Abstract
This study aimed to investigate the effects of UV-A light-emitting diodes (LEDs) and lamps on the growth and bioactive compounds of ice plant (Mesembryanthemum crystallinum). Three-week-old seedlings were germinated and grown at 23 °C air temperature, 60% relative humidity, 1000 μmol mol−1 CO2, 200 μmol m−2 s−1 (white LEDs), and a photo-period of 12 h for 3 weeks in a plant factory with artificial lighting. Plants were supplementally irradiated with different peak wavelengths of UV-A LEDs (395, 385, 375, and 365 nm) of 30 W m−2 and UV-A lamps of 15.5 W m−2 continuously for 7 days. Treatment with 395, 385, and 375 nm increased shoot fresh and dry weights than the control at 5 and 7 days of treatment. Fv/Fm value was significantly decreased at 12 h of all UV-A treatments and consistently showed lower levels compared to the control during the entire period. The photosynthetic rates of the 395 and 385 nm treatments were significantly higher than those of the other treatments. UV-A treatment enhanced total phenolic content and antioxidant capacity compared to those of the control after 3 days of treatment. Phenylalanine ammonia-lyase activity was also increased by UV-A light exposure. The content of pinitol, myo-inositol, and sucrose was increased by UV-A radiation, and the highest values were observed in the 395 nm treatment at 5 and 7 days of treatment. Our findings suggest that supplemental radiation of UV-A with a peak wavelength near 400 nm could increase the shoot biomass and antioxidant phenolics and sugar alcohols in ice plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ultraviolet (UV) light is divided into three ranges: UV-A (320–400 nm), UV-B (280–320 nm), and UV-C (100–280 nm) (Mckenzie et al. 2011). UV-C and most of the UV-B light are absorbed by the ozone layer; therefore, 98–99% of the UV radiation reaching the surface of the earth is UV-A light (Yarosh and Smiles 2009). The strong energy of UV light induces the generation of reactive oxygen species (ROS) in mitochondria, nucleus, chloroplasts, and apoplasts, and causes photooxidative stress that destroys the DNA, protein, lipid, and cell membrane in plants (Choudhury et al. 2017; Hideg et al. 2013). ROS act as signaling molecules and instantaneously activate defense mechanisms to synthesize enzymatic and non-enzymatic secondary metabolites against UV light (Hideg et al. 2002; Mittler et al. 2004). Another perception of UV radiation in plants is that UV light signals are captured by photoreceptors, which can activate the biosynthesis pathway of secondary metabolites such as hydroxycinnamic acids, flavonoids, and anthocyanin as sunscreens (Kim et al. 2011; Lee et al. 2014b; Park et al. 2020; Rodríguez et al. 2017). Most studies investigating the effects of UV light on plants have focused on UV-B because an ecosystem change caused by an increased amount of UV-B owing to ozone depletion is a critical global issue (Benca et al. 2018; Cen and Bornman 1990; Fina et al. 2017). The common conclusion is that UV-B radiation increases the content of secondary metabolites to protect plants and causes growth inhibition or visible damage to leaves.
UV-A can penetrate deeper into the leaves of plants than UV-B does (Wilson et al. 2001). UV-A radiation has been reported to promote growth (Bernal et al. 2015; Kataria et al. 2013; Tezuka et al. 1994) and increase the content of total phenolics and flavonoids in plants (Lee et al. 2014b; Rodríguez et al. 2017; Vogt et al. 1999). Lee et al. (2019) and Verdaguer et al. (2017) suggested that supplemental radiation of UV-A below the light saturation point is used as an alternative light energy for photosynthesis and contributes to plant growth promotion. Simultaneously, photoreceptors, phototropin, and cryptochrome, absorbed by UV-A light, activate the transcript levels of key enzymes related to the biosynthesis pathway of secondary metabolites (Fuglevand et al. 1996; Vogt et al. 1999). Thus, UV-A light can be used as a physical elicitor to enhance the growth and bioactive compounds of plants grown under a controlled environment.
Ice plant (Mesembryanthemum crystallinum) is a crassulacean acid metabolism plant widely distributed in Australia and the Pacific Coast of Mexico and Chile (Bohnert and Cushman 2000). The plant is used as a raw vegetable in Europe and Asia. Ice plants are known to be high-value-added plants because they are rich in bioactive compounds, such as beta-carotene, betacyanin, myo-inositol, and pinitol (Agarie et al. 2009; Vogt et al. 1999). In the human body, pinitol is converted to chiro-inositol, which stimulates the insulin pathway (Davis et al. 2000); consequently, it is considered to be effective against type 2 diabetes (Lee et al. 2014a).
To date, few studies have focused on increasing the amounts of bioactive compounds that have high nutritional value and also investigated the growth of ice plants. Most studies on UV-A have used lamps or filters, which have a broad light spectrum, and hence, determining the effect of UV-A treatment on plants is difficult. Moreover, these studies were performed under greenhouse or field conditions where the growing environment is not completely controlled (Bernal et al. 2015; Ibdah et al. 2002; Ordidge et al. 2010). We hypothesized that UV-A LEDs with a specific peak wavelength will induce the accumulation of growth and bioactive compounds of ice plants. Therefore, this study aimed to investigate the effect of supplemental UV-A light generated by light-emitting diodes (LEDs) or lamps on the growth and bioactive compounds of ice plants grown in a plant factory.
2 Materials and methods
2.1 Plant materials and growth conditions
Three-week-old ice plants (M. crystallinum) were transplanted to pots [6.5 × 6.5 × 8 cm (L × W × H)] filled with horticultural growing medium (Myung-Moon, Dongbu Hannong Co., Seoul, Korea) and six pots were placed on a sub-irrigation tray [32 × 22 × 6.5 cm (L × W × H)]. Forty-eight pots per treatment were cultivated in a plant factory equipped with visible light (white LEDs) for 4 weeks under the following conditions: 23 ℃ air temperature, 60% relative humidity, 1000 μmol mol−1 CO2 (Cha et al. 2014), 12 h photoperiod, and 200 μmol m−2 s−1 photosynthetic photon flux density (PPFD). The nutrient solution (EC 1.5 dS m−1, pH 6.0) for ice plant (Cha et al. 2016) was sub-irrigated and maintained at 1 cm depth in the sub-irrigation trays. During the cultivation period, the sub-irrigation trays were rotated clockwise once every 3 days to ensure uniformity of light distribution.
2.2 UV-A treatments
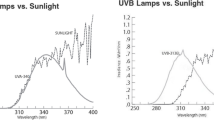
UV-A treatment started at 3 weeks after transplanting. Ice plants grown under visible LEDs without supplemental UV-A light were used as the control. UV-A LEDs (Seoul Semiconductor, Ansan, Korea) with 395 (UVA 395), 385 (UVA 385), 375 (UVA 375), and 365 (UVA 365) nm peak wavelengths were used as the supplemental UV-A radiation. UV-A lamps (F20T10BLB, Sankyo Ultraviolet, Tokyo, Japan) with a broad peak at 352 nm (UVA lamp) were also used in comparison with UV-A LEDs. The energy intensity of UV-A LEDs was measured at 12 points for each treatment using a spectroradiometer (Jaz System, Ocean Optics Inc., Dunedin, FL, USA), and the energy was set to 30 W m−2. The energy intensity of a UV-A lamp was also measured using the same method, and the average energy was 15.5 W m−2. All UV-A lights were continuously radiated on ice plants for 7 days. Light spectra of background LEDs (white LEDs), UV LEDs, and lamps were measured using a spectroradiometer (Jaz System, Ocean Optics Inc., Dunedin, FL, USA) (Fig. 1).
2.3 Growth characteristics
Growth characteristics of ice plants were measured immediately before and at 1, 3, 5, and 7 days after UV treatment. Fresh and dry weights of shoots were measured using an electronic scale (Si-234, Denver Instrument, Denver, CO, USA). Shoot samples were freeze-dried for 48 h in a freeze drier (Alpha 2–4 lsc plus, Christ, Osterode, Germany) for measurement of dry weight. Leaf area was measured using a leaf area meter (LI-3100C, Li-Cor, Lincoln, NE, USA). SPAD value, representing the chlorophyll content, was measured using a portable chlorophyll meter (SPAD-502, Konica Minolta, Tokyo, Japan).
2.4 Chlorophyll fluorescence
The maximum quantum yield of PS II (Fv/Fm) was periodically measured to verify the stress level of the whole plant subjected to UV-A radiation. Plants were adapted for 30 min to dark conditions before measuring Fv/Fm. Subsequently, chlorophyll fluorescence values generated by irradiating light at 620 nm were imaged using an image of a chlorophyll fluorescence analyzer (FC-800-O, Photon System Instruments Ltd., Brno, Czech Republic) equipped with a charge-coupled device camera and a video board. Fluor Cam software (Version 2.0, Photon System Instruments Ltd., Brno, Czech Republic) was used to analyze the image of chlorophyll fluorescence.
2.5 Photosynthetic rate
Photosynthetic rate was measured at 3 days after UV-A treatment using a portable photosynthesis system (LI-6400, Li-Cor, Lincoln, NE, USA). A leaf chamber fluorometer (6400–40, Li-Cor, Lincoln, NE, USA) was used during the day time, whereas the standard leaf chamber (6400–08, Li-Cor, Lincoln, NE, USA) was used at night. Chamber parameters were set as follows: 400 μmol s−1 air flow rate, 1,000 μmol mol−1 CO2, and 23 °C block temperature.
2.6 Total phenolic content
Total phenolic content was analyzed using a modified Folin–Ciocalteu method (Ainsworth and Gillespie 2007). The leaf tissues (approximately 0.2 g) were immediately soaked in liquid nitrogen, and the frozen samples were preserved at − 70 °C in a deep freezer. Samples were mixed with 3 mL 80% (v/v) acetone using a mortar and pestle. The extracted solution was placed into a 2 mL microtube and stored at 4 °C in a refrigerator in the dark for 12 h. The samples were then centrifuged at 3000×g for 2 min. The supernatant was analyzed as described by Son and Oh (2013). Total phenolic content was represented as gallic acid equivalent (mg) per unit fresh weight (g) (GAE mg g FW−1).
2.7 Antioxidant capacity
The antioxidant capacity of ice plant leaves was measured using the 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (Sigma-Aldrich, St. Louis, MO, USA) method (Miller and Rice-Evan 1996). Samples were prepared and extracted using the same method as that for total phenolic content, and the extracts were stored at − 20 °C in the dark for 12 h. The method described by Son and Oh (2013) was followed. Standard curves were generated using 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox) (Sigma-Aldrich, St. Louis, MO, USA) and expressed as the Trolox equivalent antioxidant capacity (mM) per unit fresh weight (g) (mM TEAC g FW−1).
2.8 Phenylalanine ammonia-lyase (PAL) activity
PAL activity was assessed according to Boo et al. (2011). Leaf tissues (approximately 1 g) were preserved at − 70 °C in a deep freezer. Samples were ground in a mortar with liquid nitrogen and then extracted with 10 mL of 25 mM borate buffer (pH 8.8) and 2 mL of 3 mM β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA). The extracted solution was centrifuged at 15,000×g for 20 min. The supernatant (5 mL) was mixed with 2.5 mL of 25 mM borate buffer (pH 8.8) and 2.5 mL of 10 mM l-phenylalanine (Sigma-Aldrich, St. Louis, MO, USA) and then reacted at 40 °C for 2 h. The reaction was terminated by adding 100 μL of 5 N HCl, and the absorbance value was read at 290 nm using a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). A standard curve was generated using trans-cinnamic acid (Sigma-Aldrich, St. Louis, MO, USA) and expressed as millimoles of trans-cinnamic acid equivalent per unit fresh weight (g) per hour (mmol t-cinnamic acid g FW−1 h−1).
2.9 Pinitol, myo-inositol, and sucrose detection
After freeze-drying, shoots were pulverized using a grinder (Tube Mill, IKA, Wilmington, NC, USA) and then, powdered samples (0.2 g) were used to detect pinitol, myo-inositol, and sucrose. The compounds were extracted with ethanol (50%, v/v) at 50 ℃ for 60 min using an ultrasonic water bath (Power Sonic 305, Hwashin Technology Co., Seoul, Korea). After sonication, extracts were centrifuged at 5000 x g for 5 min and then the supernatants were filtered through 0.2 μΜ polyvinylidene fluoride syringe filters and extraction cartridges (Oasis® MAX 3cc, Waters, MA, USA). The final solutions were analyzed using a high-performance liquid chromatograph 185 (YL9100, Young Lin Instrument Co., Ltd., Anyang, Korea) with a refractive index (RI) detector (YL9170, Young Lin Instrument Co., Ltd., Anyang, Korea). Chromatographic separation was performed using an NH2P-50 4E column (Asahipak, SHOWA DENKO, Tokyo, Japan) for 14 min. The mobile phase consisted of 60% acetonitrile in water and the flow rate was 1 mL min−1. The temperature of the column and RI detector was set to 65 and 55 ℃, respectively, and the injection volume was 20 μL. Standard curves were obtained using pinitol, myo-inositol, and sucrose (Sigma-Aldrich, St. Louis, MO, USA), and the contents of each compound were expressed as mg per unit dry weight and per shoot.
2.9.1 Statistical analysis
In this experiment, we used a completely randomized design. Five plants per treatment were used for analyzing the growth characteristics, photosynthetic rate, Fv/Fm, total phenolic content, antioxidant capacity, PAL enzyme activity, and HPLC result. The SAS program (Statistical Analysis System, 9.2 version, SAS Institute, Cary, NC, USA) was used for one-way analysis of variance, and significant differences were determined using Duncan’s multiple range test.
3 Results
3.1 Growth characteristics and photosynthetic rate
None of the UV-A treatments resulted in visible damage during the entire irradiation period (Fig. 2). The changes in shoot fresh and dry weights and leaf area showed similar trend (Fig. 3A–C). The values of most UV-A treatments were low compared to that of the control at day 1, but afterward, the growth rates of all the treatments were higher or no difference than that of the control. None of the UV-A treatments showed significant difference in shoot fresh weight during the experimental period except for the first day of treatment (Fig. 3A). The shoot dry weight of UVA 395 was 46% significantly higher than that of the control at 5 days, and UVA 395 and 375 treatments markedly increased after 7 days (Fig. 3B). In UVA 395 and UVA 375 treatments, the leaf area was 10% and 20% higher, respectively, than that of the control at 7 days (Fig. 3C). The SPAD value was not significantly different among all treatments during the whole experimental period except for 5 days (Fig. 3D).
Shoot fresh (a) and dry weight (b), leaf area (c), and SPAD value (d) of ice plant subjected to different peak wavelengths of UV-A LEDs and UV-A lamps radiation for 7 days. The UV-A treatments started at 3 weeks of transplanting. Data indicate means ± SE (n = 5). Different letters next to lines indicate significant differences at *p < 0.05 and **p < 0.01, respectively
UV-A irradiation affected the photosynthetic rate (Fig. 4). At 3 days of UV-A treatment, the photosynthetic rate under UVA 395 and UVA 385 treatments was 26% and 24% significantly higher than that of the control, respectively, during the day time, while UV-A LEDs with relatively short wavelength and UV-A lamps showed a similar level of photosynthetic rate compared to that of the control (Fig. 4A). Figure 4B shows that UV-A treatments affected the photosynthetic rate at night. The photosynthetic rate of plants grown under all UV-A LED treatments was approximately 4 μmol m−2 s−1, that under UVA lamp had a relatively low value (0.5 μmol m−2 s−1), and that under the control treatment had a negative value.
Photosynthetic rate during the day (a) and night time (b) of ice plant continuously subjected to different peak wavelengths of UV-A LEDs and UV-A lamps radiation at 3 days after treatment. The UV-A treatments started at 3 weeks of transplanting. Data indicate means ± SE (n = 5). Different letters above bars indicate significant differences at p < 0.001
3.2 Chlorophyll fluorescence
The chlorophyll fluorescence image of the maximum quantum yield of PSII (Fv/Fm) revealed the stress levels of the whole plants by color (Fig. 5A). As shown in the color bar on the right side, orange color (~ 0.8) indicates normal and healthy status of plants, and yellow to green color (< 0.8) indicates the stressed status of plants. The results of image chlorophyll fluorescence showed that as the peak wavelength was shorter and the treatment time was longer, the ice plants had lower values. Figure 5B shows the numerical change of Fv/Fm values measured via chlorophyll fluorescence images, which were calculated as the average Fv/Fm of four whole plants for each treatment. The Fv/Fm significantly started to decrease after 12 h of all UV-A treatments and consistently showed lower levels than those of the control during the treatment period. In particular, UVA 365 treatment had the lowest Fv/Fm value during the entire irradiation period. UVA 395, UVA 385, and UV-A lamps induced similar Fv/Fm values in the ice plants.
Chlorophyll fluorescence images of maximum quantum yield of PS II (Fv/Fm) value (a) and the changes of average Fv/Fm (b) of ice plant subjected to different peak wavelengths of UV-A LEDs and UV-A lamps radiation for 7 days. The UV-A treatments started at 3 weeks of transplanting. Data indicate means ± SE (n = 5). Different letters next to lines indicate significant differences at p < 0.001
3.3 Total phenolic content and antioxidant capacity
The total phenolic contents of plants treated with UVA 395, UVA 365, and UV-A lamp radiation were significantly higher than those of the control at 3 days of treatment (Fig. 6A). At 7 days of treatment, the total phenolic content of all UV treatments was at least 11% higher than that of the control plants. The highest total phenolic content was recorded for UVA 385 treatment, which had 20% higher value than the control, followed by that for UVA 365, UVA 375, UVA lamp, and UVA 395.
Total phenolic content (a) and antioxidant capacity (b) of ice plant subjected to different peak wavelengths of UV-A LEDs and UV-A lamps radiation for 7 days. The UV-A treatments started at 3 weeks of transplanting. Data indicate means ± SE (n = 5). Different letters next to lines indicate significant differences at *p < 0.05 and **p < 0.01, respectively
UV-A treatments with UVA 375, UVA 365, and UV-A lamps significantly increased antioxidant capacity compared to the control at 5 days of treatment (Fig. 6B). All UV-A treatments also showed at least 20% higher antioxidant capacity than that of the control at 7 days of treatment.
3.4 PAL activity
The PAL enzyme was activated by most UV-A treatments during the entire irradiation period (Fig. 7). UVA 375 and UVA lamp treatments showed significantly higher PAL activity than that of the control on the first day of treatment. All UV-A treatments except UVA 385 significantly increased PAL activity compared with the control at 5 days of treatment.
Phenylalanine ammonia-lyase (PAL) activity of ice plant subjected to different peak wavelengths of UV-A LEDs and UV-A lamps radiation for 7 days. The UV-A treatments started at 3 weeks of transplanting. Data indicate means ± SE (n = 5). Different letters next to lines indicate significant differences at *p < 0.05
3.5 Pinitol, myo-inositol, and sucrose
Pinitol and myo-inositol contents per unit dry weight of ice plants treated with all UV-A treatments were significantly higher than the control at 5 days (Table 1). In particular, these values under UVA 385 treatment were 1.7 and 1.9 times, respectively, higher than that of the control. At 7 days of treatment, the contents of pinitol, myo-inositol, and sucrose per unit dry weight in all UV-A LED treatments increased with increasing peak wavelengths, and the highest content was observed with UVA 395 treatment. UVA 395 treatment, which had the highest dry weight of shoot, recorded the highest contents of pinitol, myo-inositol, and sucrose per whole shoot among all the UV-A treatments and the control at both 5 and 7 days of treatment.
4 Discussion
Although the difference in peak wavelengths among the UV-A LEDs used in this study was only 10 nm, the ice plants showed distinct growth responses according to irradiation with each UV-A LED treatment. Growth inhibition of ice plants was not observed in any of the UV-A treatments (Fig. 3). Overall, UVA 365 and UVA lamps, which had short wavelengths with relatively strong energy, showed lower growth values than those of the control. In contrast, the other UV-A LEDs with a wavelength near 400 nm increased the shoot fresh and dry weight, leaf area, and SPAD value compared to those of the control. These effects of UV-A on biomass accumulation have also been reported in several previous studies (Bernal et al. 2015; Lee et al. 2019; Tezuka et al. 1994; Zhang et al. 2014). Tezuka et al. (1994) and Zhang et al. (2014) indicated that biomass accumulation was related to chlorophyll content and photosynthetic activity. Verdaguer et al. (2017) suggested that UV-A light can be absorbed by photosynthetic pigments such as chlorophylls and carotenoids, and the absorbed light can be used as an energy source for photosynthesis under low PPFD condition. In other words, UV-A light can be utilized as an alternative light energy source for photosynthesis in plants growing under low light intensity condition below the light saturation point (Lee et al. 2019). In addition, McCree (1971) reported that UV-A light near 400 nm is more efficiently used for the photosynthesis than UV-A with relatively short wavelength. In fact, in this study, the photosynthetic rates during the day and night time was increased by supplemental UV-A radiation, and shoot dry weight had a positive correlation with the photosynthetic rate (Table 2); thus, supplemental UV-A radiation may contribute to shoot biomass accumulation in ice plants.
Chlorophyll fluorescence of the maximum quantum yield of PSII (Fv/Fm) is generated during the process of releasing unnecessary energy during electron transport and is determined as the ability to move electrons (Baker and Roseqvist 2004). Thus, chlorophyll fluorescence has been measured to examine the changes in photosynthetic apparatus and activity under various environmental stresses, and it has been proven to be a fast, non-destructive, sensitive, and reliable method (Kalaji et al. 2014; Ogaya et al. 2011; Song et al. 2016). However, as chlorophyll fluorescence measures a specific leaf locally, it has a limitation in representing the value of the whole plant. In addition, it is difficult to use the conventional method because the absorbed light energy is different depending on the arrangement of the leaves and leaf position owing to the uniform light distribution of artificial lighting. In contrast, chlorophyll fluorescence images can be estimated more accurately because it can capture the chlorophyll fluorescence of the whole plant. We confirmed that the values of chlorophyll fluorescence differed according to the location of the leaves and even in a single leaf (Fig. 5A). UV-A treatment decreased the Fv/Fm value (Fig. 5B). In particular, the shorter UV-A LED wavelength with relatively high energy showed a lower Fv/Fm value. Although UV-A lamp had a peak wavelength of 352 nm, it did not respond to plants sensitively because it has a broad spectrum or lower energy (15.5 W m−2) than that of the UV LED treatment (30 W m−2). Quantum yield was reduced by UV-A light; however, the UV-A, used as an alternative energy increased the photosynthesis rate. As the UV-A LEDs used in this study contain ultraviolet and blue regions, it seems to have resulted in contradictory results.
Cryptochromes, phototropins, and UV RESISTANCE LOCUS 8 (UVR8) are known as photoreceptors that receive blue and UV-A light signals in plants. The activation of these photoreceptors promotes the biosynthesis of phenolic compounds such as anthocyanins and flavonoids in epidermal cells as a photoprotective strategy (Burchard et al. 2000). In addition, these compounds play a role as free radical scavengers owing to their antioxidant properties (Elzaawely et al. 2007). In fact, UV-A light increased the expression of PAL and CHS genes, which are important in the biosynthetic pathway of phenolic compounds and flavonoids, respectively (Lee et al. 2014b; Li et al. 2020; Mao et al. 2020). In several studies, UV-A radiation induced the accumulation of phenolic content in tomato (Guo and Wang 2010), kale (Lee et al. 2019), and Crepis japonica (Constantino et al. 2017). Similar to the previous studies, our results showed that UV-A treatment increased the total phenolic content and antioxidant capacity of ice plants (Fig. 6). The antioxidant capacity of UV-treated plants was significantly higher than that of the control at 7 days of treatment, although a significant difference was not noted among the treatments. In addition, all UV-A treatments increased PAL activity (Fig. 7). Plants treated with UVA 395 nm and UVA 385 nm showed similar variation pattern of PAL activity with total phenolic content during the irradiation period (Figs. 6A and 7). In addition, PAL activity had a positive correlation with total phenolic content, corroborating the results of this study (Table 2).
Inositol and pinitol are sugar alcohols that are known to be related to salt stress and other abiotic stresses (Sengupta et al. 2008). Sugar alcohols, which are key substances in ice plants, do not only have health-promoting effects but can also help normalize gastrointestinal function (Agarie et al. 2009; Grabitske and Slavin 2008; Kim et al. 2018; Vogt et al. 1999). Bohnert et al. (1995) reported that the transcript levels of myo-inositol 1-phosphate synthase and myo-inositol-O-methyltransferase, which are key enzymes in the biosynthesis pathway of myo-inositol and pinitol, were upregulated by abiotic stresses in ice plants. In fact, metabolite levels of glucose, sucrose, inositol, and pinitol in plants were increased by environmental stresses such as salinity, high temperature, low temperature, and drought (Bohnert et al. 1995; Guo and Oosterhuis 1995; Murakeozy et al. 2002; Nelson and Bartels 1998). Sugar alcohol has hydrogen bonding properties, which increase ionic strength, and can protect cells from the adverse effects of excessive water loss and high temperature (Sengupta et al. 2008). In this study, it was also considered that pinitol, myo-inositol, and sucrose induced by UV-A light signaling contributed to the osmoprotectant in ice plants against the adverse environment rich in ultraviolet light.
Overall, 385 and 395 nm UV-A treatments increased shoot biomass, photosynthetic rate, antioxidant phenolic compounds, PAL activity, and alcohol sugar contents of ice plants. Photosynthetic rate had a positive correlation with shoot dry weight, pinitol, and sucrose (Table 2). Sugar alcohols had a significant positive correlation with shoot biomass, and pinitol was positively correlated with antioxidant capacity. Although there were no significant differences, sucrose also had a weak positive correlation with total phenolic content and antioxidant capacity (p < 0.0911 and p < 0.0682, respectively). Pearson correlation coefficient results suggest that supplemental UV-A can be used as an alternative light energy for enhancing photosynthetic rate, thereby contributing to biomass accumulation in shoots. In addition, UV-A signal would have activated the use of enriched primary metabolites as raw sources for the biosynthesis of secondary metabolites.
In conclusion, our results suggest that the UV-A treatment used in this study did not inhibit growth but improved growth and had a positive effect on the accumulation of antioxidant phenolic compounds. Therefore, short-term supplemental UV-A radiation before harvest could be used as a cultivation technique to produce high-quality ice plants in plant factories with artificial lighting.
References
Agarie S, Kawaguchi A, Kodera A, Sunagawa H, Kojima H, Nose A, Nakahara T (2009) Potential of the common ice plant Mesembryanthemum crystallinum as a new high-functional food as evaluated by polyol accumulation. Plant Prod Sci 12:37–46. https://doi.org/10.1626/pps.12.37
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
Baker NR, Roseqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621. https://doi.org/10.1093/jxb/erh196
Benca JP, Duijnstee IAP, Looy CV (2018) UV-B–induced forest sterility: Implications of ozone shield failure in Earth’s largest extinction. Sci Adv 4:e1700618. https://doi.org/10.1126/sciadv.1700618
Bernal M, Verdaguer D, Badosa J, Abadía A, Llusià J, Peñuelas J, Olivera EN, Llorens L (2015) Effects of enhanced UV radiation and water availability on performance, biomass production, and photoprotective mechanisms of Laurus nobilis seedlings. Environ Exp Bot 109:264–275. https://doi.org/10.1016/j.envexpbot.2014.06.016
Bohnert HJ, Cushman JC (2000) The ice plant cometh: lessons in abiotic stress tolerance. J Plant Growth Regul 19:334–346. https://doi.org/10.1007/s003440000033
Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7:1099–1111. https://doi.org/10.1105/tpc.7.7.1099
Boo HO, Heo BG, Gorinstein S, Chon SU (2011) Positive effects of temperature and growth conditions on enzymatic and antioxidant status of lettuce plants. Plant Sci 181:479–484. https://doi.org/10.1016/j.plantsci.2011.07.013
Burchard P, Bilger W, Weissenböck G (2000) Contribution of hydroxycinnamates and flavonoids to epidermal shielding of UV-A and UV-B radiation in developing rye primary leaves as assessed by ultraviolet-induced chlorophyll fluorescence measurements. Plant Cell Environ 23:1373–1380. https://doi.org/10.1046/j.1365-3040.2000.00633.x
Cen YP, Bornman JF (1990) The response of bean plants to UV-B Radiation under different irradiances of background visible light. J Exp Bot 41:1489–1495. https://doi.org/10.1093/jxb/41.11.1489
Cha MK, Kim JS, Cho YY (2014) Growth model of common ice plant (Mesembryanthemum crystallinum L.) using expolinear functions in a closed-type plant production system. Korean J Hortic Sci Technol 32:493–498. https://doi.org/10.7235/hort.2014.14013
Cha MK, Park KS, Cho YY (2016) Growth characteristics of common ice plant (Mesembryanthemum crystallinum L.) on nutrient solution, light intensity, and planting distance in closed-type plant production system. Protect Hortic Plant Fact 25:89–94. https://doi.org/10.12791/KSBEC.2016.25.2.89
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress, and stress combination. Plant J 90:856–867. https://doi.org/10.1111/tpj.13299
Constantino LFS, Nascimento LBS, Casanova LM, Moreira NS, Menezes EA, Esteves RL, Costa SS, Tavares ES (2017) Responses of Crepis japonica induced by supplemental blue light and UV-A radiation. Photochem Photobiol Sci 16:238–245. https://doi.org/10.1039/C6PP00343E
Davis A, Christiansen M, Horowitz JF, Klein S, Hellerstein MK, Ostlund RE (2000) Effect of pinitol treatment on insulin action of subjects with insulin resistance. Diabetes Care 23:1000–1005. https://doi.org/10.2337/diacare.23.7.1000
Elzaawely AA, Xuan TD, Koyama H, Tawata S (2007) Antioxidant activity and contents of essential oil and phenolic compounds in flowers and seeds of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. Food Chem 104:1648–1653. https://doi.org/10.1016/j.foodchem.2007.03.016
Fina J, Casadevall R, Elgawad HA, Prinsen E, Markakis MN, Beemster GTS, Casati P (2017) UV-B inhibits leaf growth through changes in growth regulating factors and gibberellin levels. Plant Physiol 174:1110–1126. https://doi.org/10.1104/pp.17.00365
Fuglevand G, Jackson JA, Jenkins GI (1996) UV-B, UV-A, and blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in Arabidopsis. Plant Cell 8:2347–2357. https://doi.org/10.1105/tpc.8.12.2347
Grabitske HA, Slavin JL (2008) Low-digestible carbohydrates in practice. J Am Diet Assoc 108:1677–1681. https://doi.org/10.1016/j.jada.2008.07.010
Guo C, Oosterhuis DM (1995) Pinitol occurrence in soybean plants as affected by temperature and plant growth regulators. J Exp Bot 46:249–253. https://doi.org/10.1093/jxb/46.2.249
Guo J, Wang MH (2010) Ultraviolet A-specific induction of anthocyanin biosynthesis and PAL expression in tomato (Solanum lycopersicum L.). Plant Growth Regul 62:1–8. https://doi.org/10.1007/s10725-010-9472-y
Hideg E, Barta C, Kalai T, Vas I, Hideg K, Asada K (2002) Detection of singlet oxygen and superoxide with fluorescent sensors in leaves under stress by photoinhibition or UV radiation. Plant Cell Physiol 43:1154–1164. https://doi.org/10.1093/pcp/pcf145
Hideg E, Jansen MAK, Strid A (2013) UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends Plant Sci 18:107–115. https://doi.org/10.1016/j.tplants.2012.09.003
Ibdah M, Krins A, Seidlitz HK, Heller W, Strack D, Vogt T (2002) Spectral dependence of flavonol and betacyanin accumulation in Mesembryanthemum crystallinum under enhanced ultraviolet radiation. Plant Cell Environ 25:1145–1154. https://doi.org/10.1046/j.1365-3040.2002.00895.x
Kalaji HM, Oukarroum A, Alexandrov V, Kouzmanova M, Brestic M, Zivcak M, Samborska IA, Cetner MD, Allakhverdiev SI, Goltsev V (2014) Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol Biochem 81:16–25. https://doi.org/10.1016/j.plaphy.2014.03.029
Kataria S, Guruprasad KN, Ahuja S, Singh B (2013) Enhancement of growth, photosynthetic performance, and yield by exclusion of ambient UV components in C3 and C4 plants. J Photochem Photobiol B-Biol 127:140–152. https://doi.org/10.1016/j.jphotobiol.2013.08.013
Kim SY, Bae RN, Chun CH (2011) Changes in bioactive compounds contents of ‘Maehyang’ and ‘Seolhyang’ strawberry fruits by UV light illumination. Korean J Hortic Sci Technol 29:172–180
Kim YJ, Kim HM, Kim HM, Jeong BR, Lee HJ, Kim HJ, Hwang SJ (2018) Ice plant growth and phytochemical concentrations are affected by light quality and intensity of monochromatic light-emitting diodes. Hortic Environ Biotechnol 59:529–536. https://doi.org/10.1007/s13580-018-0058-3
Lee BH, Lee CC, Wu SC (2014a) Ice plant (Mesembryanthemum crystallinum) improves hyperglycaemia and memory impairments in a Wistar rat model of streptozotocin-induced diabetes. J Sci Food Agric 94:2266–2273. https://doi.org/10.1002/jsfa.6552
Lee MJ, Son JE, Oh MM (2014b) Growth and phenolic compounds of Lactuca sativa L. grown in a closed-type plant production system with UV-A, -B, or -C lamp. J Sci Food Agric 94:197–204. https://doi.org/10.1002/jsfa.6227
Lee JH, Oh MM, Son KH (2019) Short-term ultraviolet (UV)-A light-emitting diode (LED) radiation improves biomass and bioactive compounds of kale. Frontiers Plant Sci 10:1042. https://doi.org/10.3389/fpls.2019.01042
Li W, Tan L, Zou Y, Tan X, Huang J, Chen W, Tang Q (2020) The effects of ultraviolet A/B treatments on anthocyanin accumulation and gene expression in dark-purple tea cultivar ‘Ziyan’ (Camellia sinensis). Molecules 25:354. https://doi.org/10.3390/molecules25020354
Mao P, Duan F, Zheng Y, Yang Q (2020) Blue and UV-A light wavelengths positively affected accumulation profiles of healthy compounds in pak-choi. J Sci Food Agric. https://doi.org/10.1002/jsfa.10788
McCree KJ (1971) The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric Meteorol 9:191–216
Mckenzie RL, Aucamp PJ, Bais AF, Bjorn LO, Ilyas M, Madronich S (2011) Ozone depletion and climate change: impacts on UV radiation. Photochem Photobiol Sci 10:182–198. https://doi.org/10.1039/c0pp90034f
Miller NJ, Evans CAR (1996) Spectrophotometric determination of antioxidant activity. Redox Rep 2:161–171. https://doi.org/10.1080/13510002.1996.11747044
Mittler R, Vanderauwera S, Gollery M, Breusegem FV (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. https://doi.org/10.1016/j.tplants.2004.08.009
Murakeozy EP, Smirnoff N, Nagy Z, Tuba Z (2002) Seasonal accumulation pattern of pinitol and other carbohydrates in Limonium gmelini subsp. hungarica. J Plant Physiol 159:485–490. https://doi.org/10.1078/0176-1617-00617
Nelson JM, Bartels PG (1998) Irrigation effects on pinitol content of jojoba leaf blades and floral buds. Ind Crop Prod 8:159–165. https://doi.org/10.1016/S0926-6690(97)10021-8
Ogaya R, Peñuelas J, Asensio D, Llusià J (2011) Chlorophyll fluorescence responses to temperature and water availability in two co-dominant Mediterranean shrub and tree species in a long-term field experiment simulating climate change. Environ Exp Bot 71:123–127. https://doi.org/10.1016/j.envexpbot.2010.10.016
Ordidge M, Macías PG, Battey NH, Gordon MH, Hadley P, John P, Lovegrove JA, Vysini E, Wagstaffe A (2010) Phenolic contents of lettuce, strawberry, raspberry, and blueberry crops cultivated under plastic films varying in ultraviolet transparency. Food Chem 119:1224–1227. https://doi.org/10.1016/j.foodchem.2009.08.039
Park SY, Lee MY, Lee CH, Oh MM (2020) Physiologic and metabolic changes in Crepidiastrum denticulatum according to different energy levels of UV-B radiation. Int J Mol Sci 21:7134. https://doi.org/10.3390/ijms21197134
Rodríguez MM, Nair V, Benavides J, Zevallos LC, Velázquez DAJ (2017) UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 22:1065. https://doi.org/10.3390/molecules22071065
Sengupta S, Patra B, Ray S, Majumder AL (2008) Inositol methyl tranferase from a halophytic wild rice, Porteresia coarctata Roxb. (Tateoka): regulation of pinitol synthesis under abiotic stress. Plant, Cell and Environ 31:1442–1459. https://doi.org/10.1111/j.1365-3040.2008.01850.x
Son KH, Oh MM (2013) Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 48:988–995. https://doi.org/10.21273/HORTSCI.48.8.988
Song X, Zhou G, Xu Z, Lv X, Wang Y (2016) Detection of photosynthetic performance of Stipa bungeana seedlings under climatic change using chlorophyll fluorescence imaging. Front Plant Sci 6:1254. https://doi.org/10.3389/fpls.2015.01254
Tezuka T, Yamaguchi F, Ando Y (1994) Physiological activation in radish plants by UV-A radiation. J Photochem Photobiol B-Biol 24:33–40. https://doi.org/10.1016/1011-1344(94)07006-7
Verdaguer D, Jansen MA, Llorens L, Morales LO, Neugart S (2017) UV-A radiation effects on higher plants: exploring the known unknown. Plant Sci 255:72–81. https://doi.org/10.1016/j.plantsci.2016.11.014
Vogt T, Ibdah M, Schmidt J, Wray V, Nimtz M, Stracka D (1999) Light-induced betacyanin and flavonol accumulation in bladder cells of Mesembryanthemum crystallinum. Phytochemistry 52:583–592. https://doi.org/10.1016/S0031-9422(99)00151-X
Wilson KE, Thompson JE, Huner NPA, Greenberg BM (2001) Effects of ultraviolet-A exposure on ultraviolet-B-induced accumulation of specific flavonoids in Brassica napus. Photochem Photobiol 73:678–684. https://doi.org/10.1562/0031-8655(2001)0730678EOUAEO2.0.CO2
Yarosh DB, Smiles KA (2009) DNA repair and photoprotection. In: Lim HW, Draelos ZD (eds) Clinical guide to sunscreens and photoprotection. Inform Healthcare, New York, pp 169–197
Zhang L, Allen LH, Vaughan MM, Hauser BA, Boote KJ (2014) Solar ultraviolet radiation exclusion increases soybean internode lengths and plant height. Agric For Meteorol 184:170–178. https://doi.org/10.1016/j.agrformet.2013.09.011
Acknowledgements
This result was supported by “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2020RIS0282).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft preparation, J-WL; formal analysis, visualization, data curation, writing—original draft preparation, S-YP; conceptualization, writing—review and editing, supervision, project administration, M-MO.
Corresponding author
Ethics declarations
Conflict of interest
The authors can declare that they have no conflict of interest.
Additional information
Communicated by Young Yeol Cho.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, JW., Park, SY. & Oh, MM. Supplemental radiation of ultraviolet-A light-emitting diode improves growth, antioxidant phenolics, and sugar alcohols of ice plant. Hortic. Environ. Biotechnol. 62, 559–570 (2021). https://doi.org/10.1007/s13580-021-00340-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-021-00340-3