Abstract
Hypothermic machine perfusion (HMP) has been demonstrated to be more effective in mitigating ischemia–reperfusion injury (IRI) of donation after circulatory death (DCD) organs than cold storage (CS), yet the underlying mechanism remains obscure. We aimed to propose a novel therapeutic approach to ameliorate IRI in DCD liver transplantation. Twelve clinical liver samples were randomly assigned to HMP or CS treatment and subsequent transcriptomics analysis was performed. By combining in vivo HMP models, we discovered that HMP attenuated inflammation, oxidative stress, and apoptosis in DCD liver through a SEPRINA3-mediated PI3Kδ/AKT signaling cascade. Moreover, in the hypoxia/reoxygenation (H/R) model of BRL-3A, overexpression of SERPINA3 mitigated H/R-induced apoptosis, while SERPINA3 knockdown exacerbated cell injury. Idelalisib (IDE) treatment also reversed the protective effect of SERPINA3 overexpression. Overall, our research provided new insights into therapeutic strategies and identified potential novel molecular targets for therapeutic intervention against DCD liver.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Owing to the worldwide shortage of organ donors, the utilization of marginal donors has progressively emerged as a strategy to expand the donor pool [1, 2]. Moreover, patients receiving a donation after circulatory death (DCD) of the liver are more likely to suffer from severe ischemia–reperfusion injury (IRI), resulting in poor graft function and primary dysfunction, ultimately affecting the recipient survival rate [3, 4]. Therefore, it is necessary to establish effective strategies to protect the DCD grafts from IRI.
Hypothermic machine perfusion (HMP) has been extensively employed to improve organ preservation [5]. Numerous clinical trials have demonstrated that HMP, in comparison to cold storage (CS), effectively ameliorates liver function and postoperative complications. More specifically, the incidence of postoperative biliary anastomotic stenosis, post-reperfusion syndrome, and postoperative graft dysfunction was significantly lower in the HMP group than in the CS group [6]. Related animal studies have also shown that HMP can improve inflammation, oxidative stress, and apoptosis in DCD livers, which is consistent with previous research [7,8,9]. Although HMP has proven superior to CS, its underlying mechanisms of action remain less [10,11,12]. Meanwhile, owing to the large database related to rat livers, rats are optimally suited for studying HMP mechanisms [13].
SERPINA3 belongs to the SERine Proteinase Inhibitor (SERPIN) superfamily and participates in various biochemical reactions [14, 15]. More specifically, since its discovery, SERPINA3 has played a decisive role as an acute-phase reaction protein in inflammatory responses, tumors, wound healing, cardiovascular diseases, chronic obstructive pulmonary diseases, and neurodegenerative diseases [16,17,18,19,20,21]. SERPINA3 in urine can also serve as a marker of renal fibrosis and inflammation [22], whereas SERPINA3 may function to reduce inflammation and oxidative stress in the kidney, cornea, and retina [14, 23]. It activates phosphatidyl inositol-3-kinase (PI3K)δ, a gene that promotes the survival of hepatocellular carcinoma cells. [24, 25]. Collectively, these findings suggest that SERPINA3 regulates cell survival, inflammation, and oxidative stress; however, its role in liver IRI remains to be elucidated.
As a member of the PI3K IA class, PI3Kδ is involved in cellular immunity and inflammation [26, 27]. Cell proliferation, survival, inflammation, and oxidative stress are all regulated by the PI3K/protein kinase B (Akt) signaling pathway, which also plays an important role in apoptosis. Telomerase reverse transcriptase (TERT), the catalytic subunit of telomerase in eukaryotic cells regulates proliferation and differentiation. Relevant studies have shown that the PI3K/Akt pathway is involved in the regulation of TERT expression [28]. In the early stages of liver IRI, the PI3K/Akt pathway is activated to regulate liver apoptosis, inflammation, autophagy, and oxidative stress. Although PI3K/Akt is known to contribute significantly to IRI regulation, little is known about the mechanism associated with PI3Kδ in IRI.
The primary aim of the current study was to explore the roles of SERPINA3 and PI3Kδ/Akt in IRI pathogenesis, particularly in patients with DCD livers treated with HMP or CS. Thus, we hope to provide novel insights into the development of a potential new therapeutic strategy to improve IRI in DCD liver transplantation.
Materials and methods
Study design
We selected 12 cases of DCD liver grafts with CS at 4 °C or HMP in Lifeport liver transporter (Organ Recovery Systems, Itasca, IL, USA) for 2 h according to the randomization principle and then analyzed the clinical data of liver transplant patients. BGISEQ-500-RNAseq technology was used to detect differences in gene expression between the two groups of liver tissues. Besides, GO and KEGG pathway analyses were used to detect the notable pathway, and western blot was used to verify the related protein expressions of the significantly different signaling pathways. Simultaneously, we constructed a Sprague–Dawley rat DCD model and verified the expression of the aforementioned differentially expressed genes.
Organ donation and procurement
Ethics approval for this study was obtained from the Zhongnan Hospital of Wuhan University (Approval Number: 2016020), and the family members (parents, spouses, and adult children) of the donors signed a voluntary organ donation form. Human livers were procured by standard procurement from organ procurement organizations (OPOs), and organ donation and transplant procedures in China were in accordance with the National Guidelines for Donation after Cardiac Death [29, 30].
Clinical samples
Six pairs of human DCD tissue specimens were collected from the Zhongnan Hospital of Wuhan University, Wuhan, China (Table 1). Participants gave signed informed consent. According to the randomization principle, half of the DCD livers underwent HMP in the Lifeport liver transporter for 2 h without oxygenation, whereas the other half were treated with CS, and the livers were orthotopically transplanted into recipients. 1 h after the portal vein resumed blood supply, liver samples were collected from recipients.
Total human liver RNA extraction and mRNA library construction
Total RNA was extracted from the human liver tissue samples. Agilent 2100 (Agilent Technologies) and NanoDrop (Thermo Fisher Scientific) were used to quantitatively and qualitatively determine the total RNA amount, and the mRNA was purified using magnetic beads attached to Oligo (dT). Using a fragment buffer, the cleaved mRNA was fractionated into small fragments. First- and second-strand cDNA were then synthesized. After adding the a-tail mix and the RNA index adapter, the repair process was completed. Ampure XP Beads were used to purify the cDNA fragments and then dissolve them in EB liquid. The product was validated using an Agilent 2100 analyzer. The double-chain PCR product was heated and denatured and a final library was generated by circulating the splinted oligonucleotide sequence. A DNA nanosphere (DNB) was created by formatting the single-strand circular DNA (ssCir DNA) into a library. Finally, DNB was read using the BGIseq-500 platform (BGI, Shenzhen, China).
Differentially expressed genes (DEGs) and gene ontology (GO) analysis
Data were filtered using SOAPnuke (v1.5.2), where the false discovery rate (FDR) was set at 0.05, to identify differentially expressed genes. Heat maps and GO analysis maps of the top 50 DEGs were constructed.
Animals
Sprague–Dawley rats (~ 250 g) were raised in the Animal Experimental Center of Zhongnan Hospital under suitable environmental conditions with an adequate supply of nutrition (License Number:02520105C). The Wuhan University’s Ethics Committee approved the in vivo model. The experiments were performed in accordance with the regulations of the China National Science and Technology Committee, and the experimental animals were treated with humanitarian care.
Sprague–Dawley rat DCD liver and HMP models
After anesthetization, the rats were dissected along the abdominal midline without heparinization, and the diaphragm was cut to cause cardiogenic death. After cardiac arrest, the rats entered the in situ warm ischemia time and the temperature of the liver was maintained at 30 °C for 30 min. Subsequently, approximately 70 mL of histidine-tryptophan ketoglutaric (HTK) acid solution was applied to the liver via the abdominal aorta at 4 °C. After removal of the liver, it was stored in HTK for further processing. CS, CS + IDE, HMP, and HMP + IDE were treated with CS for 23 h, while HMP and HMP + IDE were treated with HMP for 1 h without oxygenation; the sham group did not receive either treatment (n = 6/group). The authors were not blinded to the group allocation. All livers were treated with isolated perfusion rat liver model (IPRL) systems for 1 h prior to sample collection. The HMP and IPRL models have been previously described [9].
Drug administration
The CS + IDE and HMP + IDE groups were intraperitoneally injected with a 28 mg/kg solution of 10% DMSO, 20% PEG300, and 70% saline dissolved in IDE (MedChemExpress, HY-13026), a specific inhibitor of PI3Kδ, for 3 days before surgery. All other groups were intraperitoneally injected with isodose solvent. BRL-3A cells were treated with 25 µM idelalisib.
Cell culture and H/R model
BRL-3A cells (Procell Life Science&Technology Co.,Ltd, Wuhan, China) were cultured in a humidified incubator (Thermo, Marietta, GA, USA) maintained at 37 °C and 5% CO2 in high-glucose Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 1% 100 U/mL penicillin G, and 100 μg/mL streptomycin. To generate the in vitro H/R model, BRL-3A cells were incubated for 12 h in a microaerophilic system (Heal Force, Shanghai, China) containing 5% CO2, 1% O2, and 94% N2 gas. Then, the cells were cultured for 6 h under normoxic conditions to allow reoxygenation.
Plasmid construction, in vitro transfection, and flow cytometry for apoptosis
The Serpina3 plasmid was obtained from MiaoLingBio, China. Serpina3 siRNA was synthesized by Genechem Biotech Inc., China. The BRL-3A cells were transfected with the relevant plasmid or the SiRNA using Lipofectamine 3000 according to the manufacturer’s instructions. Cells, as well as the culture medium, were collected and stained with Annexin V–FITC and PI (Bioworld). Cell apoptosis was determined by flow cytometry (Beckman). At least 10,000 events were recorded for each sample.
Hematoxylin–eosin (H&E) staining
After the collection of human and animal liver samples, paraformaldehyde solution (4%) was used to fix the liver tissues, which were then paraffin embedded. Each tissue section was stained with hematoxylin and eosin. Liver tissues were histopathologically scored based on the Suzuki criteria. The ImageJ software (ImageJ, Bethesda, MD, USA) was used for image analysis.
Biochemical analysis
Alanine transferase (ALT) and aspartate aminotransferase (AST) levels in the perfusate or patient serum were measured using a biochemical analyzer. Hepatic malondialdehyde (MDA) and hepatic superoxide dismutase (SOD) levels in rats were detected using kits purchased from the Nanjing Jiancheng Bioengineering Institute.
TUNEL staining and MPO immunofluorescence
We followed the instructions in the TUNEL kit (Roche, Branchburg, NJ, USA) for dewaxing, antigen repair, and staining, followed by nucleation of DAPI, image acquisition by fluorescence microscopy, and image analysis using ImageJ. For dewaxing, antigen repair, and circle and 3% BSA blocking, the sections were incubated with anti-MPO (1:100, Servicebio, GB11224) and subsequently with Cy3-conjugated goat anti-rabbit IgG (H + L; 1:100, Servicebio, GB21303). DAPI dye solution was added and the slices were mounted, observed under a fluorescence microscope, and photographed.
ROS staining
Frozen slices were prepared and dried. Histochemical pen circles were placed around the tissues, to which a spontaneous fluorescence-quenching agent was dropped. The reaction continued for 5 min, after which the slices were washed with water for 10 min. ROS staining solution drops were added to the rings and incubated at 37 °C. The sections were then rinsed three times with PBS. DAPI dye solution was added and the reaction was allowed to proceed at 25 °C. The slices were then mounted using an anti-fluorescence quenching mounting tablet, observed under a fluorescence microscope, and photographed.
Immunohistochemical (IHC) staining
After fixation with 4% paraformaldehyde and paraffin embedding, liver slices were dewaxed and hydrated. Subsequently, anti-SERPINA3 (1:500, ABclonal, A1021) was added and incubated overnight. The samples were then incubated at 37 °C, stained with 3,3-diaminobenzidine tetrahydrochloride (DAB) and hematoxylin, dehydrated with an ethanol gradient, cleared with xylene, and fixed with neutral sesame oil. Optical density analysis was performed using the ImageJ software.
Enzyme-linked immunosorbent assays (ELISA) assay
The abundance of interleukin (IL)-1β, IL-6, IL-10, and tumor necrosis factor (TNF)-α in the rat liver tissue was determined using ELISA. A moderate biotin antibody (1 ×) was then added, and the samples were resealed and incubated at 37 °C for 1 h. Subsequently, 100 μL of HRP-avidin (1 ×) was added and incubated at 37 °C for 1 h. Finally, each well was measured at 450 nm.
Western blot analysis
Total protein was extracted from the liver samples, and the liver tissue was ground and centrifuged. The supernatant was mixed with a loading buffer (Servicebio, Wuhan, China) and heated in boiling water for 10 min. Proteins were separated by SDS-PAGE, transferred to an appropriate PVDF membrane, and incubated with 5% BSA. The following antibodies were used: anti-SERPINA3 (1:1000, ABclonal, A1021), anti-PI3Kδ (1:1000, ABclonal, A19742), anti-phosphorylated Akt (1:6000, Proteintech, 66444-1-IG), anti-Akt (1:6000, Proteintech, 60203-2-IG), anti-TERT (1:1000, ABclonal, A2979), anti-Bcl-2 (1:1500, Proteintech, 26593-1-AP), anti-Bax (1:1500, Proteintech, 50599-2-IG), anti-C-caspase 3 (1:500, Proteintech, 19677-1-AP), and anti-β-actin (1:3000, Proteintech, 66009-1-IG). Protein bands were detected using the ECL kit after incubation for 2 h. The number of protein bands was determined using ImageJ software.
Statistical analysis
The data were analyzed using GraphPad Prism v8.0 (GraphPad Software, San Diego, CA, USA). All data are expressed as mean ± standard deviation (SD). To identify statistical differences among multiple groups, one-way analysis of variance (ANOVA) with Tukey’s post hoc test was performed. Statistical significance was set at P < 0.05 (two-tailed) and considered statistically significant.
Results
HMP alleviates IRI of DCD liver
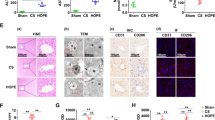
HE staining and Suzuki’s scoring of clinical specimens showed that liver tissue in clinical CS exhibited obvious edema, necrosis, vacuolation, and inflammatory cell infiltration. Meanwhile, HMP significantly improved injury severity compared to CS (Fig. 1C, D). Additionally, significant differences in serum AST and ALT levels were observed between the two patient groups on day 2 post-transplantation (P < 0.01; Fig. 1A, B). These results suggest that HMP alleviates IRI in DCD liver.
HMP promotes SERPINA3 expression
Transcriptomic analysis of clinical samples identified 17204 DEGs between the two groups. A heat map was generated using the top 50 genes, including SERPINA3 and SERPINA1 (Fig. 2A). Subsequent GO analysis revealed that SERPINA3 participates in the regulation of lipid metabolic processes (GO: 0019216), acute inflammatory response (GO:0002526), and neutrophil degranulation (GO:0043312) (Fig. 2B).
Transcriptome analysis of clinical samples showed that HMP promotes SERPINA3 expression. A Heat map of the top 50 differentially expressed genes, in which SERPINA1 and SERPINA3 were significantly upregulated; B GO enrichment analysis plot; C western blot detection of SERPINA1 and SERPINA3 in clinical samples; D: statistical analysis of western blot. ns, no significance; n = 4/group; **P < 0.01
Western blot analysis further confirmed the differential abundance of SERPINA3 in the clinical samples of the DCD and CS groups (P < 0.01); however, the abundance of SERPINA1 did not differ significantly between the groups. Therefore, SERPINA3 was selected as the target gene for the subsequent experiments.
Inhibition of PI3Kδ interferes with the protective effect of HMP
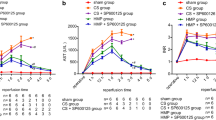
To investigate whether HMP alleviates IRI in the DCD liver via the SERPINA3/PI3Kδ pathway, we treated a proportion of rats with IDE (a specific PI3Kδ inhibitor). Transaminase levels were significantly higher in the HMP + IDE group than in the HMP group (P < 0.05). The HMP + IDE and CS groups showed no significant differences (Fig. 3A, B). Hence, the inhibition of PI3Kδ appears to prevent the protective effect of HMP on DCD liver function.
Liver function damage in the CS + IDE group was more serious than that in the CS group (P < 0.01), indicating that IDE inhibited PI3Kδ and aggravated IRI in DCD livers in rats. These results were confirmed by HE staining and Suzuki scoring (Fig. 3C, D).
HMP promotes SERPINA3 expression and related pathway proteins
Western blot analysis of the liver tissues collected from rats showed that the HMP group exhibited a significantly upregulated abundance of Serpina3, PI3Kδ, pAkt, and TERT compared with the sham group (P < 0.01) and CS group (P < 0.05). However, both CS and CS + IDE, or HMP and HMP + IDE had similar Serpina3 abundance, while PI3Kδ, pAkt, and TERT were significantly downregulated (Fig. 4B, D–G). The Serpina3 immunohistochemical results were consistent with the western blot results (Fig. 4A, C). Hence, HMP may also promote the expression of Serpina3 in Sprague–Dawley rat DCD liver models, whereas IDE can effectively inhibit the expression of PI3Kδ and its downstream molecules.
Expression of SERPINA3 and related pathway proteins after inhibition of PI3Kδ; A Immunohistochemical staining of Serpina3; B western blot analysis of Serpina3, PI3Kδ, Akt, pAkt, and TERT; C quantitative analysis of immunohistochemical staining of Serpina3, n = 6, ns, no significance; *P < 0.05; **P < 0.01; D-G: Quantitative analysis of protein expression. n = 3; ns, no significance; *P < 0.05; **P < 0.01
Inhibition of PI3Kδ aggravates oxidative stress injury
To explore the effect of PI3Kδ inhibition on oxidative stress, MDA and SOD levels were measured in the five rat groups. The MDA content in the HMP group was decreased, whereas that of SOD was increased (P < 0.01). However, no significant differences were observed between the HMP + IDE and CS groups, indicating that the inhibition of PI3Kδ counteracted the protective effect of HMP. Meanwhile, the MDA level in the CS + IDE group was further increased, and SOD levels were decreased compared with the CS group, indicating that PI3Kδ inhibition increased MDA levels and decreased SOD levels (Fig. 5C, D).
In addition, ROS staining revealed significantly lower ROS levels in the HMP group than in the CS group, whereas ROS levels in the HMP + IDE group were higher than those in the HMP group (P < 0.01; Fig. 5A, B). Hence, PI3Kδ inhibition can counteract the protective effect of HMP against DCD liver oxidative stress, thus aggravating the associated damage to the DCD liver.
Inhibition of PI3Kδ interferes with the protective effect elicited by HMP against liver inflammation
In this study, we stained neutrophil MPO in liver tissues and found that compared with that in the HMP group, neutrophil aggregation in the CS group was significantly increased (P < 0.01), while no significant difference was observed between the CS and HMP + IDE groups (Fig. 6A, B). In addition, IL-6, IL-1β, and TNF-α levels were significantly decreased, and IL-10 levels were significantly increased in the HMP group compared to those in the CS group (Fig. 6C–F). The levels of IL-1β, IL-6, TNF-α, and IL-10 did not differ significantly between the CS and HMP + IDE groups. Hence, inhibition of PI3Kδ appears to prevent the protective anti-inflammatory effect of HMP and aggravate DCD liver inflammation in Sprague–Dawley rats.
Inhibition of PI3Kδ aggravates inflammation injury in DCD liver of Sprague–Dawley rats and offsets the protective effect of HMP. A Immunofluorescence of MPO; B quantitative analysis of positive cells; C–F: ELISA analysis of IL-6, IL-10, IL-1β, and TNF-α. n = 6, ns, no significance; *P < 0.05; **P < 0.01
Liver cell apoptosis and the protective effect of HMP
Considering that IRI inevitably leads to hepatocyte apoptosis, we assessed the effect of PI3Kδ inhibition on hepatocyte apoptosis in DCD livers using TUNEL staining. Negligible levels of apoptosis were observed in the sham group, whereas significantly higher levels were detected in the hepatic cells of the CS group than in the HMP group. The number of apoptotic cells in the HMP + IDE group was also greater than that in the HMP group (P < 0.01); no differences were observed between the CS and HMP + IDE groups (Fig. 7A, B).
Inhibition of PI3Kδ aggravated liver cell apoptosis in Sprague–Dawley rats and counteracted protective effect of HMP. A TUNEL staining; B quantitative analysis of TUNEL staining, n = 6; ns no significance; *P < 0.05; **P < 0.01; C, D: expression of Bax, Bcl-2 and C-caspase 3 quantitative analysis. n = 3; ns no significance; *P < 0.05; **P < 0.01
Western blot analysis further revealed that Bax and C-caspase 3 expression were downregulated and Bcl-2 expression was upregulated in the HMP group (P < 0.01). Moreover, compared with the HMP group, Bax and C-caspase 3 were further elevated in the HMP + IDE group, while Bcl-2 was further reduced (P < 0.05; Fig. 7C, D), but no significant difference was observed between the CS and HMP + IDE groups. These results suggest that the inhibition of PI3Kδ could counteract the protective effect of HMP and increase hepatocyte apoptosis in the DCD liver.
Serpina3 attenuates hepatocyte H/R-induced injury by regulating the PI3Kδ/Akt pathway
Potential activation of the Serpina3-associated alternative pathway to counteract the exacerbation of hepatic H/R injury was assessed in in vitro. Results revealed that overexpression of Serpina3 ameliorated hypoxia/reoxygenation injury in BRL-3A cells, whereas the SI group exhibited an opposite trend compared to the OV group (P < 0.01) (Fig. 8E, F). Concurrently, overexpression of Serpina3 further activated the PI3Kδ/Akt pathway (Fig. 8A, B). IDE reversed the protective effect of Serpina3 overexpression, accompanied by a decrease in the expression of PI3Kδ compared to the OV group (P < 0.01) (Fig. 8C, D). These findings suggest that Serpina3 alleviates H/R injury in rat hepatocytes by activating the PI3Kδ/Akt pathway, which is consistent with in vivo experiment outcomes.
SERPINA3 attenuates hepatocyte H/R-induced injury by regulating the PI3Kδ/Akt pathway. A, C Western blot analysis of Serpina3, PI3Kδ, Akt, and pAkt; B, D quantitative analysis of proteins expression, n = 3; ns no significance; *P < 0.05; **P < 0.01; E the apoptosis of BRL-3A cells was measured by flow cytometry; F the statistical results of apoptosis were expressed by histogram. n = 3; ns no significance; *P < 0.05; **P < 0.01
Discussion
The large discrepancy between the supply and demand of donated organs represents the primary issue in organ donation [31]. Moreover, the variable and unpredictable warm ischemia time during DCD can increase the incidence of primary non-function and biliary tract complications while decreasing patient survival rates [32]. IRI of donor livers is complex and involves myriad pathophysiological processes. Although previous studies have shown that HMP, compared with CS, can significantly reduce IRI in DCD livers [33,34,35], the mechanism remains unclear and was the focus of the current study. Our results highlight the pivotal modulatory role of SERPINA3 in this process. Specifically, upon DCD liver IRI, SERPINA3 directly activates PI3Kδ to ameliorate oxidative stress, inflammatory responses, and apoptosis.
We found that HMP ameliorated IRI in the DCD liver in clinical samples, as evidenced by the attenuation of pathological injury. Moreover, our RNA-seq results showed that SERPINA3 was among the top 50 DEGs between DCD and CS patient groups. Through GO analysis, we found that SERPINA3 plays a significant role in the regulation of lipid metabolic processes (GO: 0019216), acute inflammatory response (GO: 0002526), and neutrophil degranulation (GO: 0043312). These pathophysiological processes are strongly associated with IRI.
Subsequently, focused on SERPINA3 by combining the transcriptome and western blot results. During DCD liver IRI, the cell oxygen supply is reduced, cell homeostasis is unbalanced, and Na+ and Ca2+ levels are increased, causing cell damage, apoptosis, and the accumulation of ROS. This not only causes an excessive oxidative stress reaction, but also promotes the production of arachidonic acid [36], inflammatory cytokines, and excessive activation of the inflammatory response, resulting in irreversible damage to the donor liver [37]. Zhang et al. demonstrated that SERPINA3K protects against oxidative stress by blocking intracellular calcium overload through the inhibition of phospholipase C (PLC) activation [14]. Similarly, in the present study, we found that SERPINA3 activated PI3Kδ/Akt to protect the liver from oxidative stress, inflammation, and apoptosis.
Although few studies have reported the role of SERPINA3 in IRI, SERPINA3 is reportedly critical for heart ischemic disease, cerebral ischemia–reperfusion injury, regulation of inflammation, thrombosis, tumors, and other related diseases [16,17,18,19,20,21]. Moreover, Ko et al. showed that oxidative modification of SERPINA3 promotes hepatocellular carcinoma cell survival by activating the PI3Kδ/Akt/TERT pathway, while inhibiting PI3Kδ and SERPINA3 leads to tumor cell death [25]. In our in vivo experiment, SERPINA3 was increased in the CS group and further upregulated after 1 h of HMP. We postulate that SERPINA3, an acute reaction protein, increases stress during the early stages of DCD liver IRI. HMP can further promote the expression of SERPINA3 while eliminating metabolic wastes and toxins in the donor liver, thereby initiating the protective effect of SERPINA3 and inhibiting inflammation, oxidative stress, and apoptosis.
Previous studies have shown that hypothermic machine perfusion can activate Akt and Kruppel-like factor 2 (KLF2) and downregulate matrix metalloproteinase 9 (MMP9) to relieve IRI. However, the specific mechanisms have not been fully defined. Ko et al. reported that SERPINA3 is oxidized to activate hnRNP-K and upregulate its transcription. However, Zhou et al. found that the expression of SERPINA3 induced by H2O2 can reduce oxidative stress [38]. Meanwhile, others have shown that SERPINA3 secretion is closely associated with vascular endothelial cells, vascular smooth muscle cells, and fibroblasts [39]. Additionally, HMP stimulates liver endothelial and vascular smooth muscle cells through fluid shear stress to induce eNOS and thrombomodulin to combat inflammation and atherosclerosis [40]. SERPINA3 has also been shown to effectively inhibit MMP9 activation [41], which is crucial for wound healing and inflammation. More specifically, MMP9 can damage the integrity of blood vessels and endothelial cells by degrading TJP1 in vascular endothelial cells and aggravate IRI [42]. In summary, we suspect that HMP may directly or indirectly stimulate the upregulation of SERPINA3 expression via one of the above pathways, acting on PI3Kδ and other pathways, thus alleviating liver IRI.
Certain limitations of this study were noted. First, our study did not use a rat liver transplantation model, but rather IPRL instead of liver transplantation [43, 44]. Second, the sampling time point of the clinical liver specimens (1 h reperfusion during transplantation) may not adequately reflect the prognosis of both treatments. Hence, further investigations are needed to explore the true long-term survival of animals and to decipher the role of SERPINA3 in protection against DCD injury.
In conclusion, our study started with clinical trials of hypothermic machine perfusion, based on RNA-seq results of clinical samples, and finally carried out relevant intervention and verification in Sprague–Dawley rats and BRL-3A cells, indicating that SERPINA3 is crucial for improving IRI in DCD livers. In particular, selective inhibition of PI3Kδ interferes with the protection offered by HMP. Overall, the SEPRINA3-mediated PI3Kδ/AKT pathway alleviates inflammation, oxidative stress, and apoptosis in the DCD liver, which is one of the protective mechanisms offered by HMP. Hence, this study provides novel insights into the design of a treatment strategy targeting potential molecular in the DCD liver.
Data availability
Data available on request from the authors.
References
Zarrinpar A, Busuttil RW. Liver transplantation: past, present and future. Nat Rev Gastroenterol Hepatol. 2013;10:434–40.
Ravaioli M, De Pace V, Angeletti A, et al. Hypothermic oxygenated new machine perfusion system in liver and kidney transplantation of extended criteria donors: first Italian clinical trial. Sci Rep. 2020;10:6063.
Giraud S, Steichen C, Allain G, et al. Dynamic transcriptomic analysis of Ischemic Injury in a porcine pre-clinical model mimicking donors deceased after circulatory death. Sci Rep. 2018;8:5986.
Ertugrul IA, van Suylen V, Damman K, et al. Donor heart preservation with hydrogen sulfide: a systematic review and meta-analysis. Int J Mol Sci. 2021;22:5737.
de Vries RJ, Tessier SN, Banik PD, et al. Subzero non-frozen preservation of human livers in the supercooled state. Nat Protoc. 2020;15:2024–40.
van Rijn R, Schurink IJ, de Vries Y, et al. Hypothermic machine perfusion in liver transplantation—a randomized trial. N Engl J Med. 2021;384:1391–401.
He W, Ye S, Zeng C, et al. Hypothermic oxygenated perfusion (HOPE) attenuates ischemia/reperfusion injury in the liver through inhibition of the TXNIP/NLRP3 inflammasome pathway in a rat model of donation after cardiac death. FASEB J. 2018.
Zhou W, Zhong Z, Lin D, et al. Hypothermic oxygenated perfusion inhibits HECTD3-mediated TRAF3 polyubiquitination to alleviate DCD liver ischemia–reperfusion injury. Cell Death Dis. 2021;12:211.
Zeng C, Hu X, He W, et al. Hypothermic machine perfusion ameliorates inflammation during ischemiareperfusion injury via sirtuin1mediated deacetylation of nuclear factorkappaB p65 in rat livers donated after circulatory death. Mol Med Rep. 2017;16:8649–56.
Monbaliu D, Brassil J. Machine perfusion of the liver: past, present and future. Curr Opin Organ Transplant. 2010;15:160–6.
Bae C, Henry SD, Guarrera JV. Is extracorporeal hypothermic machine perfusion of the liver better than the “good old icebox”? Curr Opin Organ Transplant. 2012;17:137–42.
Clarke G, Mergental H, Hann A, et al. How machine perfusion ameliorates hepatic ischaemia reperfusion injury. Int J Mol Sci. 2021;22:7523.
Zeng C, Hu X, Wang Y, et al. A novel hypothermic machine perfusion system using a LifePort kidney transporter for the preservation of rat liver. Exp Ther Med. 2018;15:1410–6.
Zhang B, Ma JX. SERPINA3K prevents oxidative stress induced necrotic cell death by inhibiting calcium overload. PLoS ONE. 2008;3:e4077.
Yiu WH, Wong DW, Wu HJ, et al. Kallistatin protects against diabetic nephropathy in db/db mice by suppressing AGE-RAGE-induced oxidative stress. Kidney Int. 2016;89:386–98.
Murohara T, Guo JP, Lefer AM. Cardioprotection by a novel recombinant serine protease inhibitor in myocardial ischemia and reperfusion injury. J Pharmacol Exp Ther. 1995;274:1246–53.
Sandford AJ, Chagani T, Weir TD, et al. Alpha 1-antichymotrypsin mutations in patients with chronic obstructive pulmonary disease. Dis Markers. 1998;13:257–60.
Kamboh MI, Minster RL, Kenney M, et al. Alpha-1-antichymotrypsin (ACT or SERPINA3) polymorphism may affect age-at-onset and disease duration of Alzheimer’s disease. Neurobiol Aging. 2006;27:1435–9.
Hoffmann DC, Textoris C, Oehme F, et al. Pivotal role for alpha1-antichymotrypsin in skin repair. J Biol Chem. 2011;286:28889–901.
Zhu H, Liu Q, Tang J, et al. Alpha1-ACT functions as a tumour suppressor in hepatocellular carcinoma by inhibiting the PI3K/AKT/mTOR Signalling pathway via activation of PTEN. Cell Physiol Biochem. 2017;41:2289–306.
Zhou ML, Chen FS, Mao H. Clinical significance and role of up-regulation of SERPINA3 expression in endometrial cancer. World J Clin Cases. 2019;7:1996–2002.
Sanchez-Navarro A, Mejia-Vilet JM, Perez-Villalva R, et al. SerpinA3 in the early recognition of acute kidney injury to chronic kidney disease (CKD) transition in the rat and its potentiality in the recognition of patients with CKD. Sci Rep. 2019;9:10350.
Sanchez-Navarro A, Murillo-de-Ozores AR, Perez-Villalva R, et al. Transient response of serpinA3 during cellular stress. FASEB J. 2022;36: e22190.
Ko E, Kim JS, Bae JW, et al. SERPINA3 is a key modulator of HNRNP-K transcriptional activity against oxidative stress in HCC. Redox Biol. 2019;24: 101217.
Ko E, Seo HW, Jung ES, et al. PI3Kdelta Is a therapeutic target in hepatocellular carcinoma. Hepatology. 2018;68:2285–300.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501.
Uehara M, McGrath MM, Ohori S, et al. Regulation of T cell alloimmunity by PI3Kgamma and PI3Kdelta. Nat Commun. 2017;8:951.
Ghareghomi S, Ahmadian S, Zarghami N, et al. Fundamental insights into the interaction between telomerase/TERT and intracellular signaling pathways. Biochimie. 2021;181:12–24.
Chinese Society of Organ Transplantation CMA. National guidelines for donation after cardiac death in China. Hepatobiliary Pancreat Dis Int. 2013;12:234–8.
Huang J, Millis JM, Mao Y, et al. A pilot programme of organ donation after cardiac death in China. Lancet. 2012;379:862–5.
Durand CM, Chattergoon MA. Bypassing the bottleneck: intentional hepatitis C transmission with organ transplant. J Clin Invest. 2019;129:3038–40.
Hrydziuszko O, Perera MT, Laing R, et al. Mass Spectrometry based metabolomics comparison of liver grafts from donors after circulatory death (DCD) and donors after brain death (DBD) used in human orthotopic liver transplantation. PLoS ONE. 2016;11:e0165884.
Zeng X, Wang S, Li S, et al. Hypothermic oxygenated machine perfusion alleviates liver injury in donation after circulatory death through activating autophagy in mice. Artif Organs. 2019;43:E320–32.
Hu X, Wang W, Zeng C, et al. Appropriate timing for hypothermic machine perfusion to preserve livers donated after circulatory death. Mol Med Rep. 2020;22:2003–11.
Zhou W, Peng S, Du P, et al. Hypothermic oxygenated perfusion combined with TJ-M2010-5 alleviates hepatic ischemia–reperfusion injury in donation after circulatory death. Int Immunopharmacol. 2022;105: 108541.
Decker K. Eicosanoids, signal molecules of liver cells. Semin Liver Dis. 1985;5:175–90.
Dar WA, Sullivan E, Bynon JS, et al. Ischaemia reperfusion injury in liver transplantation: cellular and molecular mechanisms. Liver Int. 2019;39:788–801.
Zhou T, Zong R, Zhang Z, et al. SERPINA3K protects against oxidative stress via modulating ROS generation/degradation and KEAP1-NRF2 pathway in the corneal epithelium. Invest Ophthalmol Vis Sci. 2012;53:5033–43.
Sorokin V, Woo CC. Role of Serpina3 in vascular biology. Int J Cardiol. 2020;304:154–5.
Liu Z, Zhong Z, Lan J, et al. Mechanisms of hypothermic machine perfusion to decrease donation after cardiac death graft inflammation: through the pathway of upregulating expression of KLF2 and inhibiting TGF-beta signaling. Artif Organs. 2017;41:82–8.
Cao LL, Pei XF, Qiao X, et al. SERPINA3 silencing inhibits the migration, invasion, and liver metastasis of colon cancer cells. Dig Dis Sci. 2018;63:2309–19.
Hu Q, Lan J, Liang W, et al. MMP7 damages the integrity of the renal tubule epithelium by activating MMP2/9 during ischemia–reperfusion injury. J Mol Histol. 2020;51:685–700.
Alva N, Bardallo RG, Basanta D, et al. Preconditioning-like properties of short-term hypothermia in isolated perfused rat liver (IPRL) system. Int J Mol Sci. 2018;19:1023.
Schlegel A, Muller X, Mueller M, et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine. 2020;60: 103014.
Acknowledgements
We sincerely appreciate all the students and teachers in our department for their help, as well as Shanghai Genext Medical Technology Co., Ltd., which supplied the consumables for Lifeport liver transporter.
Funding
This study was funded by the Medical Science Advancement Program (Clinical Medicine) of Wuhan University (Grant Number TFLC2018003) and the Natural Science Foundation of Hubei Province (Grant Number 2016CFA094).
Author information
Authors and Affiliations
Contributions
SP: methodology, data curation, formal analysis, writing—original draft. WJ L: data curation, formal analysis, writing—original draft. ZZ L: data curation, formal analysis, writing—original draft. SJ Y: funding acquisition, writing—review and editing. ZY P: project administration, supervision, writing—review and editing. ZB Z: project administration, supervision, writing—review and editing. QF Y: conceptualization, funding acquisition, supervision, writing—review and editing.
Corresponding authors
Ethics declarations
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical approval
This study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (Ethics Approval Number: 2016020), and all participants provided informed consent. All animal experiments were performed in accordance with the regulations of the National Science and Technology Committee of China, and the experimental animals were treated with humanitarian care (License Number: 02520105C).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, S., Liang, W., Liu, Z. et al. Hypothermic machine perfusion reduces donation after circulatory death liver ischemia–reperfusion injury through the SERPINA3-mediated PI3Kδ/Akt pathway. Human Cell 37, 420–434 (2024). https://doi.org/10.1007/s13577-023-01012-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-01012-3