Abstract
Light is a crucial signal for plant growth, development, and secondary metabolism. Exploring the effects of light on autotoxin secretion in lettuce can be helpful for improving the utilization efficiency of the nutrient solution in plant factories. The effects of white light (WL) and the combination of red (R) and blue (B) light (RB, 4R:1B) at different intensities (150, 200, and 250 μmol m−2 s−1) on the growth and root exudates of hydroponic lettuce (Lactuca sativa L.) were studied in a closed plant factory. The lettuce biomass and photosynthetic rate increased with the increasing light intensity, and the photosynthetic rate was significantly lower under WL than under RB at both 200 and 250 μmol m−2 s−1. Lettuce under WL had the longest root length and highest root surface area at 200 μmol m−2 s−1, while the root length, root surface area, and root volume under RB were the highest at 250 μmol m−2 s−1. Total organic carbon (TOC) content of root exudates in the nutrient solution based on shoot or root dry weight decreased with the increasing light intensity. With the increase in light intensity, the secretion of four autotoxins (benzoic acid, ferulic acid, gallic acid, and tannic acid) based on shoot dry weight and root dry weight decreased under WL. Compared with RB, WL significantly reduced the secretion of autotoxins at 250 μmol m−2 s−1. In conclusion, 250 μmol m−2 s−1 white light should be used for high lettuce yield, and it could also decrease the autotoxins in the nutrient solution and the occurrence of autotoxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plant roots secrete abundant compounds into their rhizosphere (Marschner 1995), and young seedlings typically release approximately 30–40% of their fixed carbon as root exudates (Lynch and Whipps 1990). Phenolic acids are the main autotoxic substances in plant root exudates and are important secondary metabolites in plants (Hao et al. 2010). The accumulation of phenolic acids in the rhizosphere could inhibit plant growth and reduce yield, causing continuous cropping challenges through allelopathic effects (Wang et al. 2010). Excessive accumulation of root exudates inhibited cucumber growth, decreased the net photosynthetic rate and stomatal conductance, and enhanced membrane lipid peroxidation (Yu and Matsui 1994; Yu et al. 2003). The allelopathic mechanisms involving phenolic acids mainly include (1) impacting the permeability of the root membrane, leading to a decrease in plant nutrient absorption and utilization and an increase in ion leakage in plants (Li and Hu 2005); (2) hindering the synthesis of Mg-carboline, thereby accelerating chlorophyll decomposition and decreasing photosynthesis (Yang et al. 2002); and (3) inhibiting the enzyme activity required for seed germination (Yan et al. 2010).
Root exudates are greatly influenced by external environmental factors (Renella et al. 2006; Dijkstra and Cheng 2007). Furthermore, the exudation of phenolic compounds was found to be highly species dependent, and the secretion of coumaric acid increased remarkably at high Cu2+ levels (Meier et al. 2012). Pramanik et al. (2000) found that the rate of phenolic acid exudation from cucumber increased with the increasing air temperature. In addition, the rate of exudation gradually increased until flowering and decreased at maturity in rice (Aulakh et al. 2001). To date, there are many studies on the effects of environmental factors such as nutrient stress and temperature on root exudates, but the effects of light on root exudates remain largely unknown.
Recently, light-emitting diode (LED) light sources with low energy consumption, adjustable light intensity, fixed wavelength, and controllable illumination have been used to study the growth, development, and morphogenesis of horticultural crops (Cao et al. 2013). Plant factory production and supplemental light from LEDs in greenhouses have become the key technologies of facility-based horticultural production (Liu et al. 2012). Goins et al. (1997) tested a series of light sources and found that a white fluorescent lamp resulted in the highest wheat yield and that wheat biomass and yield under red and blue (RB) light were similar to those under white light (WL). The combination of RB light could be treated as an alternative choice to replace the continuous spectrum for plant cultivation under artificial light in a controlled environment. Moreover, previous studies have shown that red LEDs could remarkably increase the contents of starch and soluble carbohydrates in plants by inhibiting the translocation of photosynthates out of the leaves (Sæbø et al. 1995). Blue light played an important role in regulating the physiological processes of chlorophyll formation, chloroplast development, and stomatal opening (Li and Pan 1994; Urbonaviciute et al. 2007). RB light could increase the photosynthetic rate, carbohydrate accumulation, and quality of plants (Hogewoning et al. 2010; Savvides et al. 2012). The light environment has an important influence on the growth and development of plant roots, regulating root formation and affecting the root activity and secondary metabolism in roots (Yan et al. 2016; Yang et al. 2018). Therefore, we speculate that changes in the LED light environment may reduce root exudates.
As an efficient agricultural system for continuous production, plant factories usually adopt an automatic hydroponic circulation system for the nutrient solution, which could improve the nutrient utilization efficiency and reduced the discharge of waste nutrient solution, thereby decreasing environmental impacts (Yang and Zhang 2005; Kozai 2013; Hosseinzadeh et al. 2017). However, the autotoxins secreted by roots in the nutrient solution gradually accumulate and inevitably inhibit the growth of plants (Yu and Matsui 1994; Yu et al. 2003). Continuous cropping challenges caused by autotoxin accumulation in nutrient solutions are an important issue limiting the productivity of plant factories. To solve this problem, Lee et al. (2006) found that activated carbon could effectively remove phytotoxic substance accumulation in nutrient solution and reduce the inhibitory effect on lettuce growth, but its removal capacity was limited since high-dose activated carbon treatment cannot eliminate the harmful impact of these phytotoxic substances on plant growth. A nano-TiO2 photocatalytic method effectively removed the autotoxins from the nutrient solution and increased plant yield (Sunada et al. 2008; Qiu et al. 2013a). However, the contents of available Fe, Mn, and Zn also decreased (Qiu et al. 2013b). Furthermore, Talukder et al. (2019) significantly reduced the concentration of benzoic acid in the nutrient solution and increased strawberry yield using an electrodegradation method, but this method is complicated and costly. Therefore, we aimed to reduce the secretion of autotoxins from plant roots by regulating the LED light environment and thereby improve hydroponic lettuce production.
Lettuce is a globally produced vegetable rich in nutrients, such as protein and vitamin, and is widely cultivated in facilities and gardens (Zha and Liu 2017). Previous studies have shown the effects of the LED light environment on the growth and development of lettuce (Wang et al. 2016; Chen and Yang 2018; Zha et al. 2019). Moreover, allelopathic effects of nutrients and other conditions on plants have also been reported (Pramanik et al. 2000; Renella et al. 2006; Meier et al. 2012). However, little information is available on whether and how the LED light environment impacts the root exudates of leafy vegetables hydroponically grown in plant factories. The aims of this study are to determine the effects of RB light and WL of different intensities on the (1) growth and yield, (2) organic carbon secretion, and (3) autotoxin (benzoic acid, salicylic acid, ferulic acid, gallic acid, and tannic acid) secretion of hydroponic lettuce. We clarified whether autotoxins in the nutrient solution can be reduced by changing the light quality and intensity to improve the utilization efficiency of the nutrient solution and reduce the probability of encountering continuous cropping obstacles during leafy vegetable production in plant factories.
2 Materials and methods
2.1 Plant materials and growth conditions
Lettuce (L. sativa L. cv. ‘Tiberius’) was used in the experiments, which has a high germination rate with average emergence and relatively flat leaves. Seeds were sown in sponge cubes (2.5 × 2.5 × 2.5 cm) and germinated under 200 μmol m−2 s−1 WL irradiance for 15 d in a controlled-environment plant factory. The environmental conditions in the experiment were set as follows: 23 ± 2 °C, 60% relative humidity (RH), and an atmospheric CO2 concentration. After the second leaf was fully expanded, the seedling roots were washed three times with deionized water. Then, the seedlings were randomly transplanted into hydroponic pots containing 4 L of nutrient solution. There were four hydroponic pots in each treatment, and four seedlings were planted in each pot. Lettuce seedlings were cultured in Hoagland’s solution. The electrical conductivity (EC) and pH were adjusted to 1.422 mS cm−1 and 5.8, respectively.
2.2 Light conditions and treatments
In the LED experiment, lettuce was irradiated for 16 h per day (24 h) by WL and a combination of red and blue light (RB). The LED light was 50 × 50 cm (Shenzhen Huihao Optoelectronic Co. Ltd., Shenzhen, P. R. China). The LED provides red light with a peak wavelength of 655 nm and blue light with a peak wavelength of 437 nm. The relative spectral distributions of the WL and RB light are shown in Fig. 1 and Table 1, and the ratio of red to blue light intensity was 4R:1B in RB light, which is appropriate for lettuce growth according to previous research (Zhou et al. 2013). In general, the light intensity of cultivated lettuce is 100 ~ 300 μmol m−2 s−1 in the plant factory (Zhou et al. 2013; Wang et al. 2016; Zha et al. 2019). Therefore, six light treatments were administered in the experiment, including light intensities of the three white LEDs of 150 (WL150), 200 (WL200), and 250 (WL250) μmol m−2 s−1, and light intensities of the three RB LEDs of 150 (RB150), 200 (RB200), and 250 (RB250) μmol m−2 s−1. Light intensity was measured at the canopy level by a light sensor logger (Li-1500; LI-COR, Lincoln, NE, USA).
2.3 Sampling and measurement methods
Lettuce was harvested at a younger stage (15 d after treatment) than that in commercial cultivation to reduce effects of shading among larger plants. Four lettuce plants from each treatment were randomly sampled for measurement of the leaf area, shoot and root fresh weight, and shoot and root dry weight. Leaf area was measured using a leaf area meter (LI-3100C, Li-Cor Biosciences, Lincoln, Nebraska, USA). Dry weight was determined after drying at 80 °C for 48 h.
2.3.1 Photosynthetic rate
The photosynthetic rate (Pn) of the fully expanded 2nd leaf from the top was measured under the growth light intensity using a portable photosynthesis system (LI-6400, LI-COR, Lincoln, NE, USA). The leaf temperature and CO2 concentration in the leaf chamber were 22 °C and 400 μmol mol−1, respectively.
2.3.2 Collection and determination of autotoxins
Four lettuce plants in each treatment were randomly selected from four hydroponic plots. The roots were washed three times with deionized water. Then, the plants were placed in a 100-mL triangular bottle containing 120 mL of deionized water. The triangular bottle (Bomex, Beijing, China) was covered with aluminium foil to block light and then the plants were placed under the original light treatment. After 3 d, lettuce was removed, and roots were washed three times with deionized water. Then, the rinse solution was transferred to a triangle bottle containing the residual solution, and deionized water was added to bring the volume in the triangle bottle to 120 mL. Then, the solution was filtered through a 0.45-μm membrane and stored at − 80 °C until analysis.
The concentrations of benzoic and salicylic acids were measured by ultraviolet spectrophotometry (Shimadzu UV-1800, Kyoto, Japan) (Duan 2009). First, 50-mL benzoic acid solutions were prepared at concentrations of 0, 32.8, 65.6, 98.4, 131.1, and 163.9 μmol L−1. The absorbance of each benzoic acid solution was determined at 227 nm, and a standard curve was plotted. Next, the absorbance of sample solutions was determined at 227 nm and plotted on the standard curve to calculate the concentration of benzoic acid in the samples. The concentrations of salicylic acid in the samples were measured similarly to those of benzoic acid, but the analysis wavelength for the sample solutions was 297 nm.
The concentrations of ferulic acid, gallic acid, and tannic acid were measured by FeCl3-K3[Fe(CN)6] colorimetry (Fu et al. 2006; Jiang et al. 2010). First, 0, 0.5, 1, 1.5, 2.0, and 2.5 mL of ferulic acid solution at a concentration of 103.1 μmol L−1 were placed in separate test tubes, and then the solution in each test tube was brought to 2.5 mL with deionized water. Second, 2.5 mL of 99% ethanol, 2 mL of 0.3% sodium dodecyl sulfate, and 1 mL of a 0.6% FeCl3—0.9% K3[Fe(CN)6] (volume ratio of 1:0.9) mixture were added to every tube, and the tubes were placed in the dark for 5 min. Third, 17 mL of HCl (0.1 mol L−1) was added to each test tube, and the tubes were shaken to allow complete reaction of the solution. Then, the absorbance of the solution was measured by spectrophotometry (Shimadzu UV-1800, Kyoto, Japan) at 720 nm 20 min later. The standard curve was plotted according to the absorbance and corresponding ferulic acid concentrations. The absorbance of ferulic acid in the sample solutions (2.5 mL taken from each replicate) was also measured following the second and third steps above, and the concentrations of ferulic acid in the samples were calculated according to the standard curve. The concentrations of gallic acid and tannic acid in the samples were measured similarly to that of ferulic acid. The standard curve was drawn the same as for ferulic acid, except that ferulic acid solution (103.1 μmol L−1) was replaced by gallic acid solution (117.6 μmol L−1) and tannic acid solution (11.8 μmol L−1). The absorbance of gallic acid and tannic acid was measured at 720 nm.
2.3.3 Root morphology
Root morphology was measured twice. For the first measurement, 15 d after pot cultivation in the nutrient solution under different light conditions, lettuce plants were randomly sampled and divided into shoots and roots. Fresh roots were scanned using an Epson perfection V850 photoscanner (Epson, Nagano, Japan), and the images were analyzed by WinRHIZO Pro software (version 2019, Regent Co. Ltd., Quebec, Canada). Total root length, root surface area, and root volume were determined. For the second measurement, 3 d after cultivation in deionized water, lettuce plants were divided into shoots and roots, and fresh roots were analyzed for root morphology.
2.3.4 Collection and determination of total organic carbon
Total organic carbon (TOC) was determined twice. For the first analysis, after removing the lettuce plants on the 15th day after treatments, the volume of the residual nutrient solution in the hydroponic pots were measured. The completely mixed nutrient solution was filtered through a 0.45-μm membrane and stored at − 80 °C until analysis. For the second analysis, after 3 d of cultivation in deionized water, lettuce was removed, and roots were washed three times with deionized water. Then, the rinse solution was transferred to a triangle bottle containing the residual solution, and deionized water was added to bring the volume in the bottle to 120 mL. Then, the solution was filtered through a 0.45-μm membrane and stored at − 80 °C until analysis. The TOC was determined in 10% HCl pre-acidified and 105 °C pre-dried samples by the HT 1300 high-temperature combustion system connected to a TOC analyzer (Multi N/C 3100, Analytik Jena AG, Jena, Germany).
2.4 Statistical analysis
Data were analyzed by two-way ANOVA with ‘light quality’ and ‘light intensity’ as two factors using SPSS 18.0 (International Business Machines Corporation). All data are expressed as mean ± standard error (n = 3). After variance analysis, the differences among the six treatments were determined by Duncan’s multiple range test at p < 0.05.
3 Results
3.1 Plant morphology and growth characteristics
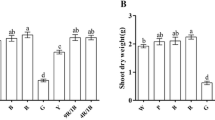
The lettuce leaf area under WL was significantly higher than that under RB light, and there was no significant difference in the same light quality at different light intensities (Table 2). The fresh weight increased with increasing light intensity. At the same light intensity, the shoot fresh weight under WL was significantly higher than that under RB, and there was no significant difference in root fresh weight between the WL and RB treatments. The shoot dry weight under WL decreased as WL250 > WL200 > WL150. At the same light intensity, the shoot dry weight under WL was also significantly higher than that under RB. Both WL150 and RB150 resulted in lower root dry weights than the other treatments did, and WL250 resulted in the highest root dry weight, which was significantly higher than that under RB250. The root dry weight under WL200 was also significantly higher than that under RB200. Light quality had a significant effect on the leaf area, shoot fresh weight, shoot dry weight, root dry weight, and root-shoot ratio, and light intensity had a significant effect on the shoot fresh weight, root fresh weight, shoot dry weight, root dry weight, and root-shoot ratio. However, there was no two-way interaction effect of light quality and light intensity on the leaf area, shoot fresh weight, root fresh weight, shoot dry weight, root dry weight, or root-shoot ratio.
3.2 Leaf photosynthesis
As shown in Fig. 2, the leaf photosynthetic rate (Pn) of both WL and RB treatments increased with light intensity. Under the same light intensity, the Pn of RB250 was 27.6% higher than that of WL250, and the Pn of RB200 was 45.3% higher than that of WL200, but the difference in Pn between RB150 and WL150 was not significant.
3.3 Root morphology
The total root length under WL200 was significantly higher than that under WL150 and WL250, while that under RB250 was significantly higher than that under RB150 and RB200 (Table 3). The root surface area under WL decreased as WL200 > WL250 > WL150, and there were significant differences in surface area among the treatments. For RB light, the root surface area decreased as RB250 > RB200 > RB150, and that under RB250 was significantly higher than that under RB200. However, the difference in surface area between RB200 and RB150 was not significant. For WL, the root volume under WL200 and WL250 was significantly higher than that under WL150, but there was no significant difference in volume between WL200 and WL250. Of the RB light intensities, RB250 resulted in the highest root volume, significantly higher than that under RB200 and RB150. These results indicated that WL at 200 μmol m−2 s−1 and RB at 250 μmol m−2 s−1 are beneficial to the development of lettuce roots. Moreover, light quality had no significant effect on total root length, root surface area, or root volume, while light intensity had a significant effect on total root length, root surface area, and root volume. There was no two-way interaction effect of light quality and light intensity on root volume.
3.4 Organic carbon content
There was no significant difference in TOC content under both WL and RB (Table 4). The TOC content/shoot dry weight decreased with increasing light intensity under both WL and RB, and that under RB was significantly higher than that under WL at the same light intensity. Similarly, the TOC content/root dry weight of WL and RB decreased with increasing light intensity, and that under RB was significantly higher than that under WL at 150 and 200 μmol m−2 s−1. The TOC content/root surface area under WL150 and WL250 was significantly higher than that under WL200, whereas this ratio decreased as RB150 > RB200 > RB250 for RB light, and the differences among the treatments were significant. Furthermore, light quality had a significant effect on the TOC content, TOC content/shoot dry weight, TOC content/root dry weight, and TOC content/root surface area, and light intensity had a significant effect on the TOC content/shoot dry weight, TOC content/root dry weight, and TOC content/root surface area, but there was no two-way interaction effect of light quality and light intensity on the TOC content, TOC content/shoot dry weight, TOC content/root dry weight, and TOC content/root surface area.
3.5 Autotoxin contents
3.5.1 Autotoxin content based on the root dry weight
Under high light intensity (250 μmol m−2 s−1), benzoic acid content/root dry weight was lower under WL than that under RB. There was no significant difference in benzoic acid content/root dry weight between RB and WL at both 150 and 200 μmol m−2 s−1, but that under RB was significantly higher than that under WL at 250 μmol m−2 s−1 (Fig. 3a). The change in salicylic acid content/root dry weight was small under high light intensity, and there was no significant difference between RB and WL (Fig. 3b). Ferulic acid/root dry weight decreased with the increase of light intensity under WL but increased slightly from 200 to 250 μmol m−2 s−1 under RB, and that under RB was significantly higher than that under WL at 250 μmol m−2 s−1 (Fig. 3c). Similarly, both gallic acid content/root dry weight and tannic acid content/root dry weight under RB were significantly higher than those under WL (Fig. 3d, E).
3.5.2 Autotoxin content based on shoot dry weight
Benzoic acid content/shoot dry weight decreased with the increase of light intensity under both WL and RB, and there was no significant difference between WL and RB (Fig. 4a). The response of salicylic acid content/shoot dry weight to light intensity and light quality was not obvious (Fig. 4b). Ferulic acid content/shoot dry weight did not change much with the increase of light intensity under RB, but decreased under WL, and that under WL was significantly lower than that under RB at 200 and 250 μmol m−2 s−1 (Fig. 4c). Gallic acid content/shoot dry weight under WL was significantly lower than that under RB at 250 μmol m−2 s−1 (Fig. 4d). At 250 μmol m−2 s−1, tannic acid content/shoot dry weight under WL were decreased and significantly lower than that under RB (Fig. 4e).
3.5.3 Proportion of carbon in the total organic carbon from the five autotoxins
The highest proportion of carbon from the five autotoxins in the TOC in lettuce root exudates was 64.7%, observed under RB150, and the lowest was 38.4%, observed under WL200. This indicated that 38.4–64.7% of the organic carbon secreted in lettuce root exudates was contributed by benzoic acid, salicylic acid, ferulic acid, gallic acid, and tannic acid (Fig. 5). Of the RB light intensities, RB150 resulted in the highest proportion, which was 61.8% and 39.7% higher than that under RB200 and RB250, respectively. In addition, the proportion of carbon in the TOC from the five autotoxins under RB light was 51.9% higher than that under WL at 150 μmol m−2 s−1, and there was no significant difference between RB and WL at 200 or 250 μmol m−2 s−1.
4 Discussion
Light not only provides basic energy for plant photosynthesis but also plays an important role in regulating plant development, morphogenesis, cell component biosynthesis, and gene expression throughout the plant life cycle (Wang et al. 2015; Son and Oh 2015). Plant growth is affected by both light intensity and light quality. Our results demonstrated that lettuce growth had different responses to the two kinds of light quality at different intensities. Lettuce under WL had a higher yield than that under RB, which might have been due to the larger leaf area under WL. Although the photosynthetic rate was lower under WL, the larger leaf area enabled the lettuce to capture more light energy. This may have resulted from the availability of 11.7% of the far-red light in the WL treatment, as plant exposure to light with a higher proportion of far-red light has been reported to promote total dry mass accumulation and stem extension (Jones 2014; Arena et al. 2016) and reduce the root-shoot ratio (Gundel et al. 2014). The shoot fresh weight, root fresh weight, shoot dry weight, and root dry weight of lettuce increased with increasing light intensity, and the photosynthetic rate increased with increasing light intensity. A higher photosynthetic rate facilitates the accumulation of photosynthates, thus enhancing the yield of lettuce (Wang et al. 2016). We found that the lettuce was shorter and stronger and that the plant was more compact under RB. However, the specific reasons for these results need to be studied further.
Light can not only regulate lateral root formation, root cell differentiation, and cell wall remodelling by changing the level of endogenous hormones in plants but also affect root morphogenesis by regulating the synthesis of photosynthates and their transport and distribution to roots (Cluis et al. 2004; Molas et al. 2006). The effects of the two kinds of light quality on the root morphology of lettuce had no obvious regularity. The maximum total root length and root surface area were observed under WL200, but there were no significant differences in fresh weight, dry weight, or root volume between WL200 and WL250. This indicated that the root diameter increased with the high light intensity of WL (250 μmol m−2 s−1) but did not have a positive effect on the accumulation of photosynthates in the root system. Increasing the RB light intensity in the range of 150 ~ 250 μmol m−2 s−1 not only increased the root length, root surface area, and root volume but also promoted the accumulation of dry matter in roots. This indicated that 200 μmol m−2 s−1 WL and 250 μmol m−2 s−1 RB light were beneficial to the growth and development of lettuce roots.
Roots are the most important organs in plants, as they efficiently obtain nutrients and water from the surrounding environment and secrete organic and inorganic substances, namely, root exudates (Satbhai et al. 2015). In this study, light intensity had no significant effect on the TOC content in the nutrient solution. Under the same light intensity, the TOC content/shoot dry weight and TOC content/root dry weight under RB light were significantly higher than those under WL. This indicated that at the same dry matter content, lettuce roots under RB light released more organic carbon into the nutrient solution than those under WL. Therefore, the high yield under WL was not only due to the larger leaf area promoting more light energy capture but also to the reduction in organic carbon secretion. Moreover, the TOC content/shoot dry weight and TOC content/root dry weight decreased with the increase in intensity of WL and RB light mainly because lettuce had higher shoot dry weight and root dry weight under high light intensity. Thus, high light intensity (250 μmol m−2 s−1) reduced the secretion of organic carbon based on a unit mass shoot dry weight or root dry weight and increased the photosynthate storage in the plants. This showed that the increase in light intensity promotes the accumulation of photosynthates in plants and reduces the secretion of root carbohydrates (Wang et al. 2015). Therefore, lettuce yield is higher under high light intensity than that under low light intensity.
Autotoxins are a major problem in continuous cropping (Wu and Wang 1999). Continuous cropping obstacles are one of the main limiting factors in agricultural production and exist not only in soil cultivation but also in hydroponic cultivation of protected horticulture. Previous studies have indicated that autotoxins are mainly phenolic acids, including benzoic acid, 4-hydroxy-benzoic acid, cinnamic acid, salicylic acid, ferulic acid, gallic acid, and tannic acid (Kil and Lee 1987; Yu and Matsui 1994; Gallet 1994; Qiu et al. 2013a, b). Our previous results confirmed that benzoic acid, salicylic acid, ferulic acid, gallic acid, and tannic acid are all present in root exudates from lettuce (Zhou et al. 2019). In this study, light quality had different effects on these five autotoxins. With the increase in light intensity, the secretion of four autotoxins (benzoic acid, ferulic acid, gallic acid, and tannic acid) based on shoot dry weight and root dry weight were obviously decreased under WL. Moreover, the secretion of benzoic acid, ferulic acid, gallic acid, and tannic acid based on root dry weight under RB were remarkably higher than those under WL at 250 μmol m−2 s−1. Similar results were observed in ferulic acid content/shoot dry weight, gallic acid content/shoot dry weight, and tannic acid content/shoot dry weight. These findings indicated that the increase in light intensity under WL not only improved photosynthesis but also reduced the secretion of organic carbon and autotoxins based on the shoot dry weight and root dry weight. Thus, lettuce yields are higher under high light intensity than under low light intensity (Fu et al. 2012). Compared with RB, WL significantly reduced the secretion of autotoxins at 250 μmol m−2 s−1, showing that lettuce had a higher yield under WL than under RB.
5 Conclusion
WL and RB with different light intensities affected both the growth and development of lettuce as well as the secretion quantities of organic carbon and autotoxins. A total of 38.4–64.7% of the organic carbon secreted by lettuce roots was contributed by benzoic acid, salicylic acid, ferulic acid, gallic acid, and tannic acid. Increasing the white light intensity from 150 to 250 μmol m−2 s−1 improved photosynthesis and reduced the secretion of organic carbon and autotoxins (benzoic acid, ferulic acid, gallic acid, and tannic acid) based on the shoot dry weight and root dry weight, thereby resulting in higher yield of lettuce. WL promoted leaf extension and reduced the organic carbon and autotoxins based on the shoot dry weight or root dry weight at 250 μmol m−2 s−1. Therefore, 250 μmol m−2 s−1 white light should be used to achieve a high yield of lettuce and reduce the secretion of autotoxins and the occurrence of autotoxicity.
References
Arena C, Tsonev T, Doneva D, De Micco V, Michelozzi M, Brunetti C, Centritto M, Fineschi S, Velikova V, Loreto F (2016) The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ Exp Bot 130:122–132. https://doi.org/10.1016/j.envexpbot.2016.05.014
Aulakh MS, Wassmann R, Bueno C, Kreuzwieser J, Rennenberg H (2001) Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol 3:139–148. https://doi.org/10.1055/s-2001-12905
Cao G, Zhang G, Yu J, Ma Y (2013) Effects of different LED light qualities on cucumber seedling growth and chlorophyll fluorescence parameters. Sci Agric Sin 46:1297–1304. https://doi.org/10.3864/j.issn.0578-1752.2013.06.024
Chen X, Yang Q (2018) Effects of intermittent light exposure with red and blue light emitting diodes on growth and carbohydrate accumulation of lettuce. Sci Hortic 234:220–226. https://doi.org/10.1016/j.scienta.2018.02.055
Cluis CP, Mouchel CF, Hardtke CS (2004) The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J 38:332–347. https://doi.org/10.1111/j.1365-313X.2004.02052.x
Dijkstra FA, Cheng W (2007) Moisture modulates rhizosphere effects on C decomposition in two different soil types. Soil Biol Biochem 39:2264–2274. https://doi.org/10.1016/j.soilbio.2007.03.026
Duan KX (2009) Experiments of instrument analysis. Chemical Industry Press, Beijing
Fu W, Li P, Wu Y (2012) Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci Hortic 135:45–51. https://doi.org/10.1016/j.scienta.2011.12.004
Fu YR, Zhang WM, Chen GM, Bai XM (2006) Determination of gallic acid and total phenolic acid in Sedum Aizoon L. Chin Tradit Pat Med 28:1016–1018
Goins GD, Yorio NC, Sanwo MM, Brown CS (1997) Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J Exp Bot 48:1407–1413. https://doi.org/10.1093/jxb/48.7.1407
Gundel PE, Pierik R, Mommer L, Ballaré CL (2014) Competing neighbors: light perception and root function. Oecologia 176:1–10. https://doi.org/10.1007/s00442-014-2983-x
Hao W, Ren L, Ran W, Shen Q (2010) Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil 336:485–497. https://doi.org/10.1007/s11104-010-0505-0
Hogewoning SW, Trouwborst G, Maljaars H, Poorter H, van Ieperen W, Harbinson J (2010) Blue light dose-esponses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J Exp Bot 61:3107–3117. https://doi.org/10.1093/jxb/erq132
Hosseinzadeh S, Verheust Y, Bonarrigo G, Van Hulle S (2017) Closed hydroponic systems: operational parameters, root exudates occurrence and related water treatment. Rev Environ Sci Bio 16:59–79. https://doi.org/10.1007/s11157-016-9418-6
Jiang NX, Liu ZM, Ren HQ, Han SY, Zhu CY (2010) Changes of phenolic acids and total phenolic acids contents in cell walls of moso bamboos at different ages. J Bamboo Res 29:24–28
Jones HG (2014) Plants and microclimate: a quantitative approach to environmental plant physiology, 3rd edn. Cambridge University Press, Cambridge
Kozai T (2013) Resource use efficiency of closed plant production system with artificial light: concept, estimation and application to plant factory. Proc Jpn Acad 89:447–461. https://doi.org/10.2183/pjab.89.447
Lee JG, Lee BY, Lee HJ (2006) Accumulation of phytotoxic organic acids in reused nutrient solution during hydroponic cultivation of lettuce (Lactuca sativa L.). Sci Hortic 110:119–128. https://doi.org/10.1016/j.scienta.2006.06.013
Li FM, Hu HY (2005) Isolation and characterization of a novel antialgal allelochemical from Phragmites communis. Appl Environ Microb 71:6545–6553. https://doi.org/10.1128/AEM.71.11.6545-6553.2005
Li SS, Pan RC (1994) Effects of blue light on the growth of rice seedlings. Chin J Rice Sci 8:115–118. https://doi.org/10.16819/j.1001-7216.1994.02.010
Liu WK, Yang QC, Wei LL (2012) Light-emitting diodes (LEDs) and their applications in protected horticulture as light sources. China agricultural science and technology press, Beijing
Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129:1–10. https://doi.org/10.1007/BF00011685
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London
Meier S, Alvear M, Borie F, Aguilera P, Ginocchio R, Cornejo P (2012) Influence of copper on root exudate patterns in some metallophytes and agricultural plants. Ecotox Environ Safe 75:8–15. https://doi.org/10.1016/j.ecoenv.2011.08.029
Molas ML, Kiss JZ, Correll MJ (2006) Gene profiling of the red light signalling pathways in roots. J Exp Bot 57:3217–3229. https://doi.org/10.1093/jxb/erl086
Pramanik MHR, Nagai M, Asao T, Matsui Y (2000) Effects of temperature and photoperiod on phytotoxic root exudates of cucumber (Cucumis sativus) in hydroponic culture. J Chem Ecol 26:1953–1967. https://doi.org/10.1023/A:1005509110317
Qiu Z, Yang Q, Liu W (2013a) Photocatalytic degradation of phytotoxic substances in waste nutrient solution by various immobilized levels of nano-TiO2. Water Air Soil Poll 224:1461. https://doi.org/10.1007/s11270-013-1461-0
Qiu Z, Yang Q, Liu W (2013b) Effect of nano-TiO2 photocatalysis on yield and quality of hydroponic lettuce. Acta Agric Boreali Sin 28:103–107
Renella G, Egamberdiyeva D, Landi L, Mench M, Nannipieri P (2006) Microbial activity and hydrolase activities during decomposition of root exudates released by an artificial root surface in Cd-contaminated soils. Soil Biol Biochem 38:702–708. https://doi.org/10.1016/j.soilbio.2005.06.021
Sæbø A, Krekling T, Appelgren M (1995) Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tiss Org 41:177–185. https://doi.org/10.1007/BF00051588
Satbhai SB, Ristova D, Busch W (2015) Underground tuning: quantitative regulation of root growth. J Exp Bot 66:1099–1112. https://doi.org/10.1093/jxb/eru529
Savvides A, Fanourakis D, van Ieperen W (2012) Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J Exp Bot 63:1135–1143. https://doi.org/10.1093/jxb/err348
Son KH, Oh MM (2015) Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic Environ Biotechnol 56:639–653. https://doi.org/10.1007/s13580-015-1064-3
Sunada K, Ding XG, Utami MS, Kawashima Y, Miyama Y, Hashimoto K (2008) Detoxification of phytotoxic compounds by TiO2 photocatalysis in a recycling hydroponic cultivation system of asparagus. J Agric Food Chem 56:4819–4824. https://doi.org/10.1021/jf8001075
Talukder MR, Asaduzzaman M, Tanaka H, Asao T (2019) Electro-degradation of culture solution improves growth, yield and quality of strawberry plants grown in closed hydroponics. Sci Hortic 243:243–251. https://doi.org/10.1016/j.scienta.2018.08.024
Urbonaviciute A, Pinho P, Samuoliene G, Duchovskis P, Vitta P, Stonkus A, Tamulaitis G, Zukauskas A, Halonen L (2007) Effect of short-wavelength light on lettuce growth and nutritional quality. Sodininkystė Ir Daržininkystė 26:157–165
Wang J, Lu W, Tong Y, Yang Q (2016) Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front Plant Sci 7:250. https://doi.org/10.3389/fpls.2016.00250
Wang XY, Xu XM, Cui J (2015) The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 53:213–222. https://doi.org/10.1007/s11099-015-0083-8
Wang X, Zhang T, Dai C (2010) Advance in mechanism and countermeasures of peanut succession monocropping obstacles. Soils 42:505–512
Wu FZ, Wang W (1999) Research on soil microflora of greenhouse tomato. North Hortic 3:1–2
Yan J, Bi HH, Liu YZ, Zhang M, Zhou ZY, Tan JW (2010) Phenolic compounds from Merremia umbellata subsp. orientalis and their allelopathic effects on Arabidopsis seed germination. Molecules 15:8241–8250. https://doi.org/10.3390/molecules15118241
Yan XH, Yu JH, Xie JM (2016) Effects of supplemental light quality and durations of illumination on growth and root activity of cucumber seedling. J Nucl Agric Sci 30:1211–1217. https://doi.org/10.11869/j.issn.100-8551.2016.06.1211
Yang CM, Lee CN, Chou CH (2002) Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: I. Inhibition of supply-orientation. Bot Bull Acad Sin 43:299–304
Yang J, Pan T, Wang J, Bao E, Zou Z (2018) Effects of different light quality on tomato seedlings growth and root. North Hortic 12:77–82. https://doi.org/10.11937/bfyy.20174675
Yang Q, Zhang C (2005) Serial interview on plant factory (IV): plant factory based cultivation system (I). Appl Eng Technol Rural Area (greenh Hortic) 8:38–39
Yu JQ, Matsui Y (1994) Phytotoxic substances in root exudates of cucumber (Cucumis sativus L.). J Chem Ecol 20:21–31. https://doi.org/10.1007/BF02065988
Yu JQ, Ye SF, Zhang MF, Hu WH (2003) Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem Syst Ecol 31:129–139. https://doi.org/10.1016/S0305-1978(02)00150-3
Zha LY, Liu WK (2017) Effect of continuous light with red and blue LED lamps on growth and characteristics of photosynthesis and chlorophyll fluorescence of five lettuce cultivars. Plant Physiol J. 53:1735–1741. https://doi.org/10.13592/j.cnki.ppj.2017.0086
Zha L, Zhang Y, Liu W (2019) Dynamic responses of ascorbate pool and metabolism in lettuce to long-term continuous light provided by red and blue LEDs. Environ Exp Bot 163:15–23. https://doi.org/10.1016/j.envexpbot.2019.04.003
Zhou W, Liu W, Yang Q (2013) Reducing nitrate content in lettuce by pre-harvest continuous light delivered by red and blue light-emitting diodes. J Plant Nutr 36:481–490. https://doi.org/10.1080/01904167.2012.748069
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (No. 31672202) and The Key R&D Projects of Ningxia, China (No. 2017BY085).
Author information
Authors and Affiliations
Contributions
WL was the recipient of funds. WL and CZ designed the experiment. CZ, QW, BL, MS, and YZ prepared the plant materials, collected samples, and undertook experiments. CZ analyzed the data and prepared the manuscript. WL revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Young Yeol Cho.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, C., Wang, Q., Liu, W. et al. Effects of red/blue versus white LED light of different intensities on the growth and organic carbon and autotoxin secretion of hydroponic lettuce. Hortic. Environ. Biotechnol. 63, 195–205 (2022). https://doi.org/10.1007/s13580-021-00394-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-021-00394-3