3 Results
3.1 Time of first grooming response
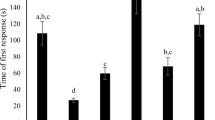
Differences in time to initiate self-grooming behavior were found between treatments (mite infestation and control), stocks (R and S), and bee ages (6, 10, and 14 days old), with significant interactions between factors (GLMM results: F1, 572 = 223.55, P < 0.001 for treatment; F1, 572 = 73.31, P < 0.001 for stock; F2, 572 = 8.70, P = 0.002 for bee age; F2, 572 = 1.75, P = 0.17 for stock × bee age interaction; F1, 572 = 22.84, P < 0.001 for stock × treatment interaction; F2, 572 = 0.91, P < 0.401 for bee age × treatment interaction; F2, 572 = 4.85, P = 0.008 for treatment × stock × bee age interaction; Figure 2).
Mean time ± SE (s) of first grooming response performed by color-marked bees when exposed to V. destructor treatment (mite infestation) and control treatment (paintbrush touch). Values are shown for the susceptible (S) and resistant (R) A. mellifera stocks at different bee ages (days). Different letters indicate significant differences between ages, stocks (genetic origin), and treatments by Fisher´s LSD (α = 0.05).
Artificially V. destructor-infested bees started to groom themselves significantly earlier than bees from the control treatment in both genetics and all analyzed ages (post hoc comparisons, Fisher’s LSD test; Figure 2). Specifically, when infested with a mite, resistant (R) bees started to groom themselves significantly earlier (mean of 8.8 s over all bee ages) than bees from the mite-susceptible (S) stock (mean of 32.4 s over all bee ages) (post hoc comparisons, Fisher’s LSD test; Figure 2).
For R stock, significant differences in the time to initiate grooming behavior toward the mite were detected between ages, with younger bees (6 days old) reacting faster to mite infestation than the older bees (10 and 14 days old) (post hoc comparisons, Fisher’s LSD test; Figure 2). In the case of the S stock, time of first grooming response was lower in 6- and 10-day-old bees compared to 14-day-old bees (Figure 2).
3.2 Grooming response level
Differences in the number of grooming attempts performed by bees were detected between treatments, stocks, and bee ages (GLMM results: F1, 569 = 138.97, P < 0.001 for treatment; F1, 569 = 84.46, P < 0.001 for stock; F2,569 = 9.115, P = 0.001 for bee age; F2, 569 = 10.47, P < 0.001 for stock × bee age interaction; F1, 569 = 5.22, P = 0.022 for stock × treatment interaction; F2, 569 = 1.77, P = 0.171 for bee age × treatment interaction; F2, 569 = 2.63, P = 0.072 for treatment × stock × bee age interaction). Post hoc comparisons revealed that a higher number of grooming attempts were performed by bees from both stocks and all ages in response to mite infestation in comparison with the control treatment (Figure 3). In addition, when stocks were compared, R bees exhibited a higher mean number of attempts against the mite than S bees, with the former showing almost twice grooming attempts at all ages (Figure 3). Regarding bee age, R bees showed a higher number of grooming attempts against the mite at younger ages (6 and 10 days old) than the older bees (14 days old) from the same stock (Figure 3). This pattern was not evident for bees of the S stock.
Mean number of grooming attempts (± SE) performed by color-marked bees during the observation time in response to V. destructor treatment (mite infestation) and control treatment (paintbrush touch). Values are shown for the susceptible (S) and resistant (R) A. mellifera stocks at different bee ages (days). Different letters indicate significant differences between ages, stocks (genetic origin), and treatments by Fisher’s LSD (α = 0.05).
Differences in leg use during the first grooming attempt were found between treatments, stocks, and bee ages, with significant interactions among the factors (GLMM results: F1, 568 = 290.23, P < 0.001 for treatment; F1, 568 = 19.88, P < 0.001 for stock; F2, 568 = 7.29, P = 0.007 for bee age; F2, 568 = 3.50, P = 0.031 for stock × bee age interaction; F1, 568 = 53.90, P < 0.001 for stock × treatment interaction; F2, 568 = 14.88, P < 0.001 for bee age × treatment interaction; F2, 568 = 13.67, P < 0.001 for treatment × stock × age interaction). The probability of using all the legs during the grooming response differed between stimuli, with control bees being 75–99% less likely to use all their legs compared to those receiving the Varroa stimulus, regardless of bee age or genetics (post hoc analysis; Supplementary File S1a). When genetic origin was compared, we observed that 6- and 10-day-old S bees were less likely to use all their legs against the mite compared to same-aged R bees (Supplementary File S1b). When bee age was considered, younger bees from R stock exhibited a higher probability of using all their legs against the mite than did older bees from the same stock (Supplementary File S1c). No difference in leg use was detected among ages for S bees (Supplementary File S1c).
No significant differences in the cleaning intensity were detected between treatments, stocks, or bee ages (Supplementary File S2).
3.3 Anxiety level
Differences in the proportion of bees that exhibited anxiety were detected between treatments, stocks, and bee ages, with a significant triple interaction among factors (GLMM results: F1, 567 = 67.10, P < 0.001 for treatment; F1, 567 = 13.05, P < 0.001 for stock; F2,567 = 1.36, P = 0.258 for age; F2, 567 = 2.92, P = 0.055 for stock × bee age interaction; F1, 567 = 1.15, P = 0.284 for stock × treatment interaction; F2, 567 = 2.55, P = 0.079 for bee age × treatment interaction; F2, 567 = 4.26, P = 0.015 for treatment × stock × age interaction; Figure 4). Post hoc comparisons revealed that bees from both stocks displayed similar anxiety levels toward the mite, but a significantly higher proportion of 10-day-old bees expressed anxiety in the R stock than in the S stock (Figure 4). In addition, twice the proportion of R bees (mean: 62% of bees of all ages) evidenced anxiety when control stimulus was applied, in comparison with S bees (mean: 32% of bees of all ages) (Figure 4).
Mean proportion (± SE) of color-marked bees that expressed anxiety in response to V. destructor treatment (mite infestation) and control treatment (paintbrush touch). Values are shown for the susceptible (S) and resistant (R) A. mellifera stocks at different bee ages (days). Different letters indicate significant differences between bee ages, stocks (genetic origin), and treatments by Fisher´s LSD (α = 0.05).
Additionally, we observed mite-parasitized bees walking around the experimental arena at different intensities, reaching and touching other companion bees. This behavior was specifically observed in approximately 50% of the resistant bees and at lower frequencies in the susceptible bees (18–20%).
3.4 Rolling behavior
An apparently higher proportion of bees from R stock (25%) displayed rolling behavior against the mite, in comparison with bees from S stock (12%), although this apparent difference was not statistically significant (GLMM results: F1, 569 = 0.06, P = 0.813 for treatment; F1, 569 = 0.02, P = 0.893 for stock; F2, 569 = 0.11, P = 0.897 for bee age; F2, 569 = 0.11, P = 0.894 for stock × bee age interaction; F1, 569 = 0.04, P = 0.841 for stock × treatment interaction; F2, 569 = 0.11, P = 0.896 for bee age × treatment interaction; F2, 569 = 0.15, P = 0.864 for treatment × stock × age interaction; Figure 5). Almost no rolling behavior was displayed by bees against the control treatment, suggesting that this reaction would be specific toward the mite (Figure 5).
3.5 Grooming effectiveness
A low proportion of bees groomed off the mites during the 3-min observation. Specifically, three out of 118 R bees (2.5%) successfully groomed off the mites placed on their bodies, while none of the S bees (0 out of 173 bees) removed the mite. This difference in grooming effectiveness between stocks was border significant (Fisher’s exact test; P = 0.0657).
3.6 Principal component analysis
The results of mite-infested bees from both stocks indicated that the two main components explain 77% of the total variability of the data (PC1: 62% and PC2: 15%; Figure 6 and Supplementary File S3). The number of attempts against the mite and the number of legs involved were negatively associated with the time of first response, and these three variables showed a significant contribution to the first component (Figure 6 and Supplementary File S3). Anxiety significantly contributed to PC2 and was negatively correlated with time of first response and positively associated with the number of attempts and legs used against the mite. In other words, those individuals that exhibited low reaction times also showed high anxiety and displayed higher attempts against the mite (Figure 6). Regarding the stock differentiation, a partial separation was found in the two-dimensional space (Figure 6). Samples of R stock tended to be distributed at higher values of both principal components and to form a relatively homogenous group where all bees exhibited intense grooming (Figure 6). Conversely, samples belonging to S stock appeared to organize in two groups, one with bees exhibiting “light” grooming reactions and another one with bees responding more intensely to mite infestation (Figure 6).
Principal component analysis (PCA) score plots based on grooming variables: time of first response, number of attempts, number of legs involved in the first attempt, and anxiety level of individual worker bees against mite infestation (V. destructor treatment). These variables were included in PCA analysis based on their statistical significance on the GLMM results. Data are shown for bees (samples) of all ages from mite-resistant (R: black dots) and susceptible (S: white dots) A. mellifera stocks.
4 Discussion
In the present study, we characterized different grooming reactions of Apis mellifera workers of different ages against Varroa destructor mites in two contrasting phenotypes: a mite-resistant and a mite-susceptible bee stock. We documented differences in the time to first response and the intensity of grooming reactions between genetic stocks and ages and suggested a relationship between traits associated with grooming behavior at the colony level (e.g., number of damaged mites) and the individual level.
Several authors have characterized the grooming reactions displayed by individual bees when infested by V. destructor. Aumeier (2001) identified eight aspects of responsive behavior of Africanized and Carnolian bees after having a mite placed on their thorax. Similarly, Invernizzi et al. (2016) recorded the occurrence of five reactions involved in the grooming behavior and compared them between European vs. Africanized bees. When these authors grouped all behaviors, they found that, in general, Africanized bees reacted more intensely to V. destructor than Italian bees. In our study, even though we compared two stocks of European honey bee populations, we detected differences between Varroa-resistant and Varroa-susceptible stocks in several grooming variables and a generally stronger response to the mite in the former. Specifically, we found that resistant bees exhibited a lower time of first response to mite infestation, were more likely to use all their legs during the first attempt, and performed a higher number of attempts throughout the observation period. These results demonstrate genotypic variability for auto-grooming response against V. destructor at the individual level, in agreement with previous studies in which variation between honey bee strains was detected for the percentage of bees that groomed and for the time to start grooming (Aumeier 2001; Vandame et al. 2002).
We found that 2.5% of the resistant bees (3 out 118) groomed off the mite during the observation time, while none of the susceptible bees could successfully remove them. Despite being low, the mite removal success observed in our R stock is similar to that reported by Invernizzi et al. (2016), who found three (two Italian and one Africanized) out of the 48 bees (6.3%) that groomed off the mite. Similarly, Guzman-Novoa et al. (2012) found 29.4% and 4.3% of “successful” bees of Africanized and Italian origin, respectively, while Aumeier (2001) obtained percentages of 18.3% and 9.1% for Africanized and Carniolan bees, respectively. Conversely, Dadoun et al. (2020) observed that more than 60% of resistant bees shed the mite, that is, a much larger percentage than that found by the present and previous studies. Differences between studies in the rates of successful mite removal could be explained by differences in the methodology used to test the grooming response (Petri dishes vs. observation frames), in the analyzed honey bee genetic stock and its propensity to perform grooming behavior, or in the collection method of mites. Kirrane et al. (2018) suggested that the grooming success of bees against V. destructor in laboratory cages was affected by the age and reproductive status of the mites. Specifically, they detected a higher mite drop for daughter mites than foundress mites. Here, we used foundress mites to artificially infest the experimental bees, which could explain the apparent low proportion of mite removal observed. It is worth noting that during the artificial infestation, we put the mite on the thorax of the color-marked bee, where it would be more difficult for this bee to reach the mite according to previous observations reported by Bak and Wilde (2016). Therefore, the location of the mite could also have affected the effectiveness of the mite removal in our assays.
The general performance of our resistant bees can be considered “intense grooming,” which consists of vigorous wiping and shaking movements and the use of more than two legs, as previously described by Guzmán-Novoa et al. (2012) for bees from resistant genotypes. We observed that the resistant bee becomes very active just after the V. destructor mite is deposited on its body and initiates the grooming response before 30 s of observation, with a mean overall ages of 26.16 s. This behavior has also been observed in selected mite-resistant stocks of Tellian bees (Dadoun et al. 2020), indicating that selection for V. destructor resistance may impact the individual behavior of worker bees toward the mite. However, it is worth noting that mite resistance that specifically emerges as a result of natural selection is currently addressed as a complex phenomenon involving multiple factors and traits at the colony level (Mondet et al. 2020).
Our results provide new information on the associations between the grooming variables and on how informative each variable is in the differentiation between our mite-resistant and susceptible stocks at the individual level. We found that the time to first response is negatively associated with both the number of legs involved in the first attempt and the total number of attempts performed against the mite, and that these three variables contributed to stock differentiation in the laboratory tests. We may speculate that a greater number of events against the mite and higher intensity in grooming events increase the probability of mite removal at the individual level. We also observed parasitized bees walking inside the experimental arena with different intensities, reaching and touching companion bees. This behavior was specifically observed in approximately 50% of the resistant bees and at lower frequencies in the susceptible bees (18–20%) and may stimulate the arena mates to help them groom, a possible sign of allogrooming behavior, which must be further evaluated. Consistently, a higher proportion of bees from the resistant stock expressed anxiety and exhibited rolling behavior across all bee ages evaluated and irrespectively of the stimulus. Globally, these observations at the individual level are in line with previous descriptions of colony behavior of the R stock in the field (Russo et al. 2020) and suggest that a “groomer colony” is composed of “groomer bees” with high responsiveness not only to the mite but also to other stimuli. This must be further evaluated by analyzing the responsiveness of the groomer bees at a genetic level, as previously explored for hygienic behavior (e.g., Boutin et al. 2015; Mondet et al. 2015; Scannapieco et al. 2017).
In addition to the stock differentiation for grooming behavior at the individual level, we found significant differences in several of the grooming variables between bees of different ages, specifically for R stock. Younger mite-resistant bees performed a higher number of attempts and used a greater proportion of legs than did older bees. As the bees aged, a decrease in the intensity of the grooming response was evident, suggesting that younger bees are more involved in cleaning activities than older ones. Recently, Dadoun et al. (2020) characterized the grooming response to V. destructor in mite-resistant and susceptible bees 4, 7, 15, and 21 days old. They found that even though workers of different ages were involved in the grooming response to mite infestation, the lowest percentage of grooming was observed in 21-day-old bees from both stocks. Unlike these authors, we detected differentiation in grooming intensity according to age specifically in our resistant bees, suggesting that a possibly more structured age division of these activities allows these colonies to be more effective in the grooming response, as observed for other sanitary traits, such as hygienic behavior (Scannapieco et al. 2016). Concerning grooming as a task-specialized behavior, Moore et al. (1995) described the behavior of “Red 93,” an A. mellifera worker bee that gradually developed into a highly specialized social groomer from day 7 of age until the end of their observations on day 15. Given these results (Moore et al. 1995) and our present observations, it would be interesting to evaluate our resistant bees throughout life to determine whether they become specialists in grooming or change their responsiveness against the mite as they get older.
The present study of honey bee behavior against V. destructor infestation using experimental arenas allowed us to identify informative components of grooming behavior to differentiate the analyzed genetic stocks. Even though individual assays can be useful to phenotype colonies in a controlled environment, their use as a proxy for grooming performance at a colony level in the frame of a breeding program is debatable due to the methodological complexity and time-consuming characteristics. Instead, this kind of assay can be useful to select bees with contrasting grooming phenotypes (while they are exhibiting the behavior) to explore the genetic basis of these differences. In this regard, advances have been made by identifying candidate genes associated with grooming behavior (Arechavaleta-Velasco et al. 2012; Hamiduzzaman et al. 2017; Morfin et al. 2020). Future research should focus on analyzing candidate genes and detecting high-throughput single-nucleotide polymorphism between our mite-resistant and susceptible genotypes. This would represent the first step to developing specific markers toward a marker-assisted selection of resistant honey bee stocks to assist local breeding programs.
Data availability
Data will be made available upon reasonable request.
Code availability
Not applicable.
References
Andino GK, Hunt GJ (2011) A scientific note on a new assay to measure honeybee mite-grooming behavior. Apidologie 42(4):481–484
Arechavaleta-Velasco ME, Guzmán-Novoa E (2001) Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies. Apidologie 32(2):157–174
Arechavaleta-Velasco ME, Alcala-Escamilla K, Robles-Rios C, Tsuruda JM, Hunt GJ (2012) Fine-scale linkage mapping reveals a small set of candidate genes influencing honey bee grooming behavior in response to Varroa mites. PLoS ONE 7(11):e47269
Aumeier P (2001) Bioassay for grooming effectiveness towards Varroa destructor mites in Africanized and Carniolan honey bees. Apidologie 32(1):81–90
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw Sl 67(1):1–48
Bąk B, Wilde J (2016) Grooming behavior by worker bees of various subspecies of honey bees to remove Varroa destructor mites. J Apic Res 54(3):207–215
Bienefeld K, Zautke F, Pronin D, Mazeed A (1999) Recording the proportion of damaged Varroa jacobsoni Oud. in the debris of honey bee colonies (Apis mellifera). Apidologie 30(4):249–256
Boecking O, Ritter W (1993) Grooming and removal behaviour of Apis mellifera intermissa in Tunisia against Varroa jacobsoni. J Apic Res 32(3–4):127–134
Boecking O, Spivak M (1999) Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 30(2–3):141–158
Boutin S, Alburaki M, Mercier PL, Giovenazzo P, Derome N (2015) Differential gene expression between hygienic and non-hygienic honeybee (Apis mellifera L.) hives. BMC Genomics 16(1):1–13
Büchler R, Drescher W, Tornier I (1992) Grooming behaviour of Apis cerana, Apis mellifera and Apis dorsata and its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps clareae. Exp Appl Acarol 16(4):313–319
Currie RW, Tahmasbi GH (2008) The ability of high-and low-grooming lines of honey bees to remove the parasitic mite Varroa destructor is affected by environmental conditions. Can J Zool 86(9):1059–1067
Dadoun N, Nait-Mouloud M, Mohammedi A, Sadeddine ZO (2020) Differences in grooming behavior between susceptible and resistant honey bee colonies after 13 years of natural selection. Apidologie 51:793–801
Dietemann V, Ellis JD, Neumann P (2013) The COLOSS BEEBOOK Volume I, Standard methods for Apis mellifera research: introduction. J Apic Res 52(4):1–4
Di Rienzo N, Montiglio PO (2016) The contribution of developmental experience vs. condition to life history, trait variation and individual differences. J. Anim. Ecol. 85(4):915–926
Fries I, Huazhen W, Wei S, Jin CS (1996) Grooming behavior and damaged mites (Varroa jacobsoni) in Apis cerana cerana and Apis mellifera ligustica. Apidologie 27(1):3–11
Guzmán-Novoa E, Emsen B, Unger P, Espinosa-Montaño LG, Petukhova T (2012) Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). J Invertebr Pathol 110(3):314–320
Hamiduzzaman MM, Emsen B, Hunt GJ, Subramanyam S, Williams CE, Tsuruda JM, Guzman-Novoa E (2017) Differential gene expression associated with honey bee grooming behavior in response to Varroa mites. Behav Genet 47(3):335–344
Invernizzi C, Zefferino I, Santos E, Sánchez L, Mendoza Y (2016) Multilevel assessment of grooming behavior against Varroa destructor in Italian and Africanized honey bees. J Apic Res 54(4):321–327
Kirrane MJ, De Guzman LI, Whelan PM, Frake AM, Rinderer TE (2018) Evaluations of the removal of Varroa destructor in Russian honey bee colonies that display different levels of Varroa sensitive hygienic activities. J Insect Behav 31(3):283–297
Mondet F, Alaux C, Severac D, Rohmer M, Mercer A, Le Conte Y (2015) Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci Rep 5:10454. https://doi.org/10.1038/srep10454
Mondet F, Beaurepaire A, McAfee A, Locke B, Alaux C, Blanchard S, Danka B, Le Conte Y (2020) Honey bee survival mechanisms against the parasite Varroa destructor: a systematic review of phenotypic and genomic research efforts. Int J Parasitol 50(6–7):433–447. https://doi.org/10.1016/j.ijpara.2020.03.005
Moore D, Angel JE, Cheeseman IM, Robinson GE, Fahrbach SE (1995) A highly specialized social grooming honey bee (Hymenoptera: Apidae). J Insect Behav 8:855–861
Moosbeckhofer R (1992) Observations on the occurrence of damaged Varroa mites in natural mite fall of Apis mellifera carnica. Apidologie 23(6):523–531
Moretto G, Gonçalves LS, De Jong D (1993) Heritability of Africanized and European honey bee defensive behavior against the mite Varroa jacobsoni. Rev Bras Genét 16:71–71
Morfin N, Given K, Evans M, Guzman-Novoa E, Hunt GJ (2020) Grooming behavior and gene expression of the Indiana “mite-biter” honey bee stock. Apidologie 51(2):267–275. https://doi.org/10.1007/s13592-019-00710-y
Morse RA, Miksa D, Masenheimer JA (1991) Varroa resistance in US honey bees. Am Bee J 131(7):433–434
Nganso BT, Fombong AT, Yusuf AA, Pirk CW, Stuhl C, Torto B (2017) Hygienic and grooming behaviors in African and European honeybees-New damage categories in Varroa destructor. PLoS ONE 12(6):e0179329
Panasiuk B, Skowronek W, Bieńkowska M, Gerula D, Węgrzynowicz P (2010) Age of worker bees performing hygienic behaviour in honeybee colony. J Apic Sci 54:109–115
Peng YS, Fang Y, Xu S, Ge L (1987) The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. J. Invertebr. Pathol. 49(1):54–60
Pritchard DJ (2016) Grooming by honey bees as a component of Varroa resistant behavior. J Apic Res 55(1):38–48
R Core Team (2017) R foundation for statistical computing; Vienna, Austria: 2018. R: A language and environment for statistical computing
Rosenkranz P, Fries I, Boecking O, Stürmer M (1997) Damaged Varroa mites in the debris of honey bee (Apis mellifera L) colonies with and without hatching brood. Apidologie 28(6):427–437
Russo RM, Liendo MC, Landi L et al (2020) Grooming behavior in naturally Varroa-resistant Apis mellifera colonies from north-central Argentina. Front Ecol Evol 8:361
Ruttner F, Hänel H (1992) Active defense against Varroa mites in a Carniolan strain of honeybee (Apis mellifera carnica Pollmann). Apidologie 23(2):173–187
Scannapieco AC, Lanzavecchia SB, Parreño MA, Liendo MC, Cladera JL, Spivak M, Palacio MA (2016) Individual precocity, temporal persistence, and task-specialization of hygienic bees from selected colonies of Apis mellifera. J Apic Sci 60(1):63–74
Scannapieco AC, Mannino MC, Soto G, Palacio MA, Cladera JL, Lanzavecchia SB (2017) Expression analysis of genes putatively associated with hygienic behavior in selected stocks of Apis mellifera L. from Argentina. Insectes Soc. 64(4):485–494
Szabo TI, Walker CRT (1995) Damages to dead Varroa jocobsoni caused by the larvae of Gal-leria mellonella. Am Bee J 135:421–422
Vandame R, Morand S, Colin ME, Belzunces L (2002) Parasitism in the social bee Apis mellifera: quantifying costs and benefits of behavioral resistance to Varroa mites. Apidologie 33:433–445
Acknowledgements
The authors thank Rodrigo Muchiut and Hernán Fain for their assistance with honey bee colony management.
Funding
This study was funded by Agencia Nacional de Promoción Científica y Tecnológica of Argentina through the project Foncyt-PICT 2016–0221 (ACS) and by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) through the project PIP 2015-0517CO (ACS). Financial support received from (i) Instituto Nacional de Tecnología Agropecuaria (INTA) through the doctoral scholarship resolution N°1126/2013 (RMR) and Grants 2019-PE-E1-I017-001 (MAP, GR) and 2019-PD-E4-I079-001 (SBL) and (ii) FONTAGRO through the project FTG/RF-16112-RG.
Author information
Authors and Affiliations
Contributions
ACS and RMR conceived and designed the research. RMR, LL, MCL, and IM conducted the experiments. IM and MCL contributed to statistical analyses. LL, HP, and JM provided field support and assistance with honey bee colony management. RR, MCL, SBL, and ACS analyzed the data and wrote the manuscript. AB, MAP, and GR contributed to the discussion of experimental design and results. All the authors read and reviewed drafts of the manuscript and approved its final version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: Peter Rosenkranz
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Russo, R.M., Landi, L., Muntaabski, I. et al. Age-performance and intensity of grooming behavior toward Varroa destructor in resistant and susceptible Apis mellifera colonies. Apidologie 53, 59 (2022). https://doi.org/10.1007/s13592-022-00971-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-022-00971-0