Abstract
This study was performed to evaluate the waterlogging tolerance of Cornell-Geneva (G11, G202, G214, G935, CG4814, and CG5087), M26, and M9 apple rootstocks. After grafting ‘Fuji’ scions on each type of rootstock, grafted trees were planted in 17-L pots and grown in a greenhouse under well-irrigated conditions. Sixteen weeks after planting, grown trees were divided into two groups: one group was drip-irrigated daily with 2 L of water (control treatment, CT), and the other group was waterlogged by repeating flooding and drainage at 1- to 3-day intervals for 4 weeks (waterlogging treatment, WT). After the cessation of flooding, trees were irrigated as in CT for 18 days. Trees grafted on G202, G214, and M9 had markedly lower leaf water potential than CT trees on the 27th day of flooding; predawn leaf water potential was − 1.26 to − 1.45 MPa in WT trees and − 0.30 to − 0.32 MPa in CT trees, and midday leaf water potential was − 2.85 to − 3.03 MPa in WT trees and − 1.83 to − 1.87 MPa in CT trees. This difference persisted until the 18th day after the cessation of flooding. The net photosynthetic rate and stomatal conductance of trees grafted on these rootstocks were also extremely low in WT trees, and they did not recover to the corresponding levels in CT trees until the 18th day after the cessation of flooding. Among WT trees, the height, trunk cross-sectional area, and dry weight of G202, G214, and M9 trees were markedly lower than those of trees grafted on other rootstocks, whereas CG4814 trees showed the least reduction in these parameters. The defoliation percentages of G202, G214, and M9 trees were 22%, 23%, and 35%, respectively, in WT trees, whereas trees grafted on other rootstocks had 4 to 9% defoliation. Thus, G202 and G214 trees showed similar sensitivity levels as M9 trees, whereas CG4814 trees were more resistant to flooding than M26 trees, and G11, G935, and CG5087 trees showed a waterlogging tolerance comparable to M26 trees.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
During the growing season, plants are repeatedly exposed to transient or long-term soil waterlogging caused by precipitation or poor soil drainage. Soil waterlogging has a major impact on plant growth, development, and survival, and is a major obstacle limiting sustainable agriculture (Parent et al. 2008). Apple trees (Malus domestica Borkh.) are somewhat resistant to transient flooding. However, because the potential yield is determined by the waterlogging tolerance of the rootstocks (Marchioretto et al. 2018), tolerance to abiotic stresses, such as waterlogging, should be considered when selecting rootstocks.
Apple trees grafted on M9 rootstock, a widely used rootstock in modern high-density planting systems, are less vigorous, presenting 15–50% of the canopy volume of those produced from seedlings (Oh 1998). This condition favors light penetration and ventilation inside the canopy, resulting in good flower bud formation, flower development, and fruit quality, and allowing for highly efficient pest control (Webster and Wertheim 2003). Trees grafted on M9 and M26 rootstocks can easily form early canopies because the given space per tree is small in high-density planting systems, and this requires reduced labor intensity (Robinson 2003). However, M9 trees are vulnerable to replant disease, wooly aphid, and cold stress, and have low waterlogging tolerance (Lee et al. 1982; Webster et al. 2000; Kviklys et al. 2016), and if soil moisture is not properly maintained in the rhizosphere, tree vigor is weakened (Lakso 2003). M26 is also precocious but has the disadvantage of weak growth under poor drainage conditions (Kim and Yoon 1998).
In order to retain similar levels of tree vigor and precocity but compensate for the weaknesses of M9 and M26 rootstocks, new dwarfing apple rootstock breeding programs are being carried out in several countries, such as the USA (G, CG, and MAC series), Canada (V series), Britain (MI series), Germany (Supp. series), Russia (B series), Poland (P series), and Japan (JM series) (Kim and Yoon 1998; Robinson 2011). In the Cornell AgriTech rootstock breeding program, several rootstocks that are highly productive and resistant to fire blight, root rot, replant disease, and cold stress have been developed since the late 1960s (Robinson et al. 2006; Auvil et al. 2011; Fazio et al. 2011). From the advanced selections, some of the G series rootstocks with high precocity and high resistance to replant disease, fire blight, and cold stress (Russo et al. 2007; Cline et al. 2010) have been commercially released, including G11, G202, and G935 in the USA, G11 in Canada, and G214 in New Zealand (Robinson et al. 2006).
In Korea, there are scattered orchards with heavy soil that do not drain well. Orchards established in bottomlands along rivers are often vulnerable to drainage because of the high groundwater level. Moreover, annual precipitation reaches 1100–1300 mm and occurs mostly during the apple growing season, which is from June to September (Korea Meteorological Administration, https://kma.go.kr/eng/index.jsp). Therefore, orchards with the abovementioned conditions may show compromised growth of M9 and M26 trees, which have low waterlogging tolerance.
This study aimed to select rootstock series with high waterlogging tolerance in terms of plant growth and physiology, using recently bred rootstocks of G and CG series and compared them with rootstocks of M9 and M26, which are the most widely used in Korea.
2 Materials and methods
2.1 Plant materials
This study was carried out in 2017 at a greenhouse of the College of Agriculture and Life Sciences, Kyungpook National University, Gunwi, Korea, using six types of the G and CG rootstock series, which were acquired from Cornell University, and M26 and M9 rootstocks, which are already widely used in Korea. The ‘Fuji’ variety was grafted as a scion onto 1-year-old G11, G202, G214, G935, CG4814, CG5087, M26, and M9 rootstocks. On March 5th, the grafted trees were planted in 17-L pots (one tree per pot) filled with a 5:3:2 (v/v) soil mixture of sand:horticultural growing media:compost. A valve with an on-and-off function was installed at the bottom of the pot to allow drainage. Before planting, roots were trimmed to minimize the differences between the dry matter of the rootstocks. Two months after planting, the entire surface of the pots was covered with a black, nonwoven fabric to retain soil moisture and prevent excessive temperature increases in the rhizosphere.
2.2 Irrigation Regimes
The experiment was divided into two blocks (CT vs. WT), and each block was divided into five plots. Within each of them, a single tree of the same rootstock was randomly placed. Water (1.0–1.5 L) was supplied to all grafted trees once or twice daily, depending on the growing season and meteorological conditions, via drip irrigation. Foliar fertilization was applied as 0.3% urea in early May, and 20 g of compound fertilizer (N:P:K = 21:17:17) per pot was applied in mid-May. The water management regime was changed after June 23rd, on the 110th day after planting. Gradual flooding for the waterlogging treatment (WT) was induced by completely flooding the root zone for 2 days, followed by 3 days of drainage during the first 10 days after the change in water management. For the following 10 days, the root zone was flooded for 3 days and drained for 2 days. Subsequently, flooding and drainage were applied on alternate days for a week. The control treatment (CT) was subjected to sufficient irrigation (2.0 L per day) during the WT period. WT was stopped on July 21st, the 27th day after the onset of flooding, and from July 22nd, trees under this treatment were irrigated in the same manner as those under CT. The normal irrigation period lasted 18 days until August 8th.
2.3 Plant growth measurement
The leaf water potential (Ѱleaf) was measured using a pressure chamber (Model 3005, Soil Moisture Equipment Corp., Goleta, CA, USA) three times: before WT, on the 27th day after the onset of flooding, and on the 18th day after the cessation of flooding. The predawn leaf water potential (Ѱpd) was measured in one leaf per replication between 04:00 and 06:00 h, and the midday leaf water potential (Ѱmd) was measured in the same way as Ѱpd between 12:00 and 14:00 h on the same day. The leaves used for Ѱleaf measurement were reflected in the leaf area and leaf dry weight data.
The net photosynthetic rate (Pn) and stomatal conductance (Gs) were measured between 11:00 and 15:00 h using a portable gas exchange system (LCI Portable Photosynthesis System, ADC BioScientific Ltd., Hoddesdon, UK) on one to two mature leaves per replication at the following time points: before WT, on the 27th day after the onset of flooding, and on the 18th day after the cessation of flooding. Photosynthetically active radiation values were fixed at 1500 μmol m−2 s−1 with a halogen lamp for photosynthesis measurements.
Tree height was recorded at weekly intervals from the start to the end of the treatments. The scion diameter was measured using a digital Vernier caliper (CD-20CPX, Mitutoyo, Kawasaki, Japan) in the lattice direction, at 5 cm above the graft union, after which the trunk cross-sectional area (TCA) was obtained.
The proportion of fallen leaves (i.e., defoliation) during the WT period, expressed as a percentage of the total number of leaves, was obtained via daily surveys. On August 9th, at the end of the 18-day recovery period, the leaf area was determined using a portable leaf area meter with conveyor belt assembly (LI-3000, Li-Cor, Inc., Lincoln, NE, USA) by measuring all the leaves attached to the tree before separating various parts of the plants. Then, the trees used for the experiments (both WT and CT) were separated into shoot, leaf, fine-root (< 2 mm diameter), and coarse-root (> 2 mm diameter) categories. Samples were then dried at 72 °C for 72 h to obtain the dry weight of each sample. Data were analyzed by one- or two-way analysis of variance (ANOVA) using SAS statistical analysis software (SAS 9.4, SAS Institute, Inc., Cary, NC, USA). Significant differences among the means were evaluated using Duncan’s multiple range test at P < 0.05.
3 Results
3.1 Leaf water potential
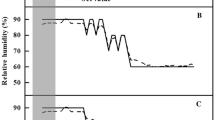
The Ѱpd of ‘Fuji’ trees measured before flooding was − 0.28 to − 0.35 MPa, regardless of the rootstock type, in both CT and WT trees, and the Ѱmd ranged from − 1.55 to − 1.89 MPa (Fig. 1), also regardless of the rootstock type. In CT trees, the Ѱpd on the 27th day after the onset of flooding ranged from − 0.30 to − 0.37 MPa for all grafted trees. Under WT, the Ѱpd of CG4814, G11, G935, CG5087, and M26 trees varied from − 0.34 to − 0.48 MPa, but was − 1.26, − 1.45, and − 1.28 MPa for G202, G214, and M9 trees, respectively; these values were markedly lower than those of trees grafted on other rootstocks. The Ѱmd of CT trees varied from − 1.65 to − 1.92 MPa, irrespective of rootstocks. Under WT, Ѱmd varied from − 2.10 to − 2.61 MPa in G11, G935, CG4814, M26, and CG5087 trees, and from − 2.85 to − 3.03 MPa in G202, G214, and M9 trees.
Changes in the predawn (a) and midday (b) leaf water potential of ‘Fuji’ nursery trees grafted on various rootstocks in CT (control treatment) and WT (waterlogging treatment); before flooding (measured on June 23rd), on the 27th day after the onset of flooding (measured on July 21st), and on the 18th day after the cessation of flooding (measured on August 8th). Vertical bars represent standard errors of the means. Bars with different letters are significantly different at P < 0.05 by Duncan’s multiple range test; ns: not significant
The Ѱpd and Ѱmd were also measured on the 18th day after the cessation of flooding. The Ѱpd of G202, G214, and M9 trees under WT ranged from − 0.72 to − 0.75 MPa, which was lower than that of the same rootstocks under CT. However, in trees on the other rootstocks, the Ѱpd range (− 0.38 to − 0.42 MPa) under WT was similar to that under CT (− 0.32 to − 0.38 MPa). The Ѱmd range (− 2.62 to − 3.15 MPa) for G202, G214, and M9 trees under WT was markedly lower compared with trees on the same rootstocks under CT, and the Ѱmd of the other trees ranged from − 2.05 to − 2.45 MPa.
3.2 Net photosynthetic rate and stomatal conductance
The Pn during the daytime before WT was 11.9–13.2 µmol m−2 s−1, and there was no noticeable difference among trees grafted on the various rootstocks (Fig. 2). However, on the 27th day after flooding started, the Pn of all CT trees was 12.5–14.4 μmol m−2 s−1. Under WT, the Pn of CG5087, G935, CG4814, and G11 trees was 4.4–5.3 μmol m−2 s−1, and that of G214, M9, G202, and M26 trees was 1.6–3.1 μmol m−2 s−1. The Pn of M26, G11, CG5087, G935, and CG4814 trees recovered to 5.2–7.0 μmol m−2 s−1 on the 18th day after the cessation of flooding. However, M9 and G214 trees had a Pn of 21% and 34% of that of M9 and G214 trees under CT (2.5 and 3.8 μmol m−2 s−1, respectively), whereas that of G202 trees was 4.8 μmol m−2 s−1 or 48% of the same trees under CT.
The net photosynthetic rate (a) and stomatal conductance (b) of ‘Fuji’ nursery trees grafted on various rootstocks in CT (control treatment) and WT (waterlogging treatment); before flooding (measured on June 23rd), on the 27th day after the onset of flooding (measured on July 21st), and on the 18th day after the cessation of flooding (measured on August 8th). Qleaf (photosynthetically-active radiation) was fixed at 1500 µmol·m−2·s−1. Vertical bars represent standard errors of the means. Bars with different letters are significantly different at P < 0.05 by Duncan’s multiple range test; ns: not significant
Changes in Gs were associated with the Ѱleaf of trees. On July 21st, the Gs value ranged from 0.03 to 0.06 mol H2O m−2 s−1 in M9, G202, and G214 trees. At the end of the trial, the Ѱleaf improvement increased the Gs value to 0.07–0.13 mol H2O m−2 s−1, which affected the increase in Pn.
3.3 Vegetative growth
At the onset of WT, the height of all grafted trees varied between 98 and 128 cm, with G202 and M26 trees being the tallest, and M9 trees being the shortest (Table 1). After 27 days of flooding and 18 days of normal irrigation, the height of trees under WT was 71% (M9) to 84% (CG5087) of that of trees under CT, indicating that tree growth was inhibited by flooding. The height of the trees grafted on other rootstocks was 75–83% of that of the same rootstocks under CT. At the end of flooding, the TCA of trees under WT was markedly decreased to 53–72% of that of trees under CT (Table 1). The TCA of G935, CG5087, and CG4814 trees was 67–72% of that of the same rootstocks under CT, which was relatively better than the TCA of G214 and M9 trees (53% and 55% of that of same rootstocks under CT, respectively).
Changes in the growth rates of trees under WT revealed that growth inhibition was relatively weak until the second week of flooding (Fig. 3). From the third week of flooding, growth rates varied significantly (P < 0.05) among the trees. Trees grafted on G935, CG5087, and CG4814 showed growth rates of 68–77% of that of the same rootstocks under CT. The growth rates of G214 and M9 trees decreased to 35% and 51%, respectively, of those of the same rootstocks under CT. By the fourth week of flooding, the growth rates of all trees under WT had further decreased. The growth rates of M26, CG5087, and CG4814 trees and of M9, G214, and G202 trees decreased to 45–55% and 18–34%, respectively, compared with the rates of the same rootstocks under CT. On August 8th, G11, CG5087, G935, and CG4814 trees showed growth rates of 26–32% of that of the same rootstocks under CT. The growth rates of M9, G214, G202, and M26 trees were 7–14% of those of the same rootstocks under CT. Thus, although flooding was terminated, its effect continued throughout the normal irrigation period.
Changes in the relative growth rate of tree height (RGRtree height = weekly growth in WT (waterlogging treatment)/weekly growth in CT (control treatment)) of ‘Fuji’ nursery trees subjected to WT. Arrows indicate the onset and end of flooding, respectively. Different letters in the same vertical column are significantly different at P < 0.05 by Duncan’s multiple range test; ns: not significant
3.4 Dry matter production
There was a significant difference (P < 0.05) between the dry weights of trees subjected to the different treatments (Table 2). G202 and M26 trees grew the most vigorously and showed similar dry weights of shoots (44.5 and 43.5 g, respectively) and fine roots (8.17 and 7.63 g, respectively), followed by G935 and G214 trees, whereas M9 trees showed shoot and fine-root dry weights of 31.70 and 5.14 g, respectively, under CT. Comparisons between the dry weights of shoots and leaves of grafted trees under both WT and CT revealed that shoots and leaves of CG4814 trees reached 79% and 74% of the dry weights of the same rootstock under CT, respectively, thereby showing the best growth under flooding conditions. Conversely, M9 trees were the most sensitive to flooding, with respectively, shoot and leaf dry weights of 40% and 30% of those of M9 trees under CT. The shoot and leaf dry weights of trees grafted on other rootstocks were 43–50% and 39–56%, respectively, of those of trees grafted on the same rootstocks under CT. Unlike that of the aboveground parts, the fine-root dry weight of CG4814 trees was 12% higher under WT than under CT. However, the fine-root dry weights of CG5087, G11, and G935 trees were 84–87%, G202, G214, and M26 were 70–74%, and that of M9 was 44% of those of the same rootstocks under CT, respectively (Table 2).
3.5 Defoliation and leaf area
The percentage of defoliation during the 27 days of flooding and 18 days of normal irrigation after the cessation of flooding, differed according to the rootstock type (Fig. 4). Defoliation progressed from the trunk near the ground, starting from the middle of the second week of flooding, but its influence continued after the flooding ended. M9 trees were the most sensitive to flooding, with 35% defoliation, followed by G214, G202, CG5087, G11, M26, G935, and CG4814 trees, with 23%, 22%, 9%, 8%, 6%, 5%, and 4% defoliation, respectively. All trees exposed to the flooding event showed reduced leaf area through defoliation and accelerated aging of the attached leaves (Fig. 4). In the submerged state, leaves were more sensitive to growth reduction than roots (Table 2). M9 trees with the highest defoliation rate in WT had the largest growth reduction in leaf area production, which was 65% of that of the same rootstock under CT, while CG4814 tree had 35%. These leaf area production differences suggest that the rhizosphere stress sensitivity caused by flooding was transmitted to the leaves.
4 Discussion
Root growth is generally more likely to be constrained by low oxygen conditions than by high carbon dioxide conditions (Kramer 1983a). At low oxygen concentrations, the hydraulic conductivity of roots is reduced because membrane proteins, which regulate the water entering and exiting the cell, inhibit water transport, subsequently affecting the Ѱleaf (Else et al. 2001; Parent et al. 2008). In this study, the Ѱpd and Ѱmd of G202, G214, and M9 trees were significantly (P < 0.05) lower than those of trees grafted on other rootstocks on the 27th day after flooding (Fig. 1), which, in turn, seems to have a direct relationship to Pn and dry matter production (Fig. 2, Table 2). Increased resistance of the roots’ water absorption–migration pathways (Watanabe et al. 2013) is believed to be more pronounced in these three trees, suggesting a difference in sensitivity to submerged stress among the trees. The Ѱleaf of trees grafted on G202, G214, and M9 rootstocks remained low even on the 18th day after the cessation of flooding, indicating that the trees could not rapidly or fully recover from root-system damage caused by waterlogging.
The extent of the stress exerted on plants by flooding can be estimated by measuring leaf gas exchange rates (Schaffer et al. 1992). Decreased photosynthesis at the initial stage of flooding has been related to Gs, However, with prolonged flooding, changes in the activities of carboxylation enzymes and the loss of chlorophyll are assumed to affect Pn (Kozlowski 1997). The decreased Pn observed in WT plants on the 27th day after flooding (Fig. 2), regardless of rootstock type, may be attributed to the decrease in hydraulic conductivity of roots caused by insufficient oxygen in the rhizosphere. In particular, the Pn of G202, G214, and M9 trees was lower than that of trees grafted on other rootstocks, presumably because their Ѱleaf decreased to − 2.85 MPa or less (Fig. 1), thereby resulting in loss of turgor pressure and closure of the stoma. On the 18th day after the cessation of flooding, the gas exchange rates of all grafted trees recovered to some extent, but these rates were still markedly lower in G214 and M9 trees under WT (Fig. 2). In these trees, the water absorption function of the roots is still degraded and is also considered to be related to the levels of hormones involved in stomatal opening, such as abscisic acid or ethylene (Blanke and Cooke 2004).
The height growth of flooded trees did not differ greatly from that of CT trees until the second week after flooding treatment, after which there was a significant difference (P < 0.05) among trees grafted with the various rootstocks (Fig. 3). This finding indicates that the apple tree is rather resistant to flooding at initial stages. Furthermore, in this study, G202, G214, M9, or M26 trees, with relatively weak resistance to flooding, seemed to lose their resistance rapidly if the flooding exceeded certain limits. However, the growth inhibition of G11, G935, CG4814, and CG5087 trees under flooding was lower than that of other trees (Fig. 3). Presumably, as the Ѱleaf value remained higher for a longer time than other trees in WT (Fig. 1), it resulted in relatively less restricted cell division and elongation and less interference with cytokinin synthesis and transport in roots (Kramer 1983b; Mielke et al. 2003), as well as the slight differences in the supply of photosynthate and nutrients caused by differences in Gs (Fig. 2), which may have affected the potential growth of trees.
Differences in the decreased range of the biomass of shoots, leaves, and roots among the trees grafted on various rootstocks could be attributed to their different Ѱleaf and Pn values during the WT period (Table 2). Repeated flooding and drainage may also have resulted in the leaching or denitrification of plant nutrients, such as nitrogen, which is essential for plant growth in the soil (Bacon et al. 1986). The decrease in transpiration caused by flooding stress would have adversely affected plant growth by limiting the flow of nutrients in the soil to the plant (Colin-Belgrand et al. 1991). Root development is influenced by many factors, such as scion and rootstock type, soil texture, soil moisture, soil air permeability, soil nutrients, and soil microorganisms (Webster 2005). In this study, the coarse-root dry weight was slightly higher in trees under WT than in those under CT, which may be because of root trimming before planting. In addition, the differences in the coarse-root dry weight between trees grafted on C or CG series and M series rootstocks seemed to be a result of rootstock characteristics (Abod and Webster 1989), given that most of the new roots of the M9 rootstock regenerated in the stem. The production of the fine-root dry matter of CG4814 tree was found to be slightly higher in WT than in CT, apparently because of the differences in the amount of coarse-root biomass attached to the trunk before planting (Table 2). Nevertheless, considering that the fine-root dry matter did not decrease in WT compared with CT, CG4814 tree appears to be relatively less sensitive to flooding.
Defoliation of flooded plants could be related to the production of ethylene (El-Beltagy and Hall 1974), which inhibits the formation of indole-3-acetic acid and blocks its transport, thereby increasing abscisic acid production and promoting leaf abscission (Addicott 1991). During the flooding period, G202, G214, and M9 trees suffered more damage than trees grafted on other rootstocks. Consequently, the stress induced by flooding may affect the rate of defoliation (Fig. 4). However, other rootstocks exhibited some resistance to flooding, as the defoliation of trees grafted on these rootstocks in response to flooding was lower than that of G202, G214, and M9 trees. In this study, the leaf area reduction caused by the increased defoliation rate (Fig. 4) is believed to be because of a physiological disorder rather than the mechanism by which plants adapt to waterlogging when affected by water absorption under anaerobic conditions of the rhizosphere (Gomez-Cadenas et al. 1996; Parolin and Wittmann 2010). Thus, it is assumed that the excessive leaf area reduction (e.g., G202, G214, and M9 trees) caused by flooding may have not only diminished the potential for plant regrowth but also affected the dry matter production and partitioning of each part of the tree as a result of decreased export of the photosynthate produced by the whole plant to sinks.
In conclusion, repeated flooding for 4 weeks had a markedly negative impact on physiological responses, dry matter production, and tree height growth. In the initial stages of flooding, all trees exhibited some extent of flooding resistance. From the third week after flooding treatment, the growth of trees drastically deteriorated as evidenced by symptoms of slowly withering leaves in some trees, and the differences in waterlogging tolerance between the rootstock types began to emerge. Even at the end of the trial, WT trees still had lower Ѱleaf and Pn than CT trees, and some individual types with large leaf area losses because of defoliation, such as G202, G214, and M9 trees, are not expected to recover within a short period. Therefore, the results of this study suggest that trees grafted on M9 rootstock were the most sensitive to flooding, and G214 and G202 trees showed similar sensitivity levels as M9 trees. Conversely, trees grafted on CG4814 rootstock were more resistant to flooding than M26 trees, and CG5087, G935, and G11 trees showed comparable tolerance to waterlogging as M26 trees.
Abbreviations
- CT:
-
Control treatment
- G s :
-
Stomatal conductance
- P n :
-
Net photosynthetic rate
- WT:
-
Waterlogging treatment
- Ѱleaf :
-
Leaf water potential
- Ѱmd :
-
Midday leaf water potential
- Ѱpd :
-
Predawn leaf water potential
References
Abod SA, Webster AD (1989) Root and shoot growth of newly transplanted apple trees as affected by rootstock cultivar, defoliation and time after transplanting. J Hort Sci 64:655–666. https://doi.org/10.1080/14620316.1989.11516005
Addicott FT (1991) Abscission: shedding of parts. In: Raghavendra AS (ed) Physiology of trees. Wiley, New York, pp 273–300
Auvil TD, Schmidt TR, Hanrahan I, Castillo F, McFerson JR, Fazio G (2011) Evaluation of dwarfing rootstocks in Washington apple replant sites. Acta Hortic 903:265–271. https://doi.org/10.17660/ActaHortic.2011.903.33
Bacon PE, Mcgarity JW, Hoult EH, Alter D (1986) Soil mineral nitrogen concentration within cycles of flood irrigation: effect of rice stubble and fertilization management. Soil Biol Biochem 18:173–178. https://doi.org/10.1016/0038-0717(86)90023-4
Blanke MM, Cooke DT (2004) Effects of flooding and drought on stomatal activity, transpiration, photosynthesis, water potential and water channel activity in strawberry stolons and leaves. Plant Growth Regul 42:153–160. https://doi.org/10.1023/B:GROW.0000017489.21970.d4
Cline JA, Norton D, Embree CG, Privé JP (2010) Performance of Jonagold, McIntosh and Novaspy on three new semi-dwarf apple rootstocks in eastern Canada. Can J Plant Sci 90:877–883. https://doi.org/10.4141/cjps09186
Colin-Belgrand M, Dreyer E, Biron P (1991) Sensitivity of seedlings from different oak species to waterlogging: effects on root growth and mineral nutrition. Ann Sci For 48:193–204. https://doi.org/10.1051/forest:19910206
El-Beltagy AS, Hall MA (1974) Effect of water stress upon endogenous ethylene levels in Vicia faba. New Phytol 73:47–60. https://doi.org/10.1111/j.1469-8137.1974.tb04605.x
Else MA, Coupland D, Dutton L, Jackson MB (2001) Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol Plant 111:46–54. https://doi.org/10.1034/j.1399-3054.2001.1110107.x
Fazio G, Aldwinckle HS, Robinson TL, Wan Y (2011) Implementation of molecular marker technologies in the Apple Rootstock Breeding program in Geneva—challenges and successes. Acta Hortic 903:61–68. https://doi.org/10.17660/ActaHortic.2011.903.3
Gomez-Cadenas A, Tadeo FR, Talon M, Primo-Millo E (1996) Leaf abscission induced by ethylene in water-stressed intact seedlings of Cleopatra mandarin requires previous abscisic acid accumulation in roots. Plant Physiol 112:401–408. https://doi.org/10.1104/pp.112.1.401
Kim KR, Yoon TM (1998) Techniques for the production of superior nursery apple trees. In: Kim KR, Yoon TM (eds) Andong National University Press. Andong, Korea, pp 28–45
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiol Monogr 1:1–29. https://doi.org/10.1093/treephys/17.7.490
Kramer PJ (1983a) Development of root systems. In: Kramer PJ (ed) Water relations of plants. Academic Press, London, pp 170–177
Kramer PJ (1983b) Water deficits and plant growth. In: Kramer PJ (ed) Water relations of plants. Academic Press, London, pp 342–389
Kviklys D, Robinson TL, Fazio G (2016) Apple rootstock evaluation for apple replant disease. Acta Hortic 1130:425–430. https://doi.org/10.17660/ActaHortic.2016.1130.63
Lakso AN (2003) Water relations of apples. In: Ferree DC, Warrington IJ (eds) Apples: botany, production and uses. CABI Publishing, Cambridge, pp 167–194
Lee CH, Sugiura A, Tomana T (1982) Effect of flooding on the growth and some physiological changes of young apple rootstock (in Japanese with English summary). J Jap Soc Hortic Sci 51:270–277. https://doi.org/10.2503/jjshs.51.270
Marchioretto LDR, Leonardo ADR, Amaral LOD, Ribeiro AMADS (2018) Tolerance of apple rootstocks to short-term waterlogging. Cienc Rural. https://doi.org/10.1590/0103-8478cr20170940
Mielke MS, Almeida AF, Gomes FP, Aguilar MAG, Mangabeira PAO (2003) Leaf gas exchange, chlorophyll fluorescence and growth responses of Genipa americana seedlings to soil flooding. Environ Exp Bot 50:221–231. https://doi.org/10.1016/s0098-8472(03)00036-4
Oh SD (1998) Emphasis on low tree & high density planting system—future prospects of research strategies in Korea. Kor J Hortic Sci Technol 16:264–268
Parent C, Capelli N, Berger A, Crèvecoeur M, Dat JF (2008) An overview of plant responses to soil waterlogging. Plant Stress 2:20–27
Parolin P, Wittmann F (2010) Struggle in the flood: tree responses to flooding stress infour tropical floodplain systems. AoB Plants. https://doi.org/10.1093/aobpla/plq003
Robinson TL (2003) Apple-orchard planting systems. In: Ferree DC, Warrington IJ (eds) Apples: botany, production and uses. CABI Publishing, Cambridge, pp 345–385
Robinson TL (2011) Advances in apple culture worldwide. Rev Bras Frutic 33:37–47. https://doi.org/10.1590/s0100-29452011000500006
Robinson TL, Fazio G, Aldwinckle HS, Hoying SA, Russo N (2006) Field performance of Geneva® apple rootstocks in the Eastern USA. Sodininkystė ir daržininkystė 25:181–191
Russo NL, Robinson TL, Fazio G, Aldwinckle HS (2007) Field evaluation of 64 apple rootstocks for orchard performance and fire blight resistance. HortScience 42:1517–1525
Schaffer B, Andersen PC, Ploetz RC (1992) Responses of fruit crops to flooding. In: Janick J (ed) Horticultural reviews, vol 13. Wiley, New York, pp 257–313
Watanabe K, Nishiuchi S, Kulichikhin K, Nakazono M (2013) Does suberin accumulation in plant roots contribute to waterlogging tolerance? Front Plant Sci 4:178. https://doi.org/10.3389/fpls.2013.00178
Webster AD (2005) Roots and root growth. In: Tromp J, Webster AD, Wertheim SJ (eds) Fundamentals of temperate zone tree fruit production. Backhuys Publishers, Leiden, pp 107–119
Webster AD, Wertheim SJ (2003) Apple rootstocks. In: Ferree DC, Warrington IJ (eds) Apples: botany, production and uses. CABI Publishing, Cambridge, pp 91–124
Webster T, Tobutt K, Evans K (2000) Breeding and evaluation of new rootstocks of apple pear and sweet cherry. The Compact Fruit Tree 33:100–104
Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through Agri-Bio Industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (315020-5).
Funding
This study was funded by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through Agri-Bio Industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA).
Author information
Authors and Affiliations
Contributions
T.M.Y. planned the experiment, developed the theoretical formalism, and verified the analytical methods; B.H.C. conducted the experiments, and wrote the paper; N.B. provided technical assistance; W.T.J., I.H.P., and S.G.H. contributed to the design and implementation of the research.
Corresponding author
Ethics declarations
Conflict of interest
Authors B.H.C., N.B., W.T.J., I.H.P., and S.G.H. have received research grants from IPET.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Ikjo Chun.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, BH., Bhusal, N., Jeong, WT. et al. Waterlogging tolerance in apple trees grafted on rootstocks from G, CG, and M series. Hortic. Environ. Biotechnol. 61, 685–692 (2020). https://doi.org/10.1007/s13580-020-00258-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-020-00258-2