Abstract
The methanol extracts of flowers obtained from 67 plant species were screened for their inhibitory activity on aldose reductase (AR). Alnus japonica, Aster spathulifolius, Chionanthus retusus, Morus bombycis, Crysanthemum boreale, Persicaria tinctoria, Platycarya strobilacea, and Serratula coronata var. insularis exhibited potent aldose reductase inhibitory (ARI) activity. HPLC-UV analysis of quercetin, an AR inhibitory flavonoid, was performed on extracts showing strong ARI activity. Quercetin was detected in C. retusus, C. boreale, P. tinctoria, and S. coronata var. insularis at concentrations of 1.33, 1.56, 0.82, and 3.37 mg g−1 extract, respectively, indicating that quercetin contributed to the ARI activity of these extracts. In the samples in which quercetin was absent, other compounds may be responsible for their potent ARI activity. These results serve as a basis for further studies regarding the bioactive components responsible for the inhibitory effects of various flower extracts on AR activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aldose reductase (AR) is an oxidoreductase that catalyzes the NADPH-dependent reduction of various aldehydes and aldoses. It is the first enzyme of the polyol pathway, which is responsible for the conversion of glucose to sorbitol (Tang et al. 2012). In hyperglycemic conditions, an increased flux of non-phosphorylated glucose into the polyol pathway results in heightened levels of reactive oxygen species and an imbalance in NADH/NAD+, which ultimately results in oxidative stress within cells (Lorenzi 2007). The cellular stress generated from the polyol pathway during such conditions is linked to the pathogenesis of complications associated with diabetic hyperglycemia such as neuropathy, retinopathy, and nephropathy (Dunlop 2000). The association between diabetes-induced tissue damage and the polyol pathway is further supported by the observation that the activity of the enzymes of the polyol pathway is increased under high glucose conditions and by their presence in most tissues and organs that are susceptible to diabetic complications such as the lenses, kidneys, and neurons (Brownlee 2004). Moreover, inhibition of AR, a rate-limiting enzyme of the polyol pathway, has been shown to delay and prevent the onset of tissue-injury in hyperglycemic animal models, indicating that this pathway plays a role in the etiology of diabetic diseases (Cheung et al. 2005). Thus, inhibition of AR is an attractive target for drug design to mitigate diabetic complications.
Many studies have reported on the potent inhibitory properties of various phytochemicals on AR including quercetin, quercitrin, epicatechin gallate, isoquercitrin, and rutin (Grewal et al. 2016). The aim of this study is to screen the AR inhibitory (ARI) activity of different flowers collected in Korea and to quantify the amount of quercetin in the extracts showing potent ARI effects. The results of this study provide preliminary information as to which plant extracts show promising ARI activity.
2 Materials and methods
2.1 Plant materials and animal specimens
Flowers from 67 plant species were used in this study. The samples were either obtained from the field and extracted with methanol (MeOH) or purchased as MeOH extracts from the Korea Research Institute of Bioscience and Biotechnology (KRIBB), Korea (Tables 1, 2, 3, 4, 5, 6). Flowers were collected in Korea during the spring season. Flowers belonging to the same species but having different colors were considered as separate samples. Prior to extraction, the flowers were dried in the shade and voucher specimens were deposited at the herbarium of the Department of Integrative Plant Science, Chung-Ang University, Korea. Seven-week-old Sprague-Dawley rats weighing 210–230 g were acquired from Koatech, Korea.
2.2 Chemicals and apparatuses
Sodium buffer, MeOH, and potassium buffer were obtained from Samchun Pure Chemical Co. (Pyeongtaek, Korea). Dimethyl sulfoxide (DMSO), 3,3′-tetramethyleneglutaric acid (TMG), dl-glyceraldehyde, and β-nicotinamide dinucleotide phosphate (NADPH) were purchased from Sigma Aldrich Chemical (St. Louis, USA). An EYELA rotary evaporator system (Japan) was used for evaporation in vacuo. An Allegra X-30R refrigerated benchtop centrifuge (Beckman Coulter™, MD, USA) and an Optizen 2120 UV spectrophotometer (Mecasys Co., Daejeon, Korea) were used for the ARI assay.
2.3 Sample preparation for the ARI assay
Six grams of dried flowers were extracted with 300 mL of methanol at 80 °C for 3 h. The resulting extracts were filtered to remove debris and evaporated to dryness under reduced pressure. The MeOH extracts were tested for their ARI activity. Samples were prepared for the assay by dissolving 1 mg of the flower extracts in 1 mL DMSO.
2.4 Preparation of AR from rat lenses
AR was prepared from rat lenses following a previously described protocol (Hayman and Kinoshita 1965). Lenses from healthy Sprague-Dawley rats were resected and homogenized in 0.1 M sodium buffer (pH 6.2), to which 0.5 mL buffer was added for every lens used. The homogenate was centrifuged at 10,000 rpm at 4 °C for 20 min, and the resulting supernatant was collected and used as the source of AR in the ARI activity assay.
2.5 Measurement of AR inhibitory activity
The ARI activity of the flower extracts was determined by spectrophotometrically measuring the decrease in NADPH absorbance at 340 nm for a period of 4 min, using dl-glyceraldehyde as a substrate for AR. The total volume of the assay solution was 1 mL and it comprised the rat lens AR, plant extract dissolved in DMSO, 25 mM dl-glyceraldehyde, 1.6 mM NADPH, 100 mM sodium buffer and 100 mM potassium phosphate buffer (pH 7.0). TMG was used as a positive control. The inhibitory activity of the samples was expressed as: (%) Inhibition = (normal enzyme activity − inhibited enzyme activity)/normal enzyme activity. The IC50 values were determined from the least-squares regression line of the logarithmic concentrations plotted against residual activity.
2.6 Standard and sample preparation for HPLC-UV analysis
HPLC samples were prepared by dissolving 10–20 mg of the flower extracts in 1 mL MeOH. A standard stock solution of quercetin was prepared by dissolving 1 mg of quercetin in 1 mL MeOH. All samples were filtered through a 0.45-µm filter prior to use.
2.7 HPLC-UV analysis of quercetin in MeOH flower extracts
The extracts with the highest inhibitory effects on AR from each flower color category were analyzed for their quercetin content. An Agilent HPLC system was used for the analysis. Chromatographic separation was performed with a reverse-phase INNO C18 (4.6 × 250 mm, 5 μm) column. A gradient elution of 0.5% acetic acid in water: acetonitrile (90:10 to 50:50 for 50 min) was followed. The flow rate and injection volume were 1 mL min−1 and 10 μL, respectively. The UV detector was set at 270 nm.
2.8 Calibration curve
The working solutions used to construct a calibration curve were obtained by diluting the quercetin stock solution to the desired concentrations. The calibration curves were used to determine the quercetin content in the samples. Linearity was assessed based on the correlation coefficient (r2).
3 Results and discussion
Diabetes is a metabolic disorder characterized by chronic hyperglycemia, and impaired fat, lipid, and carbohydrate metabolism. These attributes lead to various complications such as blindness, renal failure, and nerve damage, and thus, contribute to the overall morbidity of the disease. Studies have shown that the tissue damage caused by diabetic hyperglycemia is linked to four major mechanisms, namely, the increased flux of non-phosphorylated glucose to the polyol pathway, activation of the protein kinase C pathway, production of advanced glycation end-products, and increased activity of the hexosamine pathway (Brownlee 2001). The polyol pathway in particular has been widely studied because the inhibition of AR, a key enzyme of the pathway, prevents and sometimes reverts diabetic complications (Engerman et al. 1994; Brownlee 2004). To date, numerous AR inhibitors have been established including synthetic and naturally occurring compounds.
In this study, the inhibitory activity of MeOH extracts of various flowers on crude rat lens AR was evaluated. The samples were grouped according to their colors and the results are summarized in Tables 1, 2, 3, 4, 5 and 6. The results show that there is no apparent relationship between flower color and ARI activity of the MeOH extracts from the flowers that we screened. However, we observed that purple and darker colored flowers tend to have lower inhibitory activity against AR. Among the extracts tested, A. japonica, M. bombycis, and P. strobilacea showed the most potent ARI effects, all having an IC50 value of 0.18 μg mL−1. The potent ARI activity of these extracts can be attributed to the presence of bioactive compounds. A. japonica is abundant in diarylheptanoids, a class of compounds shown to exhibit ARI activity (Kuroyanagi et al. 2005). Diarylheptanoids, specifically gingerenones, isolated from Zingiber officinale have a high binding affinity towards AR (Antony and Vijayan 2015). This finding suggests that diarylheptanoids present in A. japonica might also have a high binding affinity towards AR, providing a possible explanation for its inhibitory effect on AR. Recent studies have shown that Morus spp. contain many bioactive compounds including flavonoids, alkaloids, and anthocyanins (Song et al. 2009). Flavonoids in particular have been shown to have strong ARI activities (Grewal et al. 2016). Kim and Oh (1999) reported that extracts from the bark, wood, and leaves of P. strobilacea moderately inhibit AR activity (Kim and Oh 1999). Here, we showed that flower extracts from P. strobilacea have potent ARI activity. These results suggest that the bioactive compounds responsible for ARI activity in P. strobilacea extracts may be distributed in higher concentrations in the flowers. These results demonstrate the potential use of MeOH extracts from the flowers of A. japonica, M. bombycis, and P. strobilacea as sources of ARIs. Further research characterizing the bioactive components of these plants will provide insights into their inhibitory activity against AR.
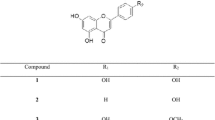
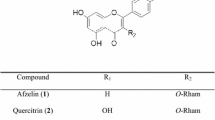
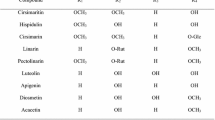
Quercetin (Fig. 1) is a flavonoid found ubiquitously in nature and is known to exhibit strong antioxidant properties and a wide range of biological activities, including anti-inflammatory, anti-diabetic, and anti-cancer effects (Deschner et al. 1991; Vessal et al. 2003; Boots et al. 2008; Yang et al. 2017; Ryu et al. 2018). The anti-diabetic properties of quercetin have been widely reported in literature in which several studies have reported quercetin as an AR inhibitor (Varma et al. 1975; Yawadio et al. 2007). The use of natural products such as quercetin not only alleviates the many disabling complications associated with diabetes but also provides protection against various diseases (e.g., cancer, osteoporosis, cardiovascular diseases) and promotes general well-being (Boots et al. 2008). Flavonoids are abundant in flowers, and quercetin has been found in many flower extracts (Fossen et al. 1999; Swaroop et al. 2005). Hence, samples showing high ARI activities were further analyzed for quercetin content. A list of the samples analyzed with their respective quercetin content is shown in Table 7. The analytical method used for the chromatographic separation showed good linearity (Fig. 2, r2 = 0.9999). Results show that quercetin was only detected in C. retusus, C. boreale, P. tinctoria, and S. coronata with concentrations of 1.33, 1.56, 0.82, and 3.37 mg g−1 extract, respectively. In the remaining plant samples, the chromatograms show that other, possibly novel compounds are present, which may be responsible for their ARI activities (Fig. 3). For example, flowers of Aster and Viola spp. are rich in terpenoids and flavonoids that have been shown to exhibit high ARI activities in several studies (Lee et al. 2005; Morikawa 2007; Lee et al. 2017a, b). Similarly, Chaenomeles, Tulipa and Carpinus spp. are abundant in polyphenolic compounds, which may explain their high AR inhibition (Huang et al. 2013; Hofmann et al. 2016). Further studies regarding the bioactive components of these plants may elucidate the mechanism of their bioactive properties.
References
Antony P, Vijayan R (2015) Identification of novel aldose reductase inhibitors from spices. PLoS ONE 10:e0138186
Boots A, Haenen GRMM, Bast A (2008) Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 585:325–337
Brownlee M (2001) The pathobiology of diabetic complications: a unifying mechanism. Nature 414:813–820
Brownlee M (2004) Biology and molecular cell biology of diabetic complications. Diabetes 54:1615–1625
Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK (2005) Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes 54:3119–3125
Deschner E, Ruperto J, Wong G, Newmark H (1991) Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis 12:1193–1196
Dunlop M (2000) Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int 58:3–12
Engerman RL, Kern TS, Larson ME (1994) Nerve conduction and aldose reductase inhibition during 5 years of diabetes or galactosaemia in dogs. Diabetologia 37:141–144
Fossen T, Larsen A, Kiremire B, Andersen O (1999) Flavonoids from blue flowers of Nymphaea caerulea. Phytochemistry 51:1133–1137
Grewal AS, Bhardwaj S, Pandita D, Lather V, Sekhon BS (2016) Update on aldose reductase inhibitors for management of diabetic complications and non-diabetic diseases. Mini Rev Med Chem 16:120–162
Hayman S, Kinoshita JJ (1965) Isolation and properties of lens aldose reductase. Biol Chem 240:877–882
Hofmann T, Nehebaj E, Albert L (2016) Antioxidant properties and detailed polyphenol profiling of European hornbeam (Carpinus betulus L.) leaves by multiple antioxidant capacity assays and high-performance liquid chromatography/multistage electrospray mass spectrometry. Ind Crops Prod 87:340–349
Huang GH, Xi ZX, Li JL, Chen C, Yang GJ, Chen WS, Zhu HY (2013) Isolation of two new phenolic compounds from the fruit of Chaenomeles speciosa (sweet) Nakai. Phytochem Lett 6:526–530
Kim YH, Oh JH (1999) Screening of Korean forest plants for rat lens aldose reductase inhibition. Biosci Biotechnol Biochem 63:184–188
Kuroyanagi M, Shimomae M, Nagashima Y, Muto N, Okuda T, Kawahar N, Nakane T, Sano T (2005) New diarylheptanoids from Alnus japonica and their antioxidative activity. Chem Pharm Bull 53:1519–1523
Lee SO, Choi SZ, Chou SU, Lee KC, Chin YW, Kim J, Kum YC, Lee KR (2005) Labdane diterpenes from Aster spathulifolius and their cytotoxic effects on human cancer cell lines. J Nat Prod 68:1471–1474
Lee J, Rodriguez JP, Quilantang NG, Lee M-H, Cho EJ, Jacinto SD, Lee S (2017a) Determination of flavonoids from Perilla frutescens var. japonica seeds and their inhibitory effect on aldose reductase. Appl Biol Chem 60:155–162
Lee J, Rodriguez JP, Lee KH, Park JY, Kang KS, Hahm D-H, Huh CK, Lee SC, Lee S (2017b) Determination of flavonoids from Cirsium japonicum var. maackii and their inhibitory activities against aldose reductase. Appl Biol Chem 60:487–496
Lorenzi M (2007) The Polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res 2007:1–10
Morikawa TJ (2007) Search for bioactive constituents from several medicinal foods: hepatoprotective, antidiabetic, and antiallergic activities. J Nat Med 61:112–116
Ryu J, Kwon S-J, Ahn JW, Kim SH, Lee SY, Kim J-B, Jo YD, Ha B-K, Kang S-Y (2018) Development of a stem-color mutant kenaf (Hibiscus cannabinus L.) cultivar, ‘Jeokbong’, and analysis of its functional compounds. Hortic Sci Technol 36:77–84
Song W, Wang HJ, Bucheli P, Zhang PF, Wei DZ, Lu YH (2009) Phytochemical profiles of different mulberry (Morus sp.) species from China. J Agric Food Chem 57:9133–9140
Swaroop A, Gupta P, Sinha K (2005) Simultaneous determination of quercetin, rutin, and coumaric acid in flowers of Rhododendron arboreum by HPTLC. Chromatographia 62:649–652
Tang WH, Martin K, Hwa J (2012) Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol 3:1–8
Varma SD, Mikuni I, Kinoshita JH (1975) Flavonoids as inhibitors of lens aldose reductase. Science 188:1215–1216
Vessal M, Hemmati M, Vasei M (2003) Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C 135:357–364
Yang H, Kim Y-J, Shin Y (2017) Antioxidant compounds and activities of different organs of potted Cymbidium spp. grown in Korea. Hortic Sci Technol 35:647–655
Yawadio R, Tanimori S, Morita N (2007) Identification of phenolic compounds isolated from pigmented rices and their aldose reductase inhibitory activities. Food Chem 101:1616–1625
Acknowledgements
This research was supported by a grant from Seoul Science High School (2017) and the Chung-Ang University Research Grants in 2017, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quilantang, N.G., Ryu, S.H., Park, S.H. et al. Inhibitory activity of methanol extracts from different colored flowers on aldose reductase and HPLC-UV analysis of quercetin. Hortic. Environ. Biotechnol. 59, 899–907 (2018). https://doi.org/10.1007/s13580-018-0072-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-018-0072-5