Abstract
Over the past decades, stem cell therapy has been investigated as a promising approach towards various diseases, including neurodegenerative disorders. Stem cells show the capability to differentiate into neuronal progenitor cells in vitro. In the present study, the differentiation potential of human-induced pluripotent stem cells (hiPSCs) into neural lineages was examined under the efficient induction media containing forskolin and 3-isobutyl-1-methyl-xanthine (IBMX) in the presence of nisin (Ni), non-essential amino acids (NEAA) and combination of those (NEAA-Ni) in vitro. The optimum concentrations of these factors were obtained by MTT assay and acridine orange (AO) staining. The effect of Ni and NEAA on the expression rate of neural-specific markers including NSE, MAP2, and ß-tubulin III was studied via immunocytochemistry (ICC) and real-time RT-PCR analyses. Our results indicated that the induction medium containing Ni or NEAA increased the gene and protein expression of NSE, MAP2, and β-tubulin III on the 14th differentiation day. On the other hand, NEAA-Ni showed a less-differentiated hiPSCs compared to Ni and NEAA alone. In conclusion, the obtained results illustrated that Ni and NEAA could be applied as effective factors for neural differentiation of hiPSCs in the future.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decades, notable advances have been made in stem cell research and used for regenerative medicine worldwide. For this purpose, stem cell types have provided an attractive cell source for curing various kinds of diseases and injuries as well as making apparent clinical cures [1]. Additionally, the application of specific stem cells has been remarkably promoted as an alternative treatment for neurodegenerative diseases and neuronal disorders. Improvement of the neurologic functions using stem cells has been seen in an animal model of spinal cord injury [2, 3]. Recently, numerous studies have reported tissue regeneration potentials of embryonic stem cells (ESCs) and mesenchymal stem cells (MSCs), such as bone marrow-derived MSCs, adipose tissue-derived MSCs, and umbilical cord-derived MSCs in vivo [4,5,6]. Although these cells could be good candidates for clinical purposes and efficiently generate specific cell lineages in vitro and in vivo, moral issues and immune rejection problems have caused alternative strategies to be considered [7].
For the first time in 2006, induced pluripotent stem cells (iPSCs) were introduced as an exciting new technology of stem cell research to exhibit the primate ESC properties. These cells were obtained from mouse fibroblasts by concurrently introducing four critical transcription factors such as Klf4, Sox2, Oct4, and c-Myc [8]. Further studies showed successfully reprogramming of human somatic cells into iPSCs, which could form colonies by unlimited proliferation and pluripotency capacity. Human iPSCs (hiPSCs) have shown the same characteristics as ESCs and the same genetic as donor’s adult fibroblasts [9, 10]. The iPSCs technology is aimed at providing accessible and numerous pluripotent cell sources, which can differentiate into all three germ layers and various types of targeted cells, while overcoming the ethical concerns that surround other stem cells [11, 12]. Furthermore, iPSCs provide a significant potential in vitro model system for clinical cell therapy, such as disease modeling, drug screening [13], and regenerative medicine [14]. Given that iPSCs can be obtained directly from the patient, the possibility of immune transplant rejection is eliminated [15]. Following this discovery, a great deal of research works considerable amount of studies have been conducted on the potential therapeutic activities of iPSCs on many neurodegenerative disorders such as Alzheimer’s disease [16], Parkinson’s [17], and Huntington's [18]. Moreover, some studies have revealed that iPSC-derived cells could be successfully transplanted into spinal cord injury models [19].
It is crucial to define methods for obtaining various types of mature neurons involving in neurodegenerative processes [20]. Improving iPSC culture medium and develop differentiation and induction protocols for applications in biomedical modeling or transplantation therapy have been an extensive area of research fields [21]. Appropriate neural differentiation compounds promote differentiation of iPSCs into masses of early neural progenitors and then into mature neurons, glia, and neural crest cells that can be used in specific disease modeling [22]. Some studies have shown that 3-isobutyl-1-methyl-xanthine (IBMX) and forskolin are effective growth factors which can promote, improve, and enhance neural differentiation of various types of stem cells [23,24,25]. In the present investigation, IBMX and forskolin factors were applied in neural culture to induce iPSCs differentiation. Moreover, the neural induction potential of nisin (Ni) and non-essential amino acids (NEAA) as well as the synergic effect of Ni in combination with NEAA (NEAA-Ni) were examined.
Ni is produced by some strains of Lactococcus lactis bacterium as a cationic peptide with 34 amino acid residue [26]. This bacteriocin contains unusual amino acids and has antimicrobial activity against an extensive range of Gram-positive bacteria [27]. Due to its non-toxicity effects in humans, US Food and Drug Administration (FDA) has accepted it as a safe antimicrobial peptide. Hence, it has been extensively used as a natural food preservative in the food industry throughout the past 40 years [28]. There have been reports that Ni could be applied for therapeutic purposes and it was significantly effective in cell tumor genesis inhibition [29, 30]. Amino acids (AA) not only are the building blocks of proteins but also participate in many compounds of cells including nucleotide and lipid biosynthesis. In fact, specific amounts of AAs are required for growth, health, and development of the body [31, 32]. The AAs are classified as essential and non-essential depending on endogenous synthesizing. Non-essential means that animals and human can sufficiently synthesize them for normal growth and optimal health and they are also critical during development [33]. NEAAs include alanine, arginine, asparagine, aspartate, cysteine, glutamate, glutamine, glycine, proline, serine, taurine, and tyrosine [31]. Several NEAA-targeted therapies have been studied in various types of diseases and some of them have been measured by clinical trials [33, 34].
It is necessary to improve the neural culture for obtaining a high percentage of neural progenitors and disease-derived mature neurons which can be used for functional analysis. Although previous works have reported several protocols for neural induction of stem cells to generate neural progenitors, the low differentiation efficiency of such protocols highlight the need for different techniques for achieving the high yield of neural differentiation. The current evaluation was aimed at investigating the possible role of NEAA, Ni, and Ni-NEAA in the differentiation of hiPSCs into neural cells.
Material and methods
MTT assay
3-(4,5-dimethyl 2 thiazolyl)-2,5- diphenyl-2H-tetrazolium bromide (MTT) is a yellow tetrazolium dye that responds to metabolic activity. This method was applied to determine the optimal concentration of NEAA and NEAA-Ni in 3 days. For initiation, SNL76/7, which was purchased from the cell bank of Stem Cell Technology Research Center Bonyakhte (Tehran, Iran), were seeded at a density of 1 × 104 cells/ well on 48-well plates. Then cells were exposed to various concentrations of NEAA (1, 2.5, 5, and 10% (v/v)) and different concentrations of NEAA (1, 2.5, 5, and 10% (v/v)) in the combination of 800 IU/ml Ni which has recently been evaluated the optimal concentration (unpublished data) as NEAA-Ni, then were maintained in an incubator at 37 °C and 5% CO2 for 2, 4, and 6 days. At the end of each time point, after removal of the culture solution, the stock of MTT solution (Sigma-Aldrich) was added to the culture medium of determined wells. The cell culture plate was allowed to incubate for 4 h at 37 °C. After removal of the supernatant, 100 µl of dimethyl sulfoxide (DMSO, Sigma-Aldrich) was added to extract the MTT formazan crystals. As a final point, the each well’s absorbance was detected at the wavelength of 570 nm by a microplate reader (BioTek Instrument, USA).
Acridine orange staining
This method is for assessing cell viability using duel fluorescent staining solution (1 μl) containing 100 μg/ml acridine orange (AO) and 100 μg/ml ethidium bromide (EB) (AO/EB, Sigma‐Aldrich). The cultured cells in 48-well plates were exposed to various concentrations of NEAA (1, 2.5, 5, and 10% (v/v)) and NEAA-Ni (1, 2.5, 5, and 10% (v/v) of NEAA and 800 IU/ml Ni) in time periods of 2, 4, and 6 days. At the end of each time point, an amount of 200 μL of AO/EB mixture was purred to each sample, which was washed with PBS previously. After 40 min, each well was observed with a fluorescent microscope (Nikon, Japan).
iPS cell culture
The hiPSC line from adult dermal fibroblasts was purchased from the cell bank of Stem Cell Technology Research Center Bonyakhte (Tehran, Iran) and cell characterization of the cells have been assessed [35]. These cells were maintained in hiPSC culture medium containing DMEM/F12 culture medium, 10% FBS-ESC qualified, 0.1 mmol/l β-mercaptoethanol, 0.1 mmol/l non-essential amino acids, 20 ng/ml bFGF, 1 mmol/l L-glutamine, and 1% penicillin/streptomycin (all from Invitrogen Co. USA). These pluripotent cells were cultured on inactivated feeder layers of SNL76/7 cells in 6 cm Petri dishes (SPL Life Sciences Co. Korea) coated with 20 µg/ml laminin in PBS (Invitrogen). Every three days, the cells were passaged with iPSC medium and the medium was replaced every other day. The iPS colonies were isolated with 0.1% collagenase IV (Invitrogen), and then, kept in inactivated SNL76/7 cells for expansion.
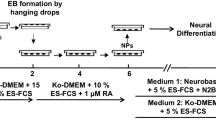
Embryoid body formation
Initially, hiPS colonies were dissociated from SNL76/7 cells with 0.1% collagenase IV. Then the colonies kept in low-adhesion non-treated six-well plates (Jet Biofil, Japan). The aggregates were suspended in hiPS medium without bFGF to generate embryoid bodies (EBs). The cells were kept for spontaneous aggregation for three days. Finally, the colonies formed as EB-like cells in the culture medium.
Differentiation of hiPSC into neuronal lineages
The EBs were collected and seeded onto laminin (20 µg/ml) coated six-well plates with neural induction media composed of Knockout DMEM/F12, 0.5 mM IBMX (Sigma), 2.5 mM forskolin (MP Biomedicals INC. CO, USA), and various concentrations of FBS (10, 5 and 2% during 1–4, 5–10 and 11–14 days of differentiation, respectively) [23]. The following day, the media were exposed to the given concentration of the NEAA, Ni, and NEAA-Ni. The cells were maintained in incubator with 5% CO2 at 37 °C for 14 days while the culture was replaced every other day. The differentiated hiPSCs were assessed for analysis of neural expression.
Quantitative gene expression analysis
On 14th day, the neural differentiation of the iPSCs was evaluated by real-time RT-PCR assay. In the present study, the transcription level of neural key genes, including NSE, MAP-2, and β-tubulin III, was assessed. First, total ribonucleic acid (RNA) was isolated and purified from cultured iPSCs using the Qiazol (Mxcell) according to the manufacturer’s instructions. The quality and quantity of RNA were examined by NanoDrop One spectrophotometer (Thermo Scientific). Next, cDNA synthesis was synthesized using the Revert Aid First Strand cDNA Synthesis kit (Gene All, Seoul, Korea) according to the manufacturer's protocol. Real-time PCR was carried out using Maxima TM SYBRGreen/ROX qPCR Master Mix (Fermentas) and Rotor-gene Q real-time analyzer (Corbett, Australia) in three-step with the following thermal setting: initial enzyme activation at 95 °C for 3 min followed by 40 cycles of amplification (95 °C for 5 s, 58 °C for 20 s and 72 °C for 30 s with fluorescence detection) and a final step of melting curve analysis. Each sample was run in duplicate and the average values were examined. HPRT1was utilized as endogenous control to calculate the relative quantitative model. The resulting gene expression data were analyzed with the 2−ΔCt method. The primers are listed in Table 1.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde (Sigma) for 20 min after washing twice with PBS and 0.4% Triton X100 in PBS was used to permeabilize for 10 min at room temperature. Next, 5% goat serum/PBS-tween-20 (Sigma) was added to block the fixed cells for 30 min at 37 °C, then incubated at 4 °C overnight in the presence of NSE (1:100 Santa Cruz biotechnology), MAP-2 (1:300 Santa Cruz biotechnology, INC), and β-tubulin III (1:50 Chemicon). Next, the cells were washed three times with PBS tween-20 (0.1%) and the phycoerythrin PE-conjugated anti-mouse IgG (1:100 Sigma) was used as the secondary antibody to incubate the cells for 1 h at room temperature. After washing the cells, the nuclei were stained with DAPI (Sigma), then photomicrographs were taken with a fluorescence microscope (Nikon, Japan).
Statistical analysis
Analysis of variance (One-way ANOVA) was used to evaluate cell proliferation and biological parameters. p ≤ 0.05 was considered statistically significant. All biological experiments were repeated at least three times independently. Data are reported as mean ± standard deviation (SD).
Results
Cell viability and cytotoxicity of NEAA and NEAA-Ni
The cytotoxicity effects of NEAA and NEAA-Ni on SNL76/7 cells were analyzed using MTT assay and the results are shown in Fig. 1. Due to the fact that on the first day, the cells were cultured on all surfaces in equal amounts, the results of culture demonstrated that none of the concentrations of NEAA and NEAA-Ni (1%, 2.5%, 5%, and 10%) had remarkable cytotoxicity in comparison with TCPS (tissue culture polystyrene or control sample) and NEAA had more proliferation than TCPS on 6th day (Fig. 1a, b). Therefore, by examining this evidence, the optimum concentration of the NEAA and NEAA-Ni were obtained.
AO/EB staining as a qualitative test was performed to determine the cytotoxicity of NEAA and NEAA-Ni. Staining assay was carried out in mentioned concentrations of NEAA and NEAA-Ni by a fluorescent microscope in time periods of 2, 4, and 6 days. As evident from the images shown in Fig. 2a, b, the treated cells had normal morphology with a defined nucleus and cytoskeleton compared to TCPS after 6 days of cell seeding. Hence, 1% NEAA, 800 IU/ml of Ni, and 1%-800 IU/ml of NEAA-Ni as final concentrations were selected for further neural differentiation studies.
Real-time RT-PCR analysis
The expression of neural-specific genes (NSE, MAP-2, and β-tubulin III) were assayed by real-time RT-PCR analysis after treatment with Ni, NEAA, and NEAA-Ni. Overall, as shown in Fig. 3, after two weeks, the neural gene expression of differentiated iPSC in all media was significantly higher than those in TCPS. In particular, real-time RT-PCR analysis demonstrated Ni and NEAA has significantly upregulated the mentioned genes in the neural differentiation process (Fig. 3). On the other hand, despite increasing in the expression of MAP-2 in Ni and NEAA media, low expression of MAP-2 was detected in iPSCs in NEAA-Ni medium on the 14th day. These data showed that the presence of Ni and NEAA in the induction of culture medium contributes to effective neural differentiation of iPSCs.
Investigation of neuronal gene expression NSE, MAP-2, and β-tubulin III in differentiated iPSCs. Real time RT-PCR analysis indicates the expression of neural markers in the presence of Ni, NEAA, and NEAA-Ni on 14th day. HPRT1 was used as a control for RNA sample quality. All experiments were repeated three times. Results are presented as mean ± SD. Significant levels are *p ≤ 0.05, ** P ≤ 0.00, and ***P ≤ 0.0001
Immunofluorescence staining for NSE, MAP-2, and β-tubulin III
Immunofluorescence staining as qualitative assay was performed to check the cellular expression of NSE, MAP-2, and β-tubulin III as neural-specific protein markers and the effect of Ni, NEAA, and NEAA-Ni was assayed during neural differentiation of hiPSCs after two weeks of the induction period. Expression of mentioned neural proteins corroborated their presence in differentiated hiPSCs in all induction media. These results showed that the protein expression of NSE, MAP-2, and β-tubulin III increased during neural differentiation of iPSCs in culture media containing Ni, NEAA, and NEAA-Ni compared to TCPS (Fig. 4 a–c).
Immunofluorescence staining of differentiated iPSCs, which were exposed to Ni, NEAA, and NEAA-Ni for 14 days. The cells were analyzed for the expression of neural markers including a NSE, b MAP-2, and c β-tubulin III (a, d, g, j), DAPI staining (b, e, h, k), merging DAPI and markers (c, f, i, l) (Magnification × 100)
Discussion
Numerous investigations have suggested that stem cells hold great promise in the remedy of various disease and disorder models. Nonetheless, ethical tensions of using the ESCs, the safety of applying MSCs, and lower self-renewal capability of adult stem cells (ASCs) are arguably the limiting factors for the clinical trial of those cells. These controversies regarding stem cell-based therapy have made studies turn towards generating iPSCs supplying great advantages and opportunities for researchers into the treatment of neurodegenerative diseases and finding clinical therapies [36, 37]. Since animal-based models do not regularly follow human physiology and sometimes are defective to predict human body response, iPSCs provide an alternative method for the costly and time-consuming experiments on animal modeling trials in vitro [38]. Although considerable efforts have been done to study the effective technique in neural differentiation of iPSCs in vitro, there are still profound challenges and considerable struggles to find simple and reproducible protocols for generating efficient differentiated neurons. Current neural protocols have not yet been used for large scale and rapid production of functional neural progenitors and mature neurons. The various studies have shown that it is necessary to attain the appropriate growth factors and effective small molecules which recapitulate neural induction [39]. Consequently, this study afforded to establish a new procedure to reach a simple, rapid, and efficient neural protocol.
First, EBs were successfully generated from iPSC clones that have been widely used in neural differentiation methods [40, 41]. Additionally, this study applied the efficient protocol containing forskolin and IBMX, which have been frequently indicated as neural differentiation factors to induce various stem cells as well as iPSCs [23, 25, 42]. Forskolin and IBMX raise the intracellular concentration of the second messenger, cyclic adenosine monophosphate (cAMP) [42]. The increase of cAMP signals through protein kinases activates the specific transcription factors. These factors significantly facilitate neuronal differentiation and morphological maturation and then increase general neural markers such as NSE, MAP-2, and β-tubulin III [24, 25, 43]. This study evaluated the influence of Ni, NEAA, and Ni-NEAA on the differentiation of iPSCs and the obtained data demonstrated the role of Ni and NEAA in iPSCs differentiation into neuronal lineages in vitro. IPSCs were differentiated for two weeks using media containing forskolin and IBMX in the presence of Ni, NEAA, and Ni-NEAA. The results of the present study showed that 1% NEAA and 800 IU/ml of Ni increased the expression of NSE, MAP-2, and β-tubulin III. The NSE as a neuron-specific, is a late event during neural differentiation, making it an influential indicator of neural maturation [44]. The MAP-2 plays significant functional roles in the outgrowth of neuronal processes [45]. In addition, β-tubulin III is an element of microtubules, which are dynamic components of the intracellular cytoskeleton and also acts as an established marker of proliferative and terminally differentiated neurons [46].
The change in the number of differentiated iPSCs in treated groups of NEAA showed that NEAA might increase the neural differentiation of iPSCs. The concentration of 1% NEAA significantly increased the expression of NSE, MAP-2, and β-tubulin III in particular. Previous studies reported that NEAA as small molecules were essential during embryonic development [47]. Grander (1998) found that AA were one of the most considerable nutrients to develop mammalian preimplantation embryo in vitro. Moreover, adding glutamine as well as other NEAA induced blastocyst formation during laboratory culture, while the removal of these AA prevented the growth of the embryo at the cellular stage [48]. In another study, the authors explained that low levels of AA as critical factors influenced the formation of specific cell lineages during differentiation of mouse EBs in the culture through alteration in DNA and histone methylation [49]. Hou et al. (2015) indicated that NEAA managed energy metabolism in a cell-specific manner; therefore, it could improve the efficiency of nutrient utilization [31]. Additionally, several studies demonstrated that NEAA was applied as one of the neuronal medium components in motor neuron induction media and made a positive expression of motor-neuron-specific factors [50,51,52]. Thus, there is a growing support for the concept that AAs, especially NEAA, can lead neural lineages and our results have provided that NEAA is a potentially effective strategy for differentiation of iPSCs to neurons based on visual morphology and differentiation markers.
In addition, the effect of Ni on neural differentiation of iPSCs was evaluated. Although numerous examinations have reported about the properties and therapeutic effects of Ni, no data are still available on the function of Ni on differentiation. In this research, for the first time, the impact of Ni on differentiation efficiency of iPSCs was investigated and Ni, as a safe natural food preservative imposed a beneficial effect on the differentiation of iPSCs into neural lineages. In the present study, Ni increased the expression of NSE, MAP-2, and β-tubulin III, as specific protein and gene markers during neural induction. Some studies reported the therapeutic capability of Ni in cancer cells as well as its potential applications in medicine over the last decade [29, 30, 53]. The significant increase in the expression levels of NSE, MAP-2, and β-tubulin III by Ni indicated that this bacteriocin was able to induce neural differentiation of iPSCs during 14 days in vitro. As a product of probiotics, Ni caused improvement of differentiation in iPSCs to neural lineages. Specific types of bacteria are classified as probiotics. These microorganisms have a variety of effects on cells and different probiotics may act in different ways. Several studies have shown the positive impact of probiotics on stem cells. To illustrate the point, Bui et al. (2015) showed that probiotic bacteria play a role in modulating the function of natural killer (NK) cells in healthy stem cell differentiation of apical papillae. These probiotic bacteria signal NK cells to secrete high amounts of IFN-γ and TNF-α, which are able to improve differentiation of the cells [54]. Han et al. (2020) reported that a balance between oral pathogenic bacteria and probiotics could activate the functions of MSCs, which had the potential to promote wound healing to the multilineage differentiation and self-renewal properties [55]. So far, there has been no available study within the database concerning the biological effects of Ni on neural differentiation, especially in iPSCs and the present study is the first work in this field. Some studies have reported several mechanisms, including changing the level of intracellular ions (e.g., calcium) and altering the transmembrane potential by Ni in the cells and then inducing the cell cycle. Ni becomes plunged in the cell membrane through the cationic portions of the amino acids which causes Ni to mediate phospholipid reorganization and permits the influx of ions [53, 56, 57]. Presumably, the capacity of Ni to change the transmembrane potential and membrane composition of cells leads to differential effects on cells. There is a need for more research works to investigate the function of Ni on the differentiation of iPSCs.
The real-time RT-PCR results of differentiated iPSCs in Ni-NEAA medium have shown a high expression level of neural markers. It seemed that Ni in combination with NEAA induced common signaling pathways in neural differentiation of iPSCs. According to the results, the Ni-NEAA medium slightly exhibited less effects on the differentiation of iPSCs compared to Ni and NEAA. Although it is reported that the activity of Ni depends on the chemical composition of the AA and how they interact with each other in the structure [58], there is no evidence of how these combinations act on each other. Zhou et al. (2016) showed that the activity of Ni changed when an eight amino acids tail from an anti-gram-negative peptide was fused to it [59]. Possibly, NEAA may modify the function of the molecular structure of Ni, which is composed of AA and then lead to less effect on neural differentiation of iPSCs. Future studies can investigate the signaling pathways involved in the effects of Ni-NEAA.
Conclusions
In conclusion, the results suggested that Ni and NEAA promote the differentiation of iPSCs into neuronal lineages. Accordingly, for the first time, this project successfully demonstrated that Ni and NEAA have a greater influence on the neural induction of iPSCs in vitro. The results of the present study open a window to investigate the functional mechanisms of Ni and NEAA in the differentiation of iPSCs. The physicochemical properties must be studied for more extensive application in a wide variety of settings and it is noteworthy that prior to in vivo assessments, more evaluations such as flow cytometry and Western blot are essential.
Abbreviations
- AO:
-
Acridine orange
- AA:
-
Amino acids
- cAMP:
-
Cyclic adenosine monophosphate
- ESCs:
-
Embryonic stem cells
- EB:
-
Ethidium Bromide
- hiPSCs:
-
Human induced pluripotent stem cells
- IBMX:
-
3-Isobutyl-1-methyl-xanthine
- MSCs:
-
Mesenchymal stem cells
- MAP-2:
-
Microtubule-associated protein-2
- NSE:
-
Neuron-specific enolase
- Ni:
-
Nisin
- NEAA-Ni:
-
Ni in combination with NEAA
- NEAA:
-
Non-essential amino acids
- RNA:
-
Ribonucleic acids
References
Lu P. Stem cell transplantation for spinal cord injury repair. Prog Brain Res. 2017;231:1–32.
Yuan T, Liu Q, Kang J, Gao H, et al. High-dose neural stem/progenitor cell transplantation increases engraftment and neuronal distribution and promotes functional recovery in rats after acutely severe spinal cord injury. Stem Cells Int. 2019;2019:9807978.
Silvestro S, Bramanti P, Trubiani O, Mazzon E. Stem cells therapy for spinal cord injury: an overview of clinical trials. Int J Mol Sci. 2020;21(2):659.
Pittenger MF, Discher DE, Péault BM, Phinney DG, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4(1):22.
Mukhamedshina YO, Gracheva OA, Mukhutdinova DM, Chelyshev YA, et al. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen Res. 2019;14(2):227–37.
Wang YH, Guo YC, Wang DR, Liu JY, et al. Adipose stem cell-based clinical strategy for neural regeneration: a review of current opinion. Stem Cells Int. 2019;2019:8502370.
Sugaya K, Vaidya M. Stem cell therapies for neurodegenerative diseases. Adv Exp Med Biol. 2018;1056:61–84.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2(12):3081–9.
Ghaedi M, Niklason LE. Human pluripotent stem cells (iPSC) generation, culture, and differentiation to lung progenitor cells. Methods Mol Biol. 2019;1576:55–92.
Nakao S, Ihara D, Hasegawa K, Kawamura T. Applications for induced pluripotent stem cells in disease modelling and drug development for heart diseases. Eur Cardiol. 2020;15:1–10.
Castro-Viñuelas R, Sanjurjo-Rodríguez C, Piñeiro-Ramil M, Hermida-Gómez T, et al. Generation and characterization of human induced pluripotent stem cells (iPSCs) from hand osteoarthritis patient-derived fibroblasts. Sci Rep. 2020;10(1):4272.
Genova E, Cavion F, Lucafò M, Leo L, et al. Induced pluripotent stem cells for therapy personalization in pediatric patients: Focus on drug-induced adverse events. World J Stem Cells. 2019;11(12):1020–44.
Zhang H, Shao X, Peng Y, Teng Y, et al. A novel machine learning based approach for iPS progenitor cell identification. PLoS Comput Biol. 2019;15(12):e1007351.
Bertucci TB, Dai G. Biomaterial engineering for controlling pluripotent stem cell fate. Stem Cells Int. 2018;2018:9068203.
Zhang FQ, Jiang JL, Zhang JT, Niu H, et al. Current status and future prospects of stem cell therapy in Alzheimer’s disease. Neural Regen Res. 2020;15(2):242–50.
Oh Y. Patient-specific pluripotent stem cell-based Parkinson’s disease models showing endogenous alpha-synuclein aggregation. BMB Rep. 2019;52(6):349–59.
Rindt H, Tom CM, Lorson CL, Mattis VB. Optimization of trans-Splicing for Huntington’s Disease RNA Therapy. Front Neurosci. 2017;11:544.
Csobonyeiova M, Polak S, Zamborsky R, Danisovic L. Recent progress in the regeneration of spinal cord injuries by induced pluripotent stem cells. Int J Mol Sci. 2019;20(15):3838.
Verpelli C, Carlessi L, Bechi G, Fusar PE, et al. Comparative neuronal differentiation of self-renewing neural progenitor cell lines obtained from human induced pluripotent stem cells. Front Cell Neurosci. 2013;7:175.
Wu YY, Chiu FL, Yeh CS, Kuo HC. Opportunities and challenges for the use of induced pluripotent stem cells in modelling neurodegenerative disease. Open Biol. 2019;9(1):180177.
Denham M, Dottori M. Neural differentiation of induced pluripotent stem cells. Methods Mol Biol. 2011;793:99–110.
Salimi A, Nadri S, Ghollasi M, Khajeh K, et al. Comparison of different protocols for neural differentiation of human induced pluripotent stem cells. Mol Biol Rep. 2014;41(3):1713–21.
Thompson R, Casali C, Chan C. Forskolin and IBMX induce neural transdifferentiation of MSCs through downregulation of the NRSF. Sci Rep. 2019;9(1):2969.
Shahbazi A, Safa M, Alikarami F, Kargozar S, et al. Rapid induction of neural differentiation in human umbilical cord matrix mesenchymal stem cells by cAMP-elevating agents. Int J Mol Cell Med. 2016;5(3):167–77.
Małaczewska J, Kaczorek-Łukowska E, Wójcik R, Siwicki AK. Antiviral effects of nisin, lysozyme, lactoferrin and their mixtures against bovine viral diarrhoea virus. BMC Vet Res. 2019;15(1):318.
Garcia-Gutierrez E, O’Connor PM, Saalbach G, Walsh CJ, et al. First evidence of production of the lantibiotic nisin P. Sci Rep. 2020;10(1):3738.
Ge X, Teng K, Wang J, Zhao F, et al. Ligand determinants of nisin for its induction activity. J Dairy Sci. 2016;99(7):5022–31.
Kamarajan P, Hayami T, Matte B, Liu Y, et al. Nisin ZP, a bacteriocin and food preservative, inhibits head and neck cancer tumorigenesis and prolongs survival. PLoS ONE. 2015;10(7):e0131008.
Zainodini N, Hassanshahi G, Hajizadeh M, Khanamani F-P, et al. Nisin induces cytotoxicity and apoptosis in human asterocytoma cell line (SW1088). Asian Pac J Cancer Prev. 2018;19(8):2217–22.
Hou Y, Yin Y, Wu G. Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp Biol Med (Maywood). 2015;240(8):997–1007.
Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1–17.
Choi BH, Coloff JL. The diverse functions of non-essential amino acids in cancer. Cancers (Basel). 2019;11(5):675.
Bonfili L, Cecarini V, Cuccioloni M, Angeletti M, et al. Essential amino acid mixtures drive cancer cells to apoptosis through proteasome inhibition and autophagy activation. Febs j. 2017;284(11):1726–37.
Lahmy R, Soleimani M, Sanati MH, Behmanesh M, et al. MiRNA-375 promotes beta pancreatic differentiation in human induced pluripotent stem (hiPS) cells. Mol Biol Rep. 2014;41(4):2055–66.
McComish SF, Caldwell MA. Generation of defined neural populations from pluripotent stem cells. Philos Trans R Soc Lond B Biol Sci. 2018. https://doi.org/10.1098/rstb.2017.0214.
Volarevic V, Markovic BS, Gazdic M, Volarevic A, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15(1):36–45.
Tukker AM, Wijnolts FMJ, de Groot A, Westerink RHS. Human iPSC-derived neuronal models for in vitro neurotoxicity assessment. Neurotoxicology. 2018;67:215–25.
Yap MS, Nathan KR, Yeo Y, Lim LW, et al. Neural differentiation of human pluripotent stem cells for nontherapeutic applications: toxicology, pharmacology, and in vitro disease modeling. Stem Cells Int. 2015;2015:105172.
Sheridan SD, Surampudi V, Rao RR. Analysis of embryoid bodies derived from human induced pluripotent stem cells as a means to assess pluripotency. Stem Cells Int. 2012;2012:738910.
Zhu L, Gomez-Duran A, Saretzki G, Jin S, et al. The mitochondrial protein CHCHD2 primes the differentiation potential of human induced pluripotent stem cells to neuroectodermal lineages. J Cell Biol. 2016;215(2):187–202.
Lepski G, Jannes C, Nikkhah G, Bischofberger J. cAMP promotes the differentiation of neural progenitor cells in vitro via modulation of voltage-gated calcium channels. Front Cell Neurosci. 2013;7:155.
Pan Y, Chen X, Wang S, Yang S, et al. In vitro neuronal differentiation of cultured human embryonic germ cells. Biochem Biophys Res Commun. 2005;327(2):548–56.
Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125–43.
Sanchez C, Diaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol. 2000;61(2):133–68.
Chacon J, Rogers CD. Early expression of Tubulin Beta-III in avian cranial neural crest cells. Gene Expr Patterns. 2019;34:119067.
Hu Q, Agarwal U, Bequette BJ. Gluconeogenesis, non-essential amino acid synthesis and substrate partitioning in chicken embryos during later development. Poult Sci. 2017;96(2):414–24.
Gardner DK. Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology. 1998;49(1):83–102.
Shan J, Hamazaki T, Tang TA, Terada N, et al. Activation of the amino acid response modulates lineage specification during differentiation of murine embryonic stem cells. Am J Physiol Endocrinol Metab. 2013;305(3):E325–35.
Bianchi F, Malboubi M, Li Y, George JH, et al. Rapid and efficient differentiation of functional motor neurons from human iPSC for neural injury modelling. Stem Cell Res. 2018;32:126–34.
Corti S, Nizzardo M, Simone C, Falcone M, et al. Genetic correction of human induced pluripotent stem cells from patients with spinal muscular atrophy. Sci Transl Med. 2012;4(165):165ra2.
Zhang M, Ngo J, Pirozzi F, Sun YP, et al. Highly efficient methods to obtain homogeneous dorsal neural progenitor cells from human and mouse embryonic stem cells and induced pluripotent stem cells. Stem Cell Res Ther. 2018;9(1):67.
Joo NE, Ritchie K, Kamarajan P, Miao D, et al. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 2012;1(3):295–305.
Bui VT, Tseng HC, Kozlowska A, Maung PO, et al. Augmented IFN-γ and TNF-α induced by probiotic bacteria in NK cells mediate differentiation of stem-like tumors leading to inhibition of tumor growth and reduction in inflammatory cytokine release; regulation by IL-10. Front Immunol. 2015;6:576.
Han N, Jia L, Guo L, Su Y, et al. Balanced oral pathogenic bacteria and probiotics promoted wound healing via maintaining mesenchymal stem cell homeostasis. Stem Cell Res Ther. 2020;11(1):61.
Moll GN, Clark J, Chan WC, Bycroft BW, et al. Role of transmembrane pH gradient and membrane binding in nisin pore formation. J Bacteriol. 1997;179(1):135.
Giffard CJ, Ladha S, Mackie AR, Clark DC, et al. Interaction of nisin with planar lipid bilayers monitored by fluorescence recovery after photobleaching. J Membr Biol. 1996;151(3):293–300.
Bartholomae M, Baumann T, Nickling JH, Peterhoff D, et al. Expanding the genetic code of lactococcus lactis and Escherichia coli to incorporate non-canonical amino acids for production of modified lantibiotics. Front Microbiol. 2018;9:657.
Zhou L, van Heel AJ, Montalban-Lopez M, Kuipers OP. Potentiating the activity of nisin against Escherichia coli. Front Cell Dev Biol. 2016;4:7.
Funding
The work was not supported by any Department.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The hiPSCs were purchased from The Stem Cell Technology Research Center.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eftekhari, E., Ghollasi, M., Halabian, R. et al. Nisin and non-essential amino acids: new perspective in differentiation of neural progenitors from human-induced pluripotent stem cells in vitro. Human Cell 34, 1142–1152 (2021). https://doi.org/10.1007/s13577-021-00537-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00537-9