Abstract
The bacterium Paenibacillus larvae and the mite Varroa destructor are two of the most severe biotic stressors affecting honeybees and are responsible for American foulbrood and varroosis respectively. To control these pathogens, beekeepers regularly apply synthetic acaricides or antibiotics to parasitized hives. However, antibiotic and acaricide overuse over time leads to resistance in bacteria strains and mite populations respectively, not to mention the residual contamination of bee products with these chemicals. The development of alternative and effective control methods of bee diseases is therefore crucial. In recent years, natural substances from plant extracts have emerged as the basis of suitable control methods to treat bee colonies parasitized by both P. larvae and V. destructor. Our aim was to evaluate the bioactivity of ethanolic and methanolic hop leaf extract (species: Humulus lupulus L, varieties: Victoria, Spalt, and Cascade) against P. larvae, V. destructor, and A. mellifera. The bactericidal activity against P. larvae was evaluated by the broth microdilution method. Topical administration protocols were used to determine the bioactivity of hop extracts on V. destructor and A. mellifera. Total polyphenols, flavonoids, saponins, and antioxidant capacity were determined for each hop leaf extract tested. The Victoria extract had the highest concentration of phenolic compounds, whereas Cascade and Victoria extracts had higher concentrations of the glycoside saponin. All hop extracts presented low toxicity against A. mellifera bees after 48 h of topical administration (except for Cascade ethanolic extract which reached a maximum of 36% of bee mortality). Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values ranged from 0.69 to 2.75 mg/kg for the Cascade variety, 1.38 to 5.5 mg/kg for the Spalt variety, and 5.5 to 11 mg/kg for the Victoria variety. After 48 h, the acaricidal activity for the ethanolic extract of the Victoria variety reached a value close to 80%, while the methanolic extract of Cascade showed an acaricidal activity close to 70%. The results reported in this study support the potential use of methanolic and ethanolic extracts of hop leaves from Argentina as promising natural alternatives for varroosis and American foulbrood control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
American foulbrood (AFB) is the most severe bacterial disease affecting honeybees and has a nearly cosmopolitan distribution (Genersch 2010). AFB only kills infected honeybee larvae; however, if left untreated, it can eventually lead to the collapse of an entire colony. American foulbrood is very contagious and is therefore considered to be a notifiable disease in most countries (Djukic et al. 2014). AFB’s causative agent is Paenibacillus larvae, a flagellated gram-positive bacterium, whose main characteristic is the formation of highly resistant endospores that affect larval and pupal stages (Genersch et al. 2006). AFB was first described in Argentina, South America, in 1989, thus, establishing the first sanitary challenge in the Argentinean apiculture. Alippi (1992) has suggested that the arrival of P. larvae to Argentina was due to the import of infected bees from the USA. AFB quickly dispersed throughout Argentinean beekeeping centers (Alippi 1996), with incidences as high as 30% in some geographic areas (Marcangeli et al. 2005). In some countries, the use of antibiotics, particularly oxytetracycline hydrochloride (OTC) is the most common method for the prevention and treatment of infected colonies (Hansen and Brødsgaard 1999; Genersch et al. 2010). However, regular antibiotic applications can negatively affect bees, and increase the emergence of resistant strains (Martel et al. 2006). Currently, the presence of OTC resistant strains has been reported in Argentina, the USA, Italy, New Zealand, and the UK (Alippi et al. 1996; Miyagi et al. 2000). Prevention and control measures of AFB in South American countries generally include vigilance for an early diagnosis, the isolation of apiaries with cases of AFB, and the multiplication of healthy colonies with hygienic queens (Harriet et al. 2013). Brazilian, Chilean, and Uruguayan authorities specifically recommend a protocol of hive burning for the colonies that show clinical signs of the disease, as an attempt to contain possible outbreaks (Harriet et al. 2013). However, the use of antibiotics against P. larvae is prohibited in most South American countries with the exception of Argentina (De la Sota and Bacci 2005).
Varroosis is a disease caused by the ectoparasitic mite Varroa destructor (Anderson and Trueman 2000). This external mite has become a serious pest for most Apis mellifera populations around the globe (Nazzi and Le Conte 2016). Varroa destructor affects immature and adult bees by feeding on body fat tissue, inflicting mechanical damage in the process (Ramsey et al. 2019). The mite is also a known vector of numerous viral diseases (Martin et al. 2012). In order to prevent colony collapse, beekeepers attempt to control mite populations in hives by the application of different acaricidal treatments. Synthetic acaricides such as pyrethroids and organophosphates are regularly applied in an attempt to control mite infestations. However, the misuse of these acaricides over time has led to the emergence of several foci of resistant in mite populations worldwide (Elzen and Westervelt 2002; Maggi et al. 2009; Maggi et al. 2010; Maggi et al. 2011; Mitton et al. 2016). In addition, the residues of synthetic acaricides persist in bee products (Maggi et al. 2016; Medici et al. 2015) used for human consumption, making them toxic, and consequently affecting their commercialization (Bogdanov 2006). These issues highlight the necessity of finding new and safer methods of Varroa control. Natural acaricides are friendlier alternatives than synthetic, because they show lower toxicity in mammals, smaller environmental effect, and more accepted by the general public (Isman et al. 2001). Given this, organic acids such as lactic, formic, and oxalic acids (Eguaras et al. 2001) and plant-derived materials, especially monoterpenoids (Blenau et al. 2012) have been studied for many years for its use in Varroa control. Evidence suggests that such use of certain natural substances could maintain a low mite infestation rate, avoiding the collapse of the colony (Imdorf et al. 1999; Damiani et al. 2010).
The development of alternative and effective control methods to fight against AFB and Varroosis diseases are crucial. It is also primordial that the emerging alternatives of control ensure low or null bee toxicity when they are applied within beehives. To reach these objectives, alternative methods proposed include the use of natural bioactive substances, which includes plant extracts (Flesar et al. 2010; Sabate et al. 2012; Boligon et al. 2013; Damiani et al. 2014), essential oils (Alippi 1996; Fuselli et al. 2006; Ansari et al. 2016; Tutun et al. 2018), pure compounds extracted from plants, bacteria, or fungus (Fuselli et al. 2006; Maggi et al. 2010; Flesar et al. 2010; Sabate et al. 2012; Brasesco et al. 2017; Khan et al. 2009), and honeybee by-products, such as propolis (Antúnez et al. 2008; Bilikova et al. 2013, Fangio et al. 2019) and royal jelly (Bilikova et al. 2013).
One candidate in mite and bacterial control is the essential oil from the hop Humulus lupulus. The hop, Humulus lupulus L., is a widely known culture; its flowers are used in the beer-brewing industry to add bitterness and aroma to beer. Hop flowers are particularly useful due to the properties of their secondary metabolites; bitterness is promoted by the commonly called alfa- and beta-acids content in flowers (Small 2016) and the aroma is provided by their essential oils content (Karabín et al. 2016). Throughout history, breweries have developed different varieties of this species, including: Victoria, Spalt, and Cascade. These varieties could lead to extracts with different properties in disease control (Moir 2000). Interestingly, the study of hop flowers is a topic of interest since their polyphenolic substances were suggested as beneficial for human health (Ceh et al. 2007). Polyphenolics and saponins are commonly occurring as secondary metabolites in plants (Abram et al. 2015; Morrissey and Osbourn 1999), playing an important role in plant defense mechanisms against biotic and abiotic external agents. Karabín et al. (2016) reported that hops exhibit a very wide spectrum of antioxidant activity, specially attributed to the polyphenol fractions. Farjan et al. (2012) observed that feeding bees with antioxidants leads to positive effects like an increase of protein uptake and a lower mortality during winter.
The application of H. lupulus extracts as an alternative for controlling bee diseases has been poorly explored. Until now, only one commercial product named Hope Guard is applied as a varroocide in beekeeping. This acaricide is based on high proportions of alfa- and beta-acids extracted from hop cones (DeGrandi-Hoffman et al. 2012). Hope Guard demonstrated successful Varroa control in infested bee colonies (Bedini et al. 2012) and bee packages during winter (Rademacher et al. 2015). Until now, no other extract from H. lupulus was tested against V. destructor and P. larvae. In addition, only the hop cones have been used in the beer-making industry, thus, hop leaves are an agricultural by-product currently discarded as waste.
Our aim was to evaluate extracts made of three varieties of hop leaves (harvested in Argentina) against V. destructor, P. larvae, and A. mellifera, and to relate their chemical composition (ethanolic- and methanolic-based) with its bioactivity.
2 Material and methods
2.1 Biological material
Paenibacillus larvae strains were isolated from beehives that exhibited clinical symptoms of AFB, located in the provinces of Buenos Aires, Córdoba, and Entre Ríos in Argentina. C1 and C2 strains were from Balcarce-Buenos Aires (37° 52′ S, 58° 15′ W), C6 from Rio Cuarto-Cordoba (33° 08′ 00″ S, 64° 21′ 00″ O), and C9 from Concordia-Entre Rios (31° 23′ 32″ S, 58° 01′ 01″ O).
The identification of each strain was performed by using the following method: Bacterial colonies were grown and maintained on 2% (w/v) MYPGP-agar plates (Mueller-Hinton broth 1% (w/v), yeast extract 1.5% (w/v), K2HPO4 0.3% (w/v), glucose 0.2% (w/v), sodium pyruvate 0.1% (w/v), and agar 2% (w/v)), and incubated at 37 °C and 10% (v/v) CO2 for 48 h. The bacterial inoculum was prepared in sterilized peptone water (peptone 0.1% (w/v) and sodium chloride 0.85% (w/v)) to a final optical density of 0.1 (OD600) determined by a UV-VIS spectrophotometer spectrum SP-1103 (Spectrum Instruments Company Ltd., Shanghai, China). Brain-heart infusion (3.7%, w/v) was used as a growth media during the broth microdilution assay. P. larvae colony development was monitored by using resazurin sodium salt.
Adult females of V. destructor and A. mellifera worker bees were collected from the experimental station of Santa Paula belonging to the Centro de Investigación en Abejas Sociales (CIAS, National University of Mar del Plata), near to the city of Mar del Plata, Buenos Aires, Argentina (38° 10′ 06′′ S, 57° 38′ 10′′ O). Adult female mites were collected from capped brood frames by the opening and inspection of each individual cell. In order to avoid starvation, mites were kept on bee larvae or pupae in Petri dishes during the collection process. Mites that showed evidence of recent molting, weakness, or any kind of abnormality were all discarded. The Varroa used is part of the Korean haplotype (Maggi et al. 2012), and is cosmopolitan in the studied area. Adult worker bees walking on brood combs were picked up from colonies and inspected for Varroa before being settled for bioassays.
Hop leaves were obtained from a local farm belonging to Granja de Lúpulo MdP, placed nearby Mar del Plata city, Buenos Aires, Argentina (38° 10′ 06′′ S, 57° 38′ 10′′ O). At least 100 g of dry leaves from each of three different hop varieties (Cascade, Spalt, and Victoria) were collected to prepare ethanolic and methanolic extracts. They were dried out at room temperature.
2.2 Ethanolic and methanolic extracts of hop leaves
The extraction of secondary metabolites coming from Victoria, Cascade, and Spalt varieties was carried out by using two solvent mixtures: methanol-water and ethanol-water (50:50). The extraction was performed by placing 1 gram of leaves (in triplicate) in 30 mL of the solvents for 3 h in an ultrasound bath at 40 °C. Then, the suspension was centrifuged for 10 min at 8000 rpm. The obtained supernatant was placed in falcon tubes (Kowalczyk et al. 2013).
2.3 Chemical characterization of hop leaf extracts
Content of total phenolic compounds
The content of the total phenolic compounds was determined by the colorimetric method of Singleton and Rossi (1965) with modifications of a 1:10 dilution of Folin-Ciocalteau reagent. Twenty microliters of the ethanolic extracts or its dilutions were placed in a 96-well microplate and left to rest for 8 min. Subsequently, 80 μL of 20% Na2CO3 were added. After 2 h in the dark, the absorbance was read at 765 nm. Gallic acid solutions between 0 and 100 μg/mL were used to construct the calibration curve. The results were expressed as milligram (mg) equivalents of gallic acid (GAE)/g of processed leaves. The values were presented as the mean of triplicated assays ± its standard deviation.
Total flavonoid content
The total content of flavonoids was determined by the method reported by Kumazawa et al. (2004) with some modifications. One and a half milliliters of 2% AlCl3 ethanolic solution was added to 1.5 mL of extract. The absorbance was measured at 420 nm after 1 h of incubation at room temperature. The total flavonoid content was calculated from a calibration curve, and the results were expressed as mg of quercetin equivalent/g of dry weight.
Saponin content
Saponin content was estimated by using two methods: the Liebermann-Buchard test and the Foam test.
Liebermann-Buchard test
The extracts were dried using a rotary evaporator and resuspended in chloroform up to 1 mg/ml. A solution of the crude extract in 1 ml of chloroform, was mixed with 10 drops of refrigerated acetic anhydride. Then drops of concentrated sulfuric acid were added through the wall contact technique. Reddish coloration can infer the presence of triterpenic saponins.
Foam test
0.50 ± 0.02 g of leaves were weighed and placed in a tube. Five milliliters of distilled water was added to the tube and vigorously shaken for 30 s. The tube was then left to rest for 30 min, before being shaken again for 20 more seconds. After standing for 30 min, the tube was shaken once more for 30 s and then left to stand for 5 min. Finally, the height of the foam was measured to an accuracy of 0.1 cm. This procedure was carried out three times for each sample. The saponins content was then calculated using the formula of Elias and Diaz (1988).
Antioxidant capacity
The diphenylpicrylhydrazyl colored radical (DPPH) in a trapping reaction measured by visible absorption through spectrophotometry, estimating the antioxidant activity of the extracts. Two milliliters of a DPPH stock solution in ethanol (0.03 mg/mL, initial maximum absorbance A518 = 0.466) were placed in a spectrophotometry glass cuvette (1 cm optical path). In each test, 1 mL of the extract was diluted in ethanol and mixed in the cuvette containing the DPPH stock solution. The temporal evolution of the absorbance at 518 nm (the maximum absorption determined for DPPH in the same solvent) was observed, and the percentage of inhibition of the radicals at a fixed time (between 400 and 1000 s after the reaction starts) was evaluated. The plot of % inhibition vs. the final concentration of the extract in the spectrophotometric cell was used to determine the IC50 parameter, i.e. the concentration of the extract able to inhibit 50% of the initial DPPH radicals. Under the same conditions, an assay for ascorbic acid in ethanol was also carried out in order to establish its IC50 as a reference value.
2.4 Antimicrobial activity
The antimicrobial activity of each extract was determined by the broth microdilution method (Cugnata et al. 2017) on four P. larvae strains (C1, C2, C6, C9). The extract concentration ranges from the minimum concentration of the extract in which in vitro bacterial growth inhibition is observed (MIC) (De Graaf et al. 2013) to the minimum inhibitory concentration of the extract when in vitro bacterial growth inhibition is not observed (MNIC). Trials involved different replica within a day (n = 3) and between days (n = 3) for each determination.
In order to obtain the minimum bactericidal concentration (MBC), an aliquot was taken from each well where no bacterial growth was observed, and its content was sown onto the surface of a MYPGP plate. This aliquot was then incubated at 37 °C for 48 h under microaerophilic conditions (O2 10% (v/v)). Then, the CFU/mL were counted, considering a turbidity of 0.5 based on the McFarland scale of 100 μL of suspension containing 1.0 × 108 CFU/mL of bacteria (McFarland 1907).
2.5 Bioactivity of hop leaves extracts against V. destructor and A. mellifera
The three varieties of hop leaves extracts were tested against Varroa mites using the complete exposure method proposed by Ruffinengo et al. (2005). The obtained solutions for each ethanolic and methanolic extract were homogeneously applied (1 mL solution/capsule) on the inner surface of a 10-cm diameter petri dish. As controls, 1 mL of ethanol:water (50:50) and methanol:water (50:50) solutions were applied. For each treatment, 5 replicates were performed. After solvent evaporation, 5 adult worker bees and 5 mites were placed in each Petri dish with a feeder made of water and candy (powdered sugar). The capsules were incubated at 30 °C and 70% RH. The mortality of mites and bees at 24 and 48 h was recorded for each treatment.
2.6 Statistic methodologies
An analysis of variance (ANOVA) with post hoc Tukey was used to compare the values for polyphenols and flavonoids content with 0.05 of significance. All the analyses were carried out by using SPSS 15.0 for Windows.
The differences between the MICs obtained for each extract against P. larvae were analyzed by means of ANOVA of one and two ways. For A. mellifera and V. destructor, a dose-response analysis and the Mantel-Cox test were performed to compare the extracts by using GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com.
3 Results
3.1 Chemical composition of hop leaves extracts
The contents of phenolic compounds in the extracts are reported in Table I. Victoria extracts had the highest volumes of phenolic compounds compared to other varieties of crops; these compounds were also higher in ethanol extractions than methanol. The Spalt variety showed higher phenolic composition than the Cascade variety (p ≤ 0.05) (Table I).
The flavonoid content in the extracts is reported in Table I. Victoria extracts had the highest volume of flavonoids content in ethanolic and methanolic extracts. These results are in line with the tendency observed for the content of phenolic compounds (p ≤ 0.05).
Qualitative analysis of ethanolic extracts showed the presence of triterpene saponins in all three hop varieties. However, triterpene saponins could only be detected in Cascade methanolic extracts (Table I; p ≤ 0.05). The content of saponins in ethanolic extracts was 120 μg/g (± 12), 257 μg/g (± 36), and 268 μg/g (± 15) for Victoria, Spalt, and Cascade varieties, respectively.
3.2 Antioxidant activity of hop leave extracts
The antioxidant activity was determined in terms of IC50 for DPPH radicals. This parameter represents the concentration needed to cause 50% inhibition of the radical probe; thus, lower IC50 values imply larger antioxidant effect. Substances that are able to perform this reaction can be considered as antioxidants and therefore radical scavengers (Brand-Williams et al. 1995). The Cascade variety had the highest values of IC50 for both alcoholic extracts. The Victoria variety had the best antioxidant activity. The rest of the varieties showed similar IC50 values among them (Table I). The IC50 for ascorbic acid, taken as the reference antioxidant, was at least two orders of magnitude inferior.
3.3 Antimicrobial activity
Table II shows the results of the MIC and MBC values obtained for the 4 strains of P. larvae tested. They ranged from 0.69 to 2.75 mg/kg for Cascade variety, 1.38 to 5.5 mg/kg for Spalt variety, and 5.5 to 11 mg/kg for Victoria variety. All these values were lower than those for OTC (10/15 mg/kg) (Alippi et al. 1996).
3.4 Bioactivity of hop leaves extracts against V. destructor and A. mellifera
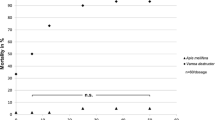
Dose-response curves were obtained for mites and bees (Figure 1). Bee and mite mortalities (Table III) were both registered after 24 and 48 h of extract exposure. Among methanolic extracts, Cascade extract was the most toxic for mites (52% and 68% at 24 h and 48 h respectively). The ethanolic extract from the Victoria variety presented the highest toxicity against V. destructor (48% and 80% at 24 h and 48 h respectively). Conversely, the ethanolic Cascade extract was the least toxic for mites (p < 0.001; Tables III and IV). The dose-response data for mites and bees (Table IV) showed that both types of hop extracts were different in mite mortality compared to controls.
After 48 h of exposure, methanolic extracts from the Cascade variety exhibited a maximum bee mortality of 18%, while ethanol-water extracts of Cascade exhibited a maximum bee mortality of 36% (Figure 1). Both types of hop extracts were different in bee mortality with respect to controls (p < 0.05; Table IV).
4 Discussion
The biological activity of extracts depends on the polyphenols and saponins chemical structure as well as on the number and nature of other components (Kaiser et al. 2013; Daglia 2012). Polyphenols and saponins regularly are secondary metabolites in plants (Abram et al. 2015; Morrissey and Osbourn 1999), playing an important role in the stress response and plant defense mechanisms against biotic and abiotic external agents (Athanasiadou and Kyriazakis 2004; Khan et al. 2008). The antimicrobial activity of polyphenols is based on the inhibition of DNA replication, in bacteria, fungi, and protozoan parasites. This antimicrobial activity is dependent upon the hydrophobic character of the compounds because of their interaction with the bacterial cell wall (Gerhäuser 2005). A large number of the biological effects of saponins on cell membranes have already been noted, including their ability to form pores in membranes which has contributed to their common use in physiological research (Choi et al. 2006; Menin et al. 2001; Plock et al. 2001; Bangham & Horne (1962)).
The antimicrobial activity reveals that MIC and MBC values obtained for the different extracts in this work were equal. Based on the classification by Duarte et al. (2007) all the extracts analyzed in this work are considered as strong inhibitors. In our study, MIC/MBC values were lower than those reported for OTC (10/15 mg/kg respectively) by Alippi et al. (1996) and similar to those obtained by Flesar (2010), where extracts of H. lupulus flowers were tested against P. larvae strains (2–4 mg/kg). Methanolic and ethanolic extracts from the Cascade variety had the best activity against P. larvae (MIC 0.69 to 2.75 mg/kg). Interestingly, Cascade extracts presented lower concentration of polyphenols but were positive for saponins (Table I). Based on our study, we think that these saponins facilitate the entrance of lethal chemical compounds in P. larvae. Further studies should explore if there is any synergistic effect among saponins and polyphenols in its bactericidal effect.
Although previous studies have tested the miticide effects of hop beta-acids against V. destructor (Bedini et al. 2012; Rademacher et al. 2015), this is the first record of leaf extract in polar solvents being tested against V. destructor, P. larvae, and A. mellifera. Beta-acids are weak organic acids produced by hop plants (Jones et al. 2003), and they have been reported as a repellent of sucking plant pests including the two-spotted spider mites (Tetranychus urticae) (Jones and Brassington 1998) and the hop aphid (Phorodon humuli) (Hampton et al. 2002; Jones et al. 2003). Our extracts were rich in polyphenols. Previous studies reported insecticidal effects for some extracts of H. lupulus, where its principal component was the polyphenol xanthohumol, as affecting adults and larvae of several insect species (Karaca and Gökçe 2014; Bedini et al. 2015). These authors have reported that such toxic effects depend on the insect species, type of extract, and dose concentration. Here, we obtained a 36% of bee mortality after 48 h of exposure for an ethanolic Cascade extract. Apart from that, no other extracts demonstrated toxicity for bees. Our results are in accordance with Flesar et al. (2010), who tested hop flower extracts against bees and reported no lethal results.
Regarding V. destructor, the dose tests of polar extracts (ethanolic and methanolic) reached toxicity values between 28 and 80%. Victoria ethanolic extract was the most toxic for V. destructor, showing 80% of mite mortality and low toxicity for bees after 48 h of trial. These results are promising since we obtained a low-dose-response in the sought effect of the leaves extracts. Previously, Damiani et al. (2010) had reported an average mortality of 56.7% of mites treated with 100.00 mg/kg of propolis solution. The same authors tested the acaricidal effects of a botanical extract of Baccharis flabellata and Minthostachys verticillata against V. destructor and reported that 11.40 and 14.40 mg/kg were needed respectively in order to kill the 50% of mites. In this context, to test a botanical extract of hop leaves in the field against V. destructor seems to be promising even considering higher extract doses.
The antioxidant activity of hop extracts reported should also be considered. The antioxidant activity is the ability of bioactive compounds to prevent, delay, and protect against oxidation of various substrates such as DNA and lipid materials, both in living organisms (e.g., humans) and in food products (Gutteridge and Halliwell 1994; Shahidi 2000; Naczk and Shahidi 2004). Although the extracts studied here presented less antioxidant activity compared with other plants (Ahmad et al. 2013; Lu et al. 2014; Miguel et al. 2014a, b) or propolis extracts (Miguel et al. 2014a, b), the values reported were similar to those reported on bee pollen (Carpes et al. 2009). In this context, bactericide and acaricide formulations based on plant extracts could produce extra benefits to bees if they are designed to be applied by oral administrations, generating pathogen mortality and ensuring better functioning of bee antioxidant systems.
The results presented here are encouraging and may highlight the role of specific chemical compounds and their mixtures on pathogen mortality. Capitalizing on the use of natural products is of paramount importance since they avoid the problems associated with the use of synthetic antibiotics, such as the prevalence of toxic residues within the hive and the appearance of resistant strains (Martel et al. 2006). Hop leaf extracts appear to be a promising alternative for bee pathogen control and demonstrate the desirable characteristics proposed by the OECD (1998): “good antimicrobial action, acaricidal activity, and specially, low toxicity for bees.” Therefore, we recommend further testing of these types of extracts in the field. Finally, we would also encourage the utilization of the leaf waste by-products of the hop industry, which are generated in large quantities and currently disposed of as waste. These could provide the materials for novel eco-friendly bee pathogen control programs.
References
Abram, V., Čeh, B., Vidmar, M., Hercezi, M., Lazić, N., et al. (2015) A comparison of antioxidant and antimicrobial activity between hop leaves and hop cones. Ind. Crop. Prod. 64, 124-134.
Ahmad, A., Husain, A., Mujeeb, M., Khan, S. A., Najmi, A. K., Siddique, N. A., et al. (2013) A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 3(5), 337-352.
Alippi, A. M. (1992) Transporte de esporas de Bacillus larvae por el ácaro Varroa jacobsoni. Rev. Fac. Agron. 68, 83-86.
Alippi, A. M. (1996) Caracterización de aislamientos de Paenibacillus larvae mediante tipo bioquímico y resistencia a oxitetraciclina. Rev. Argent. Microbiol. 28(4), 197-203.
Alippi, A. M., Ringuelet, J. A., Cerimele, E. L., Re, M. S., & Henning, C. P. (1996). Antimicrobial activity of some essential oils against Paenibacillus larvae, the causal agent of American foulbrood disease. Journal of herbs, spices & medicinal plants, 4(2), 9-16.
Anderson, D. L., Trueman, J. W. H. (2000) Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 24(3), 165-189.
Ansari, M. J., Al-Ghamdi, A., Usmani, S., Al-Waili, N., Nuru, A., Sharma, D., Khan, K. A., Kaur, M., Omer, M. (2016) In vitro evaluation of the effects of some plant essential oils on Paenibacillus larvae, the causative agent of American foulbrood, Biotechnol. Biotechnol. Equip. 30(1), 49-55. DOI: https://doi.org/10.1080/13102818.2015.1086690
Antúnez, K., Harriet, J., Gende, L., Maggi, M., Eguaras, M., Zunino, P. (2008) Efficacy of natural propolis extract in the control of American Foulbrood. Vet. Microbiol. 131(3-4), 324-331.
Athanasiadou, S., Kyriazakis, I. (2004) Plant secondary metabolites: antiparasitic effects and their role in ruminant production systems. Proc. Nutr. Soc. 63(4), 631-639.
Bangham, A. D., & Horne, R. W. (1962) Action of saponin on biological cell membranes. Nature. 196(4858), 952-953.
Bedini, S., Flamini, G., Girardi, J., Cosci, F., Conti, B. (2015) Not just for beer: evaluation of spent hops (Humulus lupulus L.) as a source of eco-friendly repellents for insect pests of stored foods. J. Pest. Sci. 88(3), 583-592.
Bilikova, K., Popova, M., Trusheva, B., Bankova, V. (2013) New anti-Paenibacillus larvae substances purified from propolis. Apidologie. 44(3), 278-285.
Blenau, W., Rademacher, E., Baumann, A. (2012) Plant essential oils and formamidines as insecticides/acaricides: what are the molecular targets?. Apidologie. 43(3), 334-347.
Bogdanov, S. (2006) Contaminants of bee products. Apidologie. 37, 1-18.
Boligon, A. A., de Brum, T. F., Zadra, M., Piana, M., dos Santos Alves, C. F., Fausto, V. P., dos Santos Barboza V. J., de Almeida Vaucher, R., Santos, R. C. V., Athayde, M. L. (2013) Antimicrobial activity of Scutia buxifolia against the honeybee pathogen Paenibacillus larvae. J. Invertebr. Pathol. 112(2), 105-107.
Brand-Williams, W., Cuvelier, M. E., Berset, C. L. W. T. (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28(1), 25-30.
Brasesco, C., Gende, L., Negri, P., Szawarski, N., Iglesias, A., Eguaras, M., Ruffinengo, S., Maggi, M. (2017) Assessing in Vitro acaricidal effect and joint action of a binary mixture between essential oil compounds (Thymol, Phellandrene, Eucalyptol, Cinnamaldehyde, Myrcene, Carvacrol) over ectoparasitic mite Varroa destructor (Acari: Varroidae). J. Apic Sci. 61(2), 203-215.
Carpes, S. T., Mourão, G. B., De Alencar, S. M., Masson, M. L. (2009) Chemical composition and free radical scavenging activity of Apis mellifera bee pollen from Southern Brazil. Braz. J. Food. Technol. 12(1/4), 220-229.
Ceh, B., Kac, M., Košir, I., Abram, V. (2007) Relationships between xanthohumol and polyphenol content in hop leaves and hop cones with regard to water supply and cultivar. Int. J. Mol. Sci. 8(9), 989-1000.
Choi, J. H., Keum, K. C., Lee, S. Y. (2006) Production of recombinant proteins by high cell density culture of Escherichia coli. Chem. Eng. Sci. 61(3), 876-885.
Cugnata, N. M., Guaspari, E., Pellegrini, M. C., Fuselli, S. R., Alonso-Salces, R. M. (2017) Optimal concentration of organic solvents to be used in the broth microdilution method to determine the antimicrobial activity of natural products against Paenibacillus larvae. J. Apic. Sci. 61(1), 37-53.
Daglia, M. (2012) Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 23(2), 174-181.
Damiani, N., Fernández, N. J., Maldonado, L. M., Álvarez, A. R., Eguaras, M. J., Marcangeli, J. A. (2010) Bioactivity of propolis from different geographical origins on Varroa destructor (Acari: Varroidae). Parasitol. Res. 107(1), 31-37.
Damiani, N., Fernández, N. J., Porrini, M.P., Gende, L. B., Alvarez, E., Buffa, F., Brasesco, C., Maggi, M., Marcangeli, J. A., Eguaras, M. J. (2014) Laurel leaf extracts for honeybee pest and disease management: antimicrobial, microsporicidal, and acaricidal activity. Parasitol. Res. 113, 701-709. https://doi.org/10.1007/s00436-013-3698-3
De Graaf, D. C., Alippi, A. M., Antúnez, K., Aronstein, K. A., Budge, G., De Koker, D., De Smet, L., Dingman, D. W., Evans, J. D., Foster, L. J., Fünfhaus, A., Garcia-Gonzalez, E., Gregore, A., Human, H., Murray, K. D., Nguyen B. K., Poppinga, L., Spivak, M., van Engelsdorp, D., Wilkins, S., Genersch, E. (2013) Standard methods for American foulbrood research. J. Apic. Res. 52(1), 1-28.
DeGrandi-Hoffman, G., Ahumada, F., Probasco, G., & Schantz, L. (2012). The effects of beta acids from hops (Humulus lupulus) on mortality of Varroa destructor (Acari: Varroidae). Experimental and Applied Acarology, 58(4), 407-421.
De la Sota, M., Bacci, M. (2005) Enfermedades de las abejas. Manual de procedimientos (SENASA). Dirección Nacional de Sanidad Anim. 3, 18-29.
Djukic, M., Brzuszkiewicz, E., Fünfhaus, A., Voss, J., Gollnow, K., Poppinga, L., Liesegang, H., Garcia-Gonzalez, E., Genersch, E., Daniel, R. (2014) How to kill the honey bee larva: genomic potential and virulence mechanisms of Paenibacillus larvae. PLoS One 9(3), e90914.
Duarte, M. C. T., Leme, E. E., Delarmelina, C., Soares, A. A., Figueira, G. M., Sartoratto, A. (2007) Activity of essential oils from Brazilian medicinal plants on Escherichia coli. J. Ethnopharmacol. 111(2), 197-201.
Eguaras, M., Del Hoyo, M., Palacio, M. A., Ruffinengo, S., Bedascarrasbure, E. L. (2001) A new product with formic acid for Varroa jacobsoni Oud. control in Argentina. I. Efficacy. J. Veterinary Med. Ser. B 48(1), 11-14.
Elias, C., Diaz, L. (1988) Determinación espectrofotométrica de ácido oleanolico y saponinas dequinua (Chenopodium quinua Willd, variedad Kancolla). Arch. Latinoam. Nutr. 1, 113-131.
Elzen, P. J., Westervelt, D. (2002) Detection of coumaphos resistance in Varroa destructor in Florida. Am. Bee J. 142(4), 291-292.
Fangio, M. F., Orallo, D. E., Gende, L. B., Churio, M. S. (2019) Chemical characterization and antimicrobial activity against Paenibacillus larvae of propolis from Buenos Aires province, Argentina. J. Apic. Res. 58(4), 626-638.
Farjan, M., Dmitryjuk, M., Lipiński, Z., Biernat-Łopieńska, E., Żółtowska, K. (2012) Supplementation of the honey bee diet with vitamin C: The effect on the antioxidative system of Apis mellifera carnica brood at different stages. J. Apic. Res. 51(3), 263-270.
Flesar, J., Havlik, J., Kloucek, P., Rada, V., Titera, D., Bednar, M., Stropnicky M., Kokoska, L. (2010) In vitro growth-inhibitory effect of plant-derived extracts and compounds against Paenibacillus larvae and their acute oral toxicity to adult honey bees. Vet. Microbiol. 145(1-2), 129-133.
Fuselli, S. R., de la Rosa, S. G., Gende, L. B., Eguaras, M. J., Fritz, R. (2006) Inhibición de Paenibacillus larvae empleando una mezcla de aceites esenciales y timol. Rev. Argent. Microbiol. 38(2), 89-92.
Genersch, E. (2010) American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol. 103, S10-S19.
Genersch, E., Forsgren, E., Pentikäinen, J., Ashiralieva, A., Rauch, S., Kilwinski, J., Fries, I. (2006) Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int. J. Syst. Evol. Microbiol. 56(3), 501-511.
Genersch, E., Von Der Ohe, W., Kaatz, H., Schroeder, A., Otten, et al. (2010) The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie. 41(3), 332-352.
Gerhäuser, C. (2005) Broad spectrum anti-infective potential of xanthohumol from hop (Humulus lupulus L.) in comparison with activities of other hop constituents and xanthohumol metabolites. Mol. Nutr. Food Res. 49(9), 827-831.
Gutteridge, J., Halliwell, B. (1994) Antioxidants in nutrition, health, and disease. Oxford University Press, Oxford.
Hampton, R., Nickerson, G., Whitney, P., Haunold, A. (2002) Comparative chemical attributes of native North American hop, Humulus lupulus var. lupuloides E. Small. Phytochemistry. 61(7), 855-862.
Hansen, H., Brødsgaard, C. J. (1999) American foulbrood: a review of its biology, diagnosis and control. Bee World. 80(1), 5-23.
Harriet, J., Campa, J., Mendoza, Y. (2013) Loque Americana: Cartilla N°22. [online] http://www.ainfo.inia.uy/digital/bitstream/item/3821/1/Cartilla-22.pdf (Accessed on 27 July 15)
Imdorf, A., Bogdanov, S., Ochoa, R. I., Calderone, N. W. (1999) Use of essential oils for the control of Varroa jacobsoni Oud. in honey bee colonies. Apidologie. 30(2-3), 209-228.
Isman, M. B., Wan, A. J., Passreiter, C. M. (2001) Insecticidal activity of essential oils to the tobacco cutworm. Spodoptera litura. Fitoterapia. 72(1), 65-68.
Jones, D. L., Brassington, D. S. (1998). Sorption of organic acids in acid soils and its implications in the rhizosphere. Eur. J. Soil Sci. 49(3), 447-455.
Jones, D. L., Dennis, P. G., Owen, A. G., Van Hees, P. A. W. (2003) Organic acid behavior in soils-misconceptions and knowledge gaps. Plant Soil 248(1-2), 31-41.
Kaiser, S., Verza, S. G., Moraes, R. C., Pittol, V., Peñaloza, E. M. C., Pavei, C., Ortega, G. G. (2013) Extraction optimization of polyphenols, oxindole alkaloids and quinovic acid glycosides from cat's claw bark by Box–Behnken design. Ind. Crop. Prod. 48, 153-161.
Karabín, M., Hudcová, T., Jelínek, L., Dostálek, P. (2016) Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 15(3), 542-567.
Karaca, İ. Ç., Gökçe, A. (2014) Toxic and behavioural effects of plant extracts to greenhouse whitefly [Trialeurodes vaporariorum (Westw.) (Hemiptera: Aleyrodidae)]. Türkiye Entomoloji Dergisi. 38(4), 459-466.
Khan, M., Singh, J., Singh, I. (2008) Plasmalogen deficiency in cerebral adrenoleukodystrophy and its modulation by lovastatin. J. Neurochem. 106(4), 1766-1779.
Khan, M. S. A., Zahin, M., Hasan, S., Husain, F. M., Ahmad, I. (2009) Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 49(3), 354-360.
Kowalczyk, D., Świeca, M., Cichocka, J., Gawlik-Dziki, U. (2013) The phenolic content and antioxidant activity of the aqueous and hydroalcoholic extracts of hops and their pellets. J. Inst. Brew. 119(3), 103-110.
Kumazawa, S., Hamasaka, T., Nakayama, T. (2004) Antioxidant activity of propolis of various geographic origins. Food Chem. 84(3), 329-339.
Lu, C. L., Zhu, W., Wang, M., Xu, X. J., Lu, C. J. (2014). Antioxidant and anti-inflammatory activities of phenolic-enriched extracts of Smilax glabra. Evid. Based Complement. Alternat. Med., https://doi.org/10.1155/2014/910438
Maggi, M. D., Sardella, N. H., Ruffinengo, S. R., Eguaras, M. J. (2009) Morphotypes of Varroa destructor collected in Apis mellifera colonies from different geographic locations of Argentina. Parasitol. Res. 105(6), 1629-1636.
Maggi, M. D., Ruffinengo, S. R., Negri, P., Eguaras, M. J. (2010) Resistance phenomena to amitraz from populations of the ectoparasitic mite Varroa destructor of Argentina. Parasitol. Res. 107(5), 1189-1192.
Maggi, M. D., Ruffinengo, S. R., Mendoza, Y., Ojeda, P., Ramallo, G., Floris, I., Eguaras, M. J. (2011). Susceptibility of Varroa destructor (Acari: Varroidae) to synthetic acaricides in Uruguay: Varroa mites’ potential to develop acaricide resistance. Parasitol. Res. 108(4), 815-821.
Maggi, M., Medici, S., Quintana, S., Ruffinengo, S., Marcángeli, J., Martinez, P. G., Fuselli, S., Eguaras, M. (2012) Genetic structure of Varroa destructor populations infesting Apis mellifera colonies in Argentina. Exp. Appl. Acarol. 56(4), 309-318.
Maggi, M., Antúnez, K., Invernizzi, C., Aldea, P., Vargas, M., et al. (2016). Honeybee health in South America. Apidologie. 47(6), 835-854.
Marcangeli, J., del Carmen García, M., Vega, C., Quiroga, A., Martín, M. L., Distéfano, L., Cano, G. (2005) Estudio sobre la eficacia a campo del Amivar® contra Varroa destructor (Mesostigmata: Varroidae) en Colmenas de Apis mellifera (Hymenoptera: Apidae). Rev. Soc. Entomol. Argent. 64(1-2), 29-33.
Martel, A. C., Zeggane, S., Drajnudel, P., Faucon, J. P., Aubert, M. (2006) Tetracycline residues in honey after hive treatment. Food Addit. Contam. 23(3), 265-273.
Martin, S. J., Highfield, A. C., Brettell, L., Villalobos, E. M., Budge, G. E., Powell, M., Nikaido, S., Schroeder, D. C. (2012) Global honey bee viral landscape altered by a parasitic mite. Science. 336(6086), 1304-1306.
McFarland, J. (1907) The nephelometer: an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J. Am. Med. Assoc. 49(14), 1176-1178.
Medici, S. K., Maggi, M. D., Sarlo, E. G., Ruffinengo, S., Marioli, J. M., Eguaras, M. J. (2015) The presence of synthetic acaricides in beeswax and its influence on the development of resistance in Varroa destructor. J. Apic. Res. 54(3), 267-274.
Menin, L., Panchichkina, M., Keriel, C., Olivares, J., Braun, U., Seppert, E. K., Saks, V. A. (2001) Macrocompartmentation of total creatine in cardiomyocytes revisited. Mol. Cell. Biochem. 220(1-2), 149-159.
Miguel, M. G., Doughmi, O., Aazza, S., Antunes, D., Lyoussi, B. (2014a) Antioxidant, anti-inflammatory and acetylcholinesterase inhibitory activities of propolis from different regions of Morocco. Food Sci. Biotechnol. 23(1), 313-322.
Miguel, M. G., Nunes, S., Dandlen, S. A., Cavaco, A. M., Antunes, M. D. (2014b) Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve, South Portugal. Food Sci. Technol. 34(1), 16-23.
Mitton, G. A., Quintana, S., Giménez Martínez, P., Mendoza, Y., Ramallo, G., Brasesco, C., Villalba, A., Eguaras, M. J., Maggi, M., Ruffinengo, S. R. (2016) First record of resistance to flumethrin in a varroa population from Uruguay. J. Apic. Res. 55(5), 422-427.
Miyagi, T., Peng, C. Y., Chuang, R. Y., Mussen, E. C., Spivak M. S. (2000) Verification of oxytetracycline-resistant American foulbrood pathogen Paenibacillus larvae in the United States. J. Invertebr. Pathol. 75(1), 95-96.
Moir, M. (2000) Hops: a millennium review. J. Am. Soc. Brew. Chem. 58(4), 131-146.
Morrissey, J. P., Osbourn, A. E. (1999) Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol. Mol. Biol. Rev. 63(3), 708-724.
Naczk, M., Shahidi, F. (2004) Extraction and analysis of phenolics in food. J. Chromatogr. A 1054(1-2), 95-111.
Nazzi, F., Le Conte, Y. (2016) Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu. Rev. Entomol. 61(1), 417-432.
OECD (1998) OECD guideline for testing of chemicals. Test No 213: Honey bees, acute oral toxicity test
Plock, A., Sokolowska-Köhler, W., Presber, W. (2001) Application of flow cytometry and microscopical methods to characterize the effect of herbal drugs on Leishmania spp. Exp. Parasitol. 97(3), 141-153.
Rademacher, E., Harz, M., Schneider, S. (2015). The development of HopGuard® as a winter treatment against Varroa destructor in colonies of Apis mellifera. Apidologie. 46(6), 748-759.
Ramsey, S. D., Ochoa, R., Bauchan, G., Gulbronson, C., Mowery, J. D., Cohen, A., Lim, D., Joklik, J., Cicero, J. M., Ellis, J. D., Hawthorne, D., Hawthorne, D. (2019) Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. 116(5), 1792-1801.
Ruffinengo, S., Eguaras, M., Floris, I., Faverin, C., Bailac, P., Ponzi, M. (2005) LD50 and repellent effects of essential oils from Argentinian wild plant species on Varroa destructor. J. Econ. Entomol. 98(3), 651-655.
Sabate, D. C., Gonzaléz, M. J., Porrini, M. P., Eguaras, M. J., Audisio, M. C., Marioli, J. M. (2012) Synergistic effect of surfactin from Bacillus subtilis C4 and Achyrocline satureioides extracts on the viability of Paenibacillus larvae. World J. Microbiol. Biotechnol. 28(4), 1415-1422.
Shahidi, F. (2000) Antioxidants in food and food antioxidants. Food/nahrung. 44(3), 158-163.
Singleton, V. L., Rossi, J. A. (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16(3), 144-158.
Small, E. (2016) 51. Hop (Humulus lupulus)–a bitter crop with sweet prospects. Biodiversity. 17(3), 115-127.
Tutun, H., Koç, N., Kart, A. (2018) Plant essential oils used against some bee diseases. Turk. J. Agric. Food Sci. Technol. 6(1), 34-45.
Acknowledgment
We want to thank to Hazel Cooley for the revision of our manuscript.
Funding
This work was supported by the PICT 2823-207 and PICT 0337-2017 of Dr. Matias Maggi. This study was also financially supported by the UNMDP, CONICET, and ANPCyT.
Author information
Authors and Affiliations
Contributions
MM, ME conceived this research and designed experiments; FMA participated in the interpretation of the data; PGM, AI, CR and FG performed experiments and analysis; AI, GM, AF and SC wrote the paper.
Corresponding author
Additional information
Manuscript editor: Monique Gauthier
Valorisation des feuilles de houblon pour le développement de pesticides écologiques pour les abeilles
Paenibacillus larvae, Varroa destructor , Apis mellifera , extraits naturels, Humulus lupulus , composition chimique
Hopfenblätter als wichtiger Beitrag für die Entwicklung bienenfreundlicher, ökologischer Pestizide
Paenibacillus larvae, Varroa destructor, Apis mellifera, natürliche Extrakte, Humulus lupulus , chemische Zusammensetzung
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iglesias, A., Gimenez Martinez, P., Ramirez, C. et al. Valorization of hop leaves for development of eco-friendly bee pesticides. Apidologie 52, 186–198 (2021). https://doi.org/10.1007/s13592-020-00808-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-020-00808-8