Abstract
The importance of the microenvironment is widely recognized as it regulates not only malignant cell behavior but also drug sensitivity. The cancer cell microenvironment is composed of biological, physical and chemical elements, and simultaneous reproduction of these three elements are important conditions investigated in cancer research. In the present study, we focused on the epidemiological and anatomical specificities of endometrioid carcinoma, obesity (biological), fluid flow (physical) and anticancer agents (chemical) to target the specific microenvironmental elements of endometrioid carcinoma. To elucidate the individual effects of these elements on endometrioid carcinoma and to investigate the relationships between these factors, we developed an adipose tissue fragments (ATFs)-embedded cell disc under a rotational culture method to generate carcinoma-stroma interactions and to create fluid flow. ATFs and fluid flow individually or synergistically influenced proliferative cellular behavior and the morphological changes underlying endometrioid carcinoma. ATFs and fluid flow also governed the expression of extracellular signal-regulated kinase and p38 signaling synergistically or individually, depending on the endometrioid carcinoma cell type. Adipose tissue induced chemoresistance to cis-diamminedichloro-platinum (CDDP) in endometrioid cancer, but the resistance effect was abolished by fluid flow. Thus, a simple reconstructed model was established to investigate three elements of the microenvironment of endometrioid carcinoma in vitro. This culture model unequivocally demonstrated the individual and synergistic effects of the three elements on endometrioid carcinoma. This new culture model is a promising tool for elucidating the mechanisms underlying endometrioid carcinoma and for developing further treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most common malignancy arising in the endometrium is endometrioid carcinoma [1]. The majority of endometrioid tumors occur in postmenopausal women, with an average age of 63 years. The incidence of endometrioid cancer in malignant tumors of the uterine body is 80% in Europe and more than 90% in the United States [1, 2]. Currently, exposure to high estrogen concentrations is postulated to be one cause of endometrioid carcinoma. Potential exposure to pathological estrogen concentrations include premature menstruation, delayed menopause, obesity, tamoxifen treatment, polycystic ovary syndrome and estrogen-producing tumors [1]. In particular, a correlation between obesity and endometrioid carcinoma has been shown epidemiologically [3, 4]. In obese patients, adipose tissue exhibits dysfunction and produces pathological amounts of adipokines including leptin, adiponectin and tumor necrosis factor-α [5, 6]. Abnormal secretion of these adipokines is known to be supplied through blood vessels or in a paracrine manner and causes various disease states in the body [7, 8]. However, no suitable culture model has been established to analyze the direct effect of adipose tissue on endometrioid carcinoma cells by simplifying the cellular components. Furthermore, the precise molecular mechanisms underlying the effect of obesity on the pathogenesis of endometrioid carcinoma remains to be elucidated.
The microenvironment is an important element that determines cell behavior not only in normal tissue but also in malignant tumors [9, 10]. It is known that the microenvironment can regulate the drug sensitivity of malignant cells in addition to regulating their proliferation and invasive activity [11, 12]. The basic elements that make up the microenvironment can be classified into three elements as follows: biological, physical and chemical. Biological elements are mainly composed of cell–cell interactions due to paracrine effects and cell–cell adhesion [13, 14]. Temperature, pressure, shear stress, etc. are the major constituents of physical elements [15, 16]. For chemical components, physiologically active substances, electrolytes, drugs, and so on are important players [17, 18]. These three types of microenvironmental constituents are also present in endometrioid carcinoma (Fig. 1A). It has been reported that there is a close intercellular interaction between endometrioid carcinoma and stroma [19, 20]. Due to the presence of numerous mucus-secreting cells and a rich vascular network in the uterus, endometrial tissue and endometrioid carcinoma arising from the endometrium are surrounded by fluid due to the flow of mucus and interstitial fluid (Fig. 1B) [21]. From an organ-level perspective, the flow of mucus and interstitial fluid is a very subtle stimulus. However, at the cellular level, these stimuli are expected to produce shear stress on cells and stimulate cell surface structures such as cilia and microvilli [22, 23]. Cis-diamminedichloro-platinum (CDDP) is usually employed as first-line chemotherapy in patients with advanced or recurrent endometrioid carcinoma [24]. After intravascular CDDP administration, systemic interstitial fluid arising from the vascular system, results in an artificially created chemical microenvironment for cells.
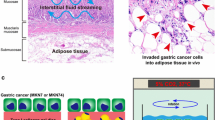
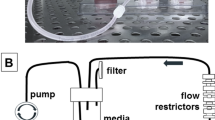
Endometrial carcinoma microenvironment and experimental design. A Biological, physical and chemical microenvironment of endometrioid carcinoma. In the biological microenvironment, abundant adipose tissue surrounds the uterus and the endometrium produces abundant mucus. Rich vascular network exists in the endometrium. B Histopathological image showing hematoxylin–eosin stained endometrial carcinoma. Arrows indicate vasculature and asterisks mucus. C A simple scheme of the cell disc method. D A simple scheme of the continuous fluid flow generation system
The importance of the microenvironment in cancer research is widely recognized. However, the synergistic effects of multiple subtypes of the microenvironment (vide supra) on cancer cells are not well understood. Unfortunately, at the present time no culture model has been established that can simultaneously and simply provide cancer cells with a biological, physical and chemical microenvironment.
In this study, a new in vitro model has been developed that reproduces the biological, physical and chemical microenvironment of endometrioid carcinoma. Using a simple in vitro culture method, the effects of the three microenvironments alone on endometrioid carcinoma behavior were analyzed. In addition, the influence of the microenvironment, altered by the interaction between the constituent elements on cell dynamics and drug sensitivity of endometrioid carcinoma, was investigated. The main purpose of the study was to establish unequivocally the individual and coordinated effects that each microenvironment component has on endometrioid cancer cells.
Materials and methods
Cells
All procedures involving animal materials were performed in accordance with the ethical guidelines of Saga University (approval number: A2022-042-0). Two human endometrioid carcinoma cell lines, HEC-265 (JCRB1142) and HEC-151 (JCRB1122), were provided by the Japanese Cancer Research Bank (JCRB, Osaka, Japan). Adipose tissue fragments (ATFs) were derived from subcutaneous adipose tissue of 1-week-old Wistar rats. Cells were cultured in RPMI-1640 medium (Fujifilm, Tokyo, Japan) containing 15% fetal bovine serum (NICHIREI BIOSCIENCES, Tokyo, Japan), 100 μg/mL streptomycin and 100 μg/mL penicillin. All cell lines were incubated in a 5% CO2 and 20% O2 gas mixture at 37 ºC in a CO2 incubator. Our previous studies have addressed the issue of species differences in humoral cross-reactivity and confirmed that rat-derived ATFs possessed cross-reactivity with human-derived cells. [25, 26]
Cell culture model
To replicate carcinoma-stroma cell interactions, the collagen cell disc method was employed (Fig. 1C) [25]. Subcutaneous adipose tissue was minced into pieces of 0.5–1.0 mm in diameter with a sharp blade. A total of 0.15 g of minced tissue was premixed homogeneously with 10 mL of collagen gel solution (Cellmatrix, Type I-A; Nitta Gelatin Co. Ltd., Osaka, Japan). Next, 1 mL aliquots of this collagen gel solution containing the minced fragments of adipose tissue were poured into 15 mm diameter 24-well plates. Cell-free discs were made from collagen gels without cells. Then, 1.0 × 10 [5] endometrioid carcinoma cells were seeded onto the surface of the collagen disc. After the epithelial cells adhered to the collagen disc surface, each cell disc was removed from the 24-well plate and used in various experiments. The collagen discs were transferred to 9 cm dishes containing 20 mL of culture medium, which was changed every 2 days. After 7 days of culture, tissues were fixed in 10% formalin and embedded in paraffin.
Fluid flow-generating system
The fluid flow-generating system used in the present study has been previously described (Fig. 1D) [25, 27]. The culture dishes were incubated in a 5% CO2 and 20% O2 atmosphere at 37 ºC in a CO2 incubator. To generate fluid flow, the culture dishes were placed on a cell shaker (CS-LR; TAITEC, Saitama, Japan) at 50 rpm throughout the culture period. In the static condition, dishes were placed in the CO2 incubator and were stationary.
Histology and immunohistochemistry
Histological examinations were performed on hematoxylin–eosin (HE)-stained sections. Cell proliferation was evaluated using a mouse monoclonal anti-Ki67 antibody (Dako, Glostrup, Denmark). The degree of cell apoptosis was determined using a rabbit monoclonal anti-cleaved caspase-3 antibody (Cell Signaling Technology [CST], Danvers, MA, USA).
Morphometric analysis
Cancer layer thicknesses were measured at 10 points in 5 randomly selected non-contiguous and non-overlapping areas (low magnification, ×10 objective). Cells were counted in 5 randomly selected non-contiguous and non-overlapping fields within the stained sections, and the percentage of Ki67-positive cells was measured to evaluate proliferation. Next, the percentage of cleaved caspase 3-positive cells was determined to evaluate the degree of apoptosis, which was also confirmed using HALO image analysis software (Indica Labs, Albuquerque, NM, USA).
Western blot analysis
Endometrioid carcinoma cells and ATFs were co-cultured using inserts with an 8-μm pore size (Falcon Cell Culture Insert; Becton Dickinson, Franklin, NJ, USA). ATFs were embedded in collagen gels and endometrioid carcinoma cells were seeded onto the cell insert surface. The inserts were placed in 9 cm dishes with 20 mL of complete medium. After 48 h of culture, the collagen gels were lysed in 300 μL of M-PER Reagent (Thermo Fisher Scientific, Waltham, MA, USA) containing Protease/Phosphatase Inhibitor Cocktail (#5872; CST). Lysates containing an equal quantity of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis in 12% Bis–Tris gels and transferred to polyvinylidene difluoride membranes. The membranes were incubated overnight at 4ºC with antibodies against extracellular signal-regulated kinase (ERK) 1/2 (#9102; CST), p-ERK (#4370; CST), p38 (#8690; CST), p-p38 (#4511; CST). The antibody-bound antigens on membranes were detected by a chemiluminescent immunodetection system (Western Breeze; Thermo Fisher Scientific). Band densities were detected using the FUSION system (Vilber-Lourmat, Eberhardzell, Germany) and analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA). Data are presented as ratios relative to control values.
Cis-diamminedichloroplatinum (CDDP)
To examine the effects of CDDP (Nichi-Iko Pharmaceutical Co., Ltd., Toyama, Japan) on endometrioid carcinoma, CDDP was added to the culture medium at final concentrations of 2.5 μg/mL or 5.0 μg/mL. The cell culture medium was exchanged every 2 days. Samples were collected and analyzed on day 7. During western blotting, the concentration of CDDP in the culture medium was 2.5 μg/mL.
Statistical analysis
In this study, the statistical analysis was performed using two-way ANOVA and multiple t tests with the Bonferroni correction as a post hoc pairwise comparison. Data were obtained from 4 to 6 independent experiments. Values are presented as means ± SD, together with the number of experiments carried out. The mean value of replicates in experiments was used to determine statistical significance; P values < 0.05 were deemed to be statistically significant findings. All statistical analyses were performed using JMP 16 for Windows (SAS, Cary, N.C., USA).
Results
Biological and physical microenvironments have specific effects on endometrioid carcinoma
To investigate the biological effect of ATFs and physical effect on carcinoma cells, 2 different human endometrioid carcinoma cell lines, HEC-265 and HEC-151, were cultured on the surfaces of collagen gel discs containing ATFs. HEC-265 and HEC-151 are well and moderately differentiated carcinoma cell types, respectively.
HEC-265 cells without ATFs were configured as 5 and 8 cell layers, with a small number of microtubular structures under the static condition (Fig. 2). Fluid flow induced coarse papillary structure formation in HEC-265 cells, but there was no significant difference in the cell layer thickness compared to the static condition. HEC-265 cells co-cultured with ATFs showed significantly thickened layers compared with the monoculture group under static condition. HEC-265 cells with ATFs under fluid flow stimulation exhibited a papillary structure, and fluid flow significantly decreased the thickness of the cellular layers of HEC-265 cells. There was no significant difference in layer thickness compared to the static and monoculture groups under the fluid flow condition.
Effects of fluid flow and adipose tissue fragments (ATFs) on the cellular kinetics of endometrioid carcinoma. A Representative histopathological images of HEC-265 and HEC-151 cells on day 7 after hematoxylin–eosin staining. Fluid flow and ATFs induced not only cell hypertrophy and thickening of the cell layer, but also tubule formation and the induction of papillary structures in endometrioid carcinoma cells. B Thickness of HEC-265 and HEC-151 cell layers. All data represent the means ± SD of 5 measurements. *P < 0.05. Mono monoculture, +ATFs HEC-265 or HEC-151 cells co-cultured with ATFs
Under the static condition, HEC-151 cells without ATFs had a configuration of 5 to 10 cell layers with mild irregularities of layer thickness; a small number of microtubular structures were observed in this group. Fluid flow significantly increased the cell layer thickness and induced papillary structures of HEC-151 cells compared to the static condition in both monoculture and co-cultured with ATFs groups. ATFs significantly promoted the thickness of the cellular layer and induced many papillary structures in HEC-151 cells compared to the monoculture groups with or without fluid flow. Fluid flow and ATFs synergistically increased the thickness of the cellular layers of HEC-151 cells, with a significant difference compared to the monoculture group under the static condition.
Biological and physical microenvironments regulate the growth but not apoptosis of endometrioid carcinoma
Immunostaining for Ki67 and cleaved caspase-3 was performed to analyze the effects of ATFs and fluid stimulation on the proliferation and apoptosis of endometrioid carcinoma cells (Fig. 3A, B).
Fluid flow and ATFs regulate the proliferation of endometrioid carcinoma. A, B Representative immunostained images for Ki67 and cleaved caspase-3 in HEC-265 or HEC-151 cells at day 7. The bar graphs show quantitative analysis of immuno-positive cells. All data represent the means ± SD of 5 measurements. *P < 0.05. Mono monoculture, +ATFs HEC-265 or HEC-151 cells co-cultured with ATFs
For the HEC-265 cells co-cultured with ATFs group, fluid flow significantly increased the Ki67 positive rate of HEC-265 cells compared to the static condition. Fluid flow and ATFs synergistically increased the Ki67 positive rate of HEC-265 cells with a significant difference found compared to the monoculture group under the static condition. Although fluid stimulation showed a tendency to decrease the cleaved caspase-3 positive rate of HEC-265 cells, no significant differences were found under all conditions investigated in the present study.
In the HEC-151 monoculture group, fluid flow stimulation increased the positive rate of Ki67. ATFs significantly increased the Ki67 positive rate of HEC-151 cells with or without fluid flow. Compared to the monoculture group under the static condition, fluid flow and ATF synergistically promoted the Ki67 positive rate of HEC-151 cells. No significant differences were found in the cleaved caspase-3 positive rate of HEC-151 cells under any of the conditions investigated in the present study.
Biological and physical microenvironments modulate ERK1/2 and p38 expression of endometrial carcinoma
To evaluate the effect of the microenvironmental component on endometrial carcinoma, we focused on the mitogen-activated protein kinase (MAPK) pathway, which is known to play an important role in controlling endometrial cancer dynamics [28].
As shown in Fig. 4A, no significant differences in the total ERK1/2 expression and the phosphorylated/total ERK1/2 ratio were found in comparisons between all HEC-265 cell groups. There was no significant difference in total p38 expression of HEC-265 cells in all experimental groups. In the monoculture group of HEC-265 cells, fluid flow increased the phosphorylated/total p38 ratio. It is noteworthy that ATFs significantly reduced the phosphorylated/total p38 ratio in HEC-265 cells under the fluid flow condition.
Effect of fluid flow and ATFs on MAPK signaling pathway in endometrioid carcinoma. MAPK family protein, ERK1/2, p-ERK1/2, p38 and p-p38 expression levels in HEC-265 (A) or HEC-151 (B) cells were evaluated by western blotting. Relative expression is depicted as the ratio of target protein expression to α/β-tubulin expression. All data are the means ± SD of 4–6 measurements. *P < 0.05. Mono monoculture, +ATFs HEC-265 or HEC-151 cells co-cultured with ATFs; S static, F fluid flow
Of note, there were no significant differences in total ERK1/2 expression of HEC-151 cells in any of the experimental groups (Fig. 4B). In contrast, fluid flow significantly reduced the phosphorylated/total ERK1/2 ratio in HEC-151 cells in the monoculture and co-culture groups. In contrast, under fluid flow, ATFs significantly upregulated the phosphorylated/total ERK1/2 ratio of HEC-151 cells. Fluid flow and ATFs synergistically downregulated the phosphorylated/total ERK1/2 ratio of HEC-151 cells with a significant difference compared to the monoculture group under the static condition. In the monoculture group, fluid flow increased the total p38 expression of HEC-151 cells. ATFs significantly upregulated total p38 expression of HEC-151 under static and fluid flow conditions. Fluid flow and ATFs synergistically upregulated the total p38 expression of HEC-151 cells with a significant difference compared to monoculture group under static condition. A trend was observed for decreased phosphorylation of p38 in HEC-151 cells under the influence of either single fluid stimulation or single ATFs co-culture, but no statistically significant difference was recognized in these changes. Fluid flow and ATFs synergistically induced downregulation of the phosphorylated/total p38 ratio of HEC-151 cells, with a significant difference compared to the monoculture group under the static condition.
Biological and physical elements altered the effect of the microenvironmental chemical element on endometrioid carcinoma
In the present study, HEC-265 and HEC-151 cells were treated with CDDP at concentrations of 2.5 µg/mL or 5.0 µg/mL (Fig. 5).
Effects of fluid flow and ATFs on the chemosensitivity of endometrioid carcinoma to CDDP. Representative histopathological images of HEC-265 or HEC-151 cells on day after treatment with CDDP determined by hematoxylin–eosin staining for each condition. The concentrations of CDDP applied to cells were 2.5 µg/mL and 5.0 µg/mL. The bar graphs show quantitative analysis of the cell layer thickness in HEC-265 or HEC-151 cells. All data represent the means ± SD of 4 measurements. *P < 0.05. Mono monoculture, +ATFs HEC-265 or HEC-151 cells co-cultured with ATFs, CDDP Cis-diamminedichloro-platinum, CDDP0 no CDDP, CDDP2.5 2.5 µg/mL of CDDP, CDDP5 5.0 µg/mL of CDDP
In the CDDP-untreated control group (CDDP0), the influence of microenvironmental elements had the same effects on both HEC-265 and HEC-151 cells as the previous experimental findings (vide supra). Under the static condition, ATFs significantly increased the thickness of the HEC-265 cell layer after the application of 2.5 µg/mL CDDP, while ATFs significantly decreased the thickness of the cell layer under fluid flow condition. The HEC-265 monoculture group under fluid flow formed significantly thicker cellular layers compared to the static condition in the presence of 2.5 µg/mL CDDP. Under the static condition, ATFs significantly thickened the HEC-265 cell layer when 5.0 µg/mL CDDP was applied. It is noteworthy that fluid flow significantly decreased the thicknesses of the HEC-265 cell layers in the co-cultured with ATFs group after exposure to 5.0 µg/mL CDDP.
The application of CDDP at a concentration of 2.5 µg/mL, ATFs caused significant thickening of the HEC-151 cell layers under the static condition. Fluid flow significantly decreased the thicknesses of the HEC-151 cellular layers of the co-cultured with ATFs group after the application of 2.5 µg/mL CDDP. No significant difference was observed among all experimental groups under CDDP 5 µg/mL administration.
Cellular, physical and chemical elements synergistically modulate ERK1/2 and p38 expression of endometrioid carcinoma
In the absence of CDDP, no differences in total ERK1/2 expression of HEC-265 and HEC-151 cells were found among all experimental groups (Fig. 4), but significant differences were detected in the total ERK1/2 expression after CDDP was applied.
As shown in Fig. 6A, the fluid flow condition significantly decreased the total ERK1/2 expression of the HEC-265 monoculture group after CDDP treatment. The CDDP treated co-cultured group with ATFs exhibited significantly lower total ERK1/2 expression of HEC-265 compared to the monoculture group under the static and fluid flow conditions. Fluid flow and ATFs synergistically downregulated total ERK1/2 expression in HEC-265 cells compared to the monoculture group under the static condition. Under the static condition, ATFs significantly increased the phosphorylated/total ERK1/2 ratio of CDDP treated HEC-265 cells. Under fluid flow, the CDDP treated co-cultured group with ATFs exhibited a lower phosphorylated/total ERK1/2 ratio compared to the static condition. CDDP did not produce a significant difference in the expression level of total p38 in all experimental groups of HEC-265 cells. The CDDP treated co-cultured group with ATFs exhibited significantly decreased phosphorylated/total p38 ratios under the static and fluid flow conditions. Fluid flow and ATFs significantly reduced phosphorylation of p38 in CDDP treated HEC-265 cells through a synergistic action.
Effect of fluid flow and ATFs on the MAPK signaling pathway in endometrioid carcinoma exposed to CDDP. MAPK family protein, ERK1/2, p-ERK1/2, p38 and p-p38 expression levels in HEC-265 (A) or HEC-151 (B) cells were evaluated by western blotting. The concentration of CDDP applied to cells was 2.5 µg/mL. Relative expression is depicted as the ratio of target protein expression to α/β-tubulin expression. All data are presented as the means ± SD of 5 measurements. *P < 0.05. Mono monoculture, +ATFs HEC-265 or HEC-151 cells co-cultured with ATFs, CDDP Cis-diamminedichloro-platinum, S static, F fluid flow
In the monoculture group, fluid flow significantly downregulated total ERK1/2 expression in CDDP treated HEC-151 cells compared to the static condition (Fig. 6B). Under fluid flow, the CDDP treated co-cultured group with ATFs exhibited a significantly upregulated expression level of total ERK1/2 compared to the monoculture group. Of considerable interest, ATFs significantly upregulated the phosphorylated/total ERK1/2 ratio of HEC-151 cells after CDDP administration compared to the monoculture group regardless of the presence or absence of fluid flow. Similarly, under these conditions the CDDP treated co-cultured with ATFs group exhibited a significantly higher expression of total p38 expression in HEC-151 cells compared to the monoculture condition. In the co-cultured group with ATFs, fluid flow significantly increased total p38 expression in the CDDP treated HEC-151 cells compared to the static condition. Fluid flow and ATFs acted synergistically to significantly increase total p38 expression in CDDP treated HEC-151 cells. Under the fluid flow condition, ATFs significantly upregulated the phosphorylated/total p38 ratio in CDDP treated HEC-151 cells compared to the monoculture group. In the CDDP treated co-cultured with ATFs group, fluid flow increased the phosphorylated/total p38 ratio of HEC-151 cells compared to the static condition. Finally, fluid stimulation and ATFs significantly increased the phosphorylated/total p38 ratio of CDDP treated HEC-151 cells through a synergistic effect.
Discussion
The importance of the microenvironment in current cancer research is widely recognized [29], but dynamic changes in the microenvironment due to interactions between the factors that constitute the microenvironment have not yet been fully elucidated. One of the reasons why research on the interactions between microenvironmental factors has not progressed is that an appropriate culture model has not been established. In the present study, a method to reproduce simultaneously biological, physical and chemical microenvironmental elements in a culture system was established using a cell disc method.
The behavior of two types of endometrioid carcinoma cells was changed by fluid flow as a physical element or co-cultured with ATFs as a biological element alone. The co-existence of the above two factors affected the tissue morphology, proliferative capacity of the endometrioid carcinomas. In this study, fluid stimulation and ATFs did not affect apoptosis of carcinoma cells, but our previous studies have shown that both factors had a significant impact on not only proliferation but also apoptosis of normal and cancer cells [25, 30]. Notably, the effect of CDDP as a chemical element was altered by the presence of the other microenvironmental elements. The biological element in the microenvironment is strongly influenced by the physical and chemical elements, so it will be necessary to clarify changes in the biological element in a future study. Unfortunately, we were not able to elucidate the detailed mechanisms underlying changes in drug sensitivity in this study, but the findings nevertheless provide important points in considering the treatment strategy of endometrioid carcinoma.
The MAPK pathway has attracted much attention as a target for the effects of obesity on endometrioid carcinoma [31, 32], and has also been reported to play a central role in the drug resistance of cancer cells [33, 34]. In the present study, HEC-265 and HEC-151 cells showed similar responses to microenvironmental influences, albeit with minor differences. There was no significant difference in the total expression level or the phosphorylation rate of ERK1/2 in HEC-256 cells in the absence of CDDP, but fluid flow and ATFs induced a statistically significant difference in the total expression level or phosphorylation rate of ERK1/2 in CDDP-treated HEC-265 cells. Furthermore, the elevation of the ERK1/2 phosphorylation ratio in CDDP treated HEC-265 cells was prevented by fluid flow. Similar phenomena were also observed in the analysis results of the thickness of the cellular layer after the application of CDDP. These findings suggest that fluid flow and ATFs may mutually regulate the CDDP chemosensitivity of endometrioid carcinoma through the MAPK pathway. However, JNK, PI3K, Myc etc. are known signals involved in drug resistance, and it will be necessary to analyze changes in these signaling systems in future studies [35, 36].
We fully recognize several limitations of our study. In general, it is common to use different cell lines to elucidate universal disease characteristics. Here, we used only one well differentiated and one moderately differentiated endometrioid carcinoma cell line. Behavioral changes in these cell lines induced by microenvironmental elements were similar but not identical. In future research, it will be necessary to clarify the commonality of behavioral changes according to the degree of differentiation, including patient samples, and the specificity of each sample. Furthermore, it was unclear whether the effect of adipose tissue on endometrioid carcinoma was due to mature adipocytes or fibroblast-like cells derived from adipose tissue. The fibroblast-like cell population includes immature adipocytes, vascular endothelial cells, fibroblasts, myofibroblasts [37, 38]. Mature and immature adipocytes have functional commonalities [39], and mature adipocytes, immature adipocyte and fibroblast-like cells are known to change into each other’s phenotype under certain conditions [40, 41]. For the reasons described above, it will be appropriate, for the analysis of the cellular effects of adipose tissue on endometrioid carcinoma, to use tissue fragments containing all cellular components.
Considering its future application to personalized medicine, the difference in drug resistance of each patient’s cell sample may also be useful for prognosis prediction. Therefore, we will endeavor to establish generalized test methods using our newly developed cell disc method.
In conclusion, a culture method that concurrently replicates the biological, physical and chemical elements of microenvironment specific to endometrioid carcinoma was developed using a collagen cell disc and fluid flow generation system. This new research method clearly demonstrated that each microenvironmental element singularity synergistically regulated the cellular behavior of endometrioid carcinoma cells. This method is a very promising research tool that will facilitate investigations into novel treatments for endometrioid carcinoma.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
World Health Organization. Female genital tumours 5th edn. Lyon, France. International Agency for Research on Cancer. Geneva, Switzerland: Print copies are distributed by WHO Press; 2020.
Clement PB, Young RH. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2002;9:145–84.
Nevadunsky NS, Van Arsdale A, Strickler HD, et al. Obesity and age at diagnosis of endometrial cancer. Obstet Gynecol. 2014;124:300–6.
Feng Y-H. The association between obesity and gynecological cancer. Gynecol Minim Invasive Ther. 2015;4:102–5.
Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48: e12997.
Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des. 2010;16:1896–901.
Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7:57–62.
Prieto-Hontoria PL, Pérez-Matute P, Fernández-Galilea M, Bustos M, Martínez JA, Moreno-Aliaga MJ. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim Biophys Acta (BBA) Bioenerg. 2011;1807:664–78.
Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–6.
Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–66.
Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–8.
Senthebane DA, Rowe A, Thomford NE, et al. The role of tumor microenvironment in chemoresistance: to survive, keep your enemies closer. Int J Mol Sci. 2017;18:1586.
Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol. 2018;6:18.
Komohara Y, Takeya M. CAFs and TAMs: maestros of the tumour microenvironment. J Pathol. 2017;241:313–5.
Mitchell MJ, King MR. Computational and experimental models of cancer cell response to fluid shear stress. Front Oncol. 2013;3:44.
Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res. 2013;1:210–6.
Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–54.
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185.
Matsumoto M, Yamaguchi Y, Seino Y, et al. Estrogen signaling ability in human endometrial cancer through the cancer–stromal interaction. Endocr Relat Cancer. 2008;15:451–63.
Arnold JT, Lessey BA, Seppälä M, Kaufman DG. Effect of normal endometrial stroma on growth and differentiation in Ishikawa endometrial adenocarcinoma cells. Can Res. 2002;62:79–88.
Mescher AL. Junqueira’s basic histology: text and atlas, Sixteenth edition, 50th anniversary edition. New York: McGraw-Hill; 2021.
Kim SW, Ehrman J, Ahn MR, et al. Shear stress induces noncanonical autophagy in intestinal epithelial monolayers. Mol Biol Cell. 2017;28:3043–56.
Lai SK, Wang Y-Y, Wirtz D, Hanes J. Micro-and macrorheology of mucus. Adv Drug Deliv Rev. 2009;61:86–100.
Van Wijk F, Van der Burg M, Burger CW, Vergote I, van Doorn HC. Management of recurrent endometrioid endometrial carcinoma: an overview. Int J Gynecol Cancer. 2009;19:314–20.
Nagase K, Akutagawa T, Rikitake-Yamamoto M, et al. Cellular and physical microenvironments regulate the aggressiveness and sunitinib chemosensitivity of clear cell renal cell carcinoma. J Pathol. 2021;254:46–56.
Aoki S, Toda S, Ando T, Sugihara H. Bone marrow stromal cells, preadipocytes, and dermal fibroblasts promote epidermal regeneration in their distinctive fashions. Mol Biol Cell. 2004;15:4647–57.
Aoki S, Makino J, Nagashima A, et al. Fluid flow stress affects peritoneal cell kinetics: possible pathogenesis of peritoneal fibrosis. Perit Dial Int. 2011;31:466–76.
Donohoe F, Wilkinson M, Baxter E, Brennan DJ. Mitogen-activated protein kinase (MAPK) and obesity-related cancer. Int J Mol Sci. 2020;21:1241.
Wilson J, Balkwill F. The role of cytokines in the epithelial cancer microenvironment. In: Seminars in cancer biology. Elsevier; 2002. p. 113–20.
Kawata K, Aoki S, Futamata M, et al. Mesenchymal cells and fluid flow stimulation synergistically regulate the kinetics of corneal epithelial cells at the air–liquid interface. Graefes Arch Clin Exp Ophthalmol. 2019;257:1915–24.
Makker A, Goel MM, Das V, Agarwal A. PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: an update. Gynecol Endocrinol. 2012;28:175–81.
Chen J. Multiple signal pathways in obesity-associated cancer. Obes Rev. 2011;12:1063–70.
Liu F, Yang X, Geng M, Huang M. Targeting ERK, an Achilles’ Heel of the MAPK pathway, in cancer therapy. Acta Pharm Sin B. 2018;8:552–62.
Housman G, Byler S, Heerboth S, et al. Drug resistance in cancer: an overview. Cancers. 2014;6:1769–92.
Tsuruo T, Naito M, Tomida A, et al. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94:15–21.
McCubrey JA, Steelman LS, Kempf CR, et al. Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. J Cell Physiol. 2011;226:2762–81.
Ramakrishnan VM, Boyd NL. The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Eng Part B Rev. 2018;24:289–99.
Wang T, Sharma AK, Wolfrum C. Novel insights into adipose tissue heterogeneity. Rev Endocr Metab Disord. 2022;23:5–12.
Lihn A, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21.
Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809.
Aoki S, Toda S, Sakemi T, Sugihara H. Coculture of endothelial cells and mature adipocytes actively promotes immature preadipocyte development in vitro. Cell Struct Funct. 2003;28:55–60.
Acknowledgements
We thank S. Nishimura, M. Nishida and S. Nakahara for excellent technical assistance.
Funding
This work was partially supported by grants from JSPS KAKENHI Grant Number 19K18468 (to MN) and 21K16773 (to MH).
Author information
Authors and Affiliations
Contributions
SM: Investigation, Writing—Original Draft. MK Investigation, Methodology. MN, TS, MH, TN: Investigation. AK: Statistical analysis. ST: Supervision. SA: Conceptualization, Methodology, Investigation, Writing—Reviewing and Editing, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures involving human or animal materials were performed in accordance with the ethical guidelines of Saga University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morito, S., Kawasaki, M., Nishiyama, M. et al. Microenvironmental elements singularity synergistically regulate the behavior and chemosensitivity of endometrioid carcinoma. Human Cell 36, 1147–1159 (2023). https://doi.org/10.1007/s13577-023-00886-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00886-7