Abstract
Ovarian cancer is a predominant gynecologic malignancy and correlated with high mortality and severe morbidity. Exosomal microRNAs (miRNAs) play crucial roles in various processes during the progression of ovarian cancer, such as cell proliferation, apoptosis, and invasion. However, the function of exosomal miR-21-5p in ovarian cancer is still unknown. Here, we found that miR-21-5p was upregulated in ovarian cancer tissues, plasma exosomes of ovarian cancer patients, and exosomes from ovarian cancer cells. MiR-21-5p was incorporated in the exosomes from the ovarian cancer cells. In addition, 5-ethynyl-2′-deoxyuridine (Edu), a marker of cancer cell proliferation, was enhanced by miR-21-5p mimic while reduced by miR-21-5p inhibitor in ovarian cancer cells. MiR-21-5p mimic could increase, but miR-21-5p inhibitor could decrease the migration and invasion of cancer cells. Ovarian cancer cell apoptosis was induced by miR-21-5p inhibitor. Moreover, miR-21-5p inhibitor could up-regulate the expression of pro-apoptotic cleaved caspase3 and Bax while downregulate the expression of anti-apoptotic Bcl2 in the cells. Exosomal miR-21-5p inhibited the expression of cyclin-dependent kinase 6 (CDK6) by targeting its 3′-untranslated region (3′-UTR) at both the mRNA and protein levels. Tumorigenicity analysis in nude mice revealed that exosomal miR-21-5p could increase tumor volume, size, and weight of ovarian cancer in vivo. Besides, miR-21-5p targeted CDK6 in tumor tissues of nude mice. In conclusion, exosomal miR-21-5p contributes to the progression of ovarian cancer by regulating CDK6. Our findings will provide novel insights into the mechanism of exosomal miR-21-5p in the development of ovarian cancer. Exosomal miR-21-5p may serve as a potential target for the therapy of ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among all gynecologic cancers, ovarian cancer is the leading cause of cancer mortality among women [1]. Although the treatment of ovarian cancer has been improved, the current therapies are restricted because of drug-resistant cancer cells [2]. Due to the lack of prominent and remarkable clinical performance at early stages and the insufficiency of screening methods, the diagnosis of ovarian cancer patients is usually at more advanced stages [3]. Current therapies cannot cure the malignancy and the 5-year survival rate of ovarian cancer is 45% [4]. Main risk factors for ovarian cancer include central adiposity [5], circulating vitamin D [6], and family history of breast cancer [7]. However, the pathogenesis of ovarian cancer is complex, therefore it is of great importance to explore the underlying mechanisms of ovarian cancer development.
MicroRNAs (miRNAs) are small non-coding RNAs (ncRNAs) with nearly 20–25 nucleotides in length and have significant impacts on numerous biological processes [8]. MiRNAs regulate gene expression post-transcriptionally by targeting mRNAs at the 3′-untranslated region (3′-UTR) [9]. MiRNAs regulate their targets that exert essential functions in many physiological and pathological processes, such as cell apoptosis, differentiation, proliferation, invasion, metastasis, and tumorigenesis [9]. The Nano-sized particles called exosomes serve as transport vesicles of biological loads, such as miRNAs, mRNAs, proteins, and long ncRNAs (lncRNAs), leading to the phenotypic impact on the receiver cells [10]. Exosomes, loaded with regulative miRNAs, play a critical role in long-distance cell communication participate in cancer development [11]. It has been well-recognized that exosomes promote cancer progression by regulating the expression of miRNAs in tumors and surrounding cells [12, 13]. Meanwhile, exosome-derived miRNAs are involved in regulating the pathogenesis of ovarian cancer. For example, ovarian cancer cell-derived exosomal miR-205 increases metastasis by enhancing angiogenesis [14]. Exosomal miR-205 participates in cell apoptosis, migration, invasion, and proliferation by mediating vascular endothelial growth factor A (VEGFA) in ovarian cancer cells [15]. Furthermore, miR-21-5p is a well-studied miRNA in multiple disease models and plays crucial roles in the development of different cancers, such as breast cancer, colorectal cancer, and non-small cell lung cancer [16,17,18]. It has been reported that miR-21-5p is abnormally expressed in ovarian cancer [19]. Inhibition of miR-21 represses the progression of ovarian cancer [20, 21]. Besides, miR-21 from exosomes is involved in the development of cancer [22]. However, the effect of exosomal miR-21-5p on ovarian cancer development remains elusive.
The function of cell-cycle kinase (CDK6) has drawn significant attention recently [23]. CDK6 has been identified as a transcriptional modulator and a representative cyclin-dependent kinase with features different from its close homolog cyclin-dependent kinase 4 (CDK4) [24]. CDK6 coordinates the transcriptional regulation of many genes, and it functions independently or dependent on its cell-cycle kinase action [25]. The transcription activity of CDK6 is essential to its function in mediating cancer progression [25]. It was reported that CDK6 preserves ovarian cancer from platinum-induced death by regulating forkhead box protein O3 (FOXO3) [26, 27]. Furthermore, it has been reported that exosomes are able to target CDK6 in cancer development. For example, CDK6 participates in exosomal PCAT-1/miR-182/miR-217 signaling-mediated Kras-associated chemoresistance and immunosuppression of lung cancer progression [28]. However, the correlation of CDK6 with exosomal miR-21-5p in the development of ovarian cancer is unclear.
In this study, we aimed to explore the role and underlying mechanism of exosomal miR-21-5p in the development of ovarian cancer. We identified a novel function of exosomal miR-21-5p in contributing to ovarian cancer progression by regulating CDK6.
Materials and methods
Ovarian cancer clinical samples
A total of 20 ovarian cancer clinical samples were obtained from 20 ovarian cancer patients who were admitted at the First Affiliated Hospital of Bengbu Medical College between June 2017 to June 2018. All patients were diagnosed by histopathological analysis. All cases were independently diagnosed and reviewed by two clinicians. Before surgery, no systemic or local therapy was initiated. Ovarian cancer tissues and corresponding para-neoplastic tissues collected from patients were immediately frozen in liquid nitrogen and stored at − 80 °C before subsequent experiments. Venous plasma samples were obtained from all participants and centrifuged, and the serum samples were stored at − 80 °C before further experiments. All patients and healthy cases signed the written consent form. This study was approved by the Ethics Committee of aforementioned hospital (No. 2765#BMC) and followed the experimental guidelines of the World Medical Association. The patient information was listed in Table 1.
Exosome isolation and analysis
The culture medium and plasma were centrifuged at 3000×g for 15 min to precipitate cells and cellular debris. Next, Exoquick exosome precipitation solution (System Biosciences, USA) was used to isolate exosomes. The exosome characteristics were analyzed by transmission electron microscopy (TEM) as previously described [29]. Exosome pellets were resuspended in PBS buffer and dropped onto a carbon-coated copper electron microscope grid [29]. A J Tecnai G2 F20 ST transmission electron microscope was used to observe the exosomes from the cell culture medium, peritoneal fluids, and ascites.
Cell culture and treatment
The IOSE-80 (human normal ovarian epithelial cells derived from human ovarian epithelium), A2780 (human ovarian cancer cell line, epithelial cell-like, derived from human skin), and SKOV-3 (human ovarian cancer cell line, epithelial cell-like, derived from human ovary) cell lines were obtained from American Type Tissue Culture Collection. Cells were cultured in DMEM (Gibco, USA) containing 100 units/mL penicillin (Gibco, USA), 0.1 mg/mL streptomycin (Gibco, USA), and 10% fetal bovine serum (Gibco, USA) at 37 °C with 5% CO2. MiR-21-5p mimic, miR-21-5p inhibitor, and the corresponding controls were constructed (GenePharma, China). Cell transfections were performed using Liposome 3000 (Invitrogen, USA) following the manufacturer's instructions. To evaluate the effect of miR-21-5p on ovarian cancer progression, exosomes were extracted from A2780 and SKOV-3 cells treated with miR-21-5p mimic, inhibitor, or the corresponding controls, and A2780 and SKOV-3 cells were further treated with the exosomes for further analysis.
Edu (5-ethynyl-2′-deoxyuridine) assay
Cell proliferation was analyzed by EdU assay using EdU detecting kit (RiboBio, China). Briefly, A2780 and SKOV-3 cells were cultured with EdU for 2 h, followed by fixation with 4% paraformaldehyde at room temperature for 30 min. Next, cells were permeabilized with 0.4% Triton X-100 for 10 min and stained with staining cocktail of EdU room temperature for 30 min in dark. The nuclear of cells was then stained with Hoechst at room temperature for 30 min. Images were analyzed using a Leica light microscope (Leica Microsystems) [30]. Cells were counted using ImageJ software (National Institutes of Health, http://rsbweb.nih.gov).
Transwell assay
For cell migration assay, cells were cultured for 24 h and resuspended in serum-free culture medium. Cells were then plated into the apical chamber of the transwell at a density of 5 × 103 cells/well. The culture medium was made up to 150 μL and the basolateral chamber was added with 600 μL culture medium. After 24 h of culturing at 37 °C with 5% CO2, cells were fixed with 4% paraformaldehyde for 10 min and stained by crystal violet dye for 20 min, followed by migration analysis using an intelligent biological navigator (Olympus, Tokyo, Japan). The migrated cells were recorded and calculated [30].
For cell invasion assay, Matrigel was melted at 4 °C overnight and diluted by pre-cold serum-free culture medium (ratio 8:1). The medium (50 μL) was placed into the transwell polycarbonate membrane with a pore diameter of 8 μm, making all the wells covered by Matrigel at 37 °C for 2 h. The cells were cultured for 24 h and resuspended in serum-free culture medium, then plated into the apical chamber of transwell with 1 × 105 cells/well, the medium was made up to 150 μL. The basolateral chamber was added with 600 μL complete medium with 50% FBS. After 24 h, cells were fixed with 4% paraformaldehyde for 15 min and stained by crystal violet dye for 10 min. The invaded cells were analyzed and calculated [30].
Cell apoptosis assay
About 2 × 105 cells were plated on 6-well plates. Cell apoptosis was measured by using the Annexin V-FITC Apoptosis Detection Kit (CST, USA) following the manufacturer’s instructions. Briefly, about 2 × 106 cells were collected and washed by binding buffer and dyed at 25 ℃, followed by flow cytometry analysis [31].
Quantitative reverse transcription-PCR (qRT-PCR)
Total RNAs were extracted from tissues and cells using RNeasy MiniKit (Qiagen company, Hilden, Germany). The first-strand cDNA was synthesized using reverse transcription kit (RR047A, Takara Bio Inc., Otsu, Shiga, Japan). For miRNAs, RNA samples were reverse transcribed into cDNA using the miRNA FirstStrand cDNA Synthesis kit (B532451-0020, Tailing Reaction, Shanghai Sangon Biotechnology Co. Ltd., Shanghai, China). qRT-PCR was performed using PremixExTaqTMII (Perfect Real Time) kit (DRR081, Takara Bio Inc., Otsu, Shiga, Japan). GAPDH and U6 were used as the internal control for mRNA and miRNA, respectively. The experiments were independently repeated for three times. Relative expression levels were calculated using the 2−ΔΔCt method. The primer sequences were as follows:
miR-21-5p F: 5′-TAGCTTATCAGACTGATG-3′
miR-21-5p R: 5′-CAGTGCGTGTCGTGGAGT-3′
CDK6 F: 5′-CGGGATCCACCATGGAGAAGGACGGCCTG-3′
CDK6 R: 5′-CGGATCCATTGCTCAGGCTGTATTCAGCTCCGA-3′
GAPDH F: 5′-AAGAAGGTGGTGAAGCAGGC-3′.
GAPDH R: 5′-TCCACCACCCAGTTGCTGTA-3′
U6 F: 5′-GCTTCGGCAGCACATATACTAA-3′
U6 R: 5′-AACGCTTCACGAATTTGCGT-3′
Luciferase reporter gene assay
The luciferase reporter gene assay was conducted using a Dual-luciferase Reporter Assay System (Promega, USA). Briefly, A2780 and SKOV-3 cells were treated with the control mimic or miR-21-5p mimic, the vector containing CDK6 or CDK6 mutant fragment were transfected into cells using Lipofectamine 3000 (Invitrogen, USA), followed by detection of luciferase activities. Renilla was used as a normalized control [30].
RNA pulldown assay
Cells were transfected with biotin-labeled bio-miR-21-5p probe and bio-NC probe for 48 h. Cells were incubated in Pierce immunoprecipitation solution. The lysate was incubated overnight with Dynabeads coated with RNase BSA and yeast tRNA. After that, cells were washed twice with precooling buffer and the supernatant was collect. MiR-21-5p was purified by Trizol method, and the enrichment of CDK6 was detected by RT-qPCR.
Western blot analysis
Total proteins were extracted from cells or mice tissues with RIPA buffer (CST, USA). Protein concentrations were measured using the BCA Protein Quantification Kit (Abbkine, USA). The same amount of (30 μg) of protein samples were separated by SDS-PAGE (12% polyacrylamide gels), followed by transferring to PVDF membranes (Millipore, USA). The membranes were blocked with 5% milk and incubated with the primary antibodies for Tsg101 (1:1,000) (ab83, Abcam, USA), CD63 (1:1,000) (ab216130, Abcam, USA), CD81 (1:1,000) (ab109201, Abcam, USA), caspase3 (1:1,000) (ab4051, Abcam, USA), Bax (1:1,000) (ab32503, Abcam, USA), Bcl2 (1:1,000) (ab194583, Abcam, USA), CDK6 (1:1,000) (ab226349, Abcam, USA), and β-actin (1:1,000) (ab8227, Abcam, USA) at 4 °C overnight. Next, the corresponding second antibodies (1:1,000) (ab7090, Abcam, USA) were used to incubate the membranes at room temperature for 1 h. Visualization was performed using an Odyssey CLx Infrared Imaging System.
Analysis of tumorigenicity in nude mice
The effect of exosomal miR-21-5p on tumor growth in vivo was analyzed in 4-week-old female Balb/c nude mice (n = 5). Mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). A2780 cells were treated with the exosomes extracted from A2780 cells transfected with miR-21-5p mimic, inhibitor, or the corresponding controls. And about 1 × 107 cells were subcutaneously injected into the mice. After 7 days of injection, tumor growth was measured every 7 days. Mice were euthanized after 28 days of injection and tumors were scaled. Tumor volume (V) was evaluated by estimating the length and width with calipers and measured with the method × 0.5. The expression levels of CDK6 and β-actin protein were analyzed by Western blot analysis in the tumor tissues. Animal care and procedures were authorized by the Animal Ethics Committee.
Statistical analysis
Data were presented as mean ± standard deviation (SD). Statistical analyses were conducted using SPSS 21.0 software. Unpaired Student’s t test was used to compare two groups, and one-way ANOVA was used to compare multiple groups. Graphs were generated by GraphPad Prism. P < 0.05 were considered as statistically significant.
Results
The expression levels of miR-21-5p were elevated in the plasma exosomes of ovarian cancer patients
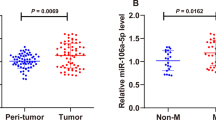
The expression levels of miR-21-5p were significantly increased in ovarian cancer patient tissues (n = 20) compared to that in adjacent normal tissues (n = 20) (P < 0.01) and normal ovarian tissues (n = 20) (P < 0.01) (Fig. 1a). To assess the potential correlation of miR-21-5p with ovarian cancer progression, the expression of miR-21-5p in plasma exosomes from ovarian cancer patients was detected. It showed that the expression levels of miR-21-5p were elevated in the plasma exosomes of ovarian cancer patients (n = 20) compared to that of normal cases (n = 20) (P < 0.01) (Fig. 1b), indicating that miR-21-5p may be correlated with the clinical development of ovarian cancer. Western blot analysis revealed the existence of the exosome markers, such as CD63, CD81, and Tsg101, of the exosome of serum samples from patients with ovarian cancer (Fig. 1c). Together these data suggested that miR-21-5p was upregulated in the plasma exosomes and tumor tissues of ovarian cancer patients.
The expression levels of miR-21-5p are elevated in the plasma exosome of ovarian cancer patients. a The expression levels of miR-21-5p were measured by qPCR assays in the ovarian cancer patient tissues (n = 20), the adjacent normal tissues (n = 20), and the normal ovarian tissues (n = 20). b The expression levels of miR-21-5p were measured by qPCR assays in the plasma exosome from ovarian cancer patients (n = 20) and normal cases (n = 20). c The expression of CD63, CD81, Tsg101, and β-actin was assessed by Western blot analysis in the exosomes from ovarian cancer patients. The experiments were independently repeated for at least three times. Data are presented as mean ± SD. Statistic significant differences were indicated: **P < 0.01
MiR-21-5p is transported by the exosomes from ovarian cancer cells
Next, whether miR-21-5p was loaded in the exosomes of the ovarian cancer cells was investigated. The expression levels of miR-21-5p were enhanced in ovarian cancer cells (A2780 and SKOV-3) compared to that in normal IOSE-80 cells (P < 0.01) (Fig. 2a). The expression levels of the exosome markers, such as CD63, CD81, and Tsg101, were enriched in exosomes extracted from A2780 and SKOV-3 cells (Fig. 2b). In addition, the characteristics of the exosomes were identified by transmission electron microscopy (TEM) (Fig. 2c). SKOV-3 and A2780 cells were treated with miR-21-5p mimic, inhibitor, or the corresponding controls, and the treatment efficiency was validated in the exosomes from these cells (P < 0.001) (Fig. 2d). Together these data indicated that miR-21-5p was upregulated in the exosomes from ovarian cancer cells.
MiR-21-5p is transported by the exosomes from the ovarian cancer cells. a The expression levels of miR-21-5p were measured by qPCR assays in the IOSE-80, SKOV-3, and A2780 cells. b The expression of CD63, CD81, Tsg101, and β-actin was assessed by Western blot analysis in the exosomes from ovarian cancer patients. c The characteristics of exosomes were analyzed by transmission electron microscopy (TEM) in SKOV-3 and A2780 cells. (D) SKOV-3 and A2780 cells were treated with miR-21-5p mimic, miR-21-5p inhibitor, or the corresponding controls, and the treatment efficiency was validated by qPCR assays in the exosomes from the cells. The experiments were independently repeated for at least three times. Data are presented as mean ± SD. Statistic significant differences were indicated: *P < 0.05, **P < 0.01, ***P < 0.01
Exosomal miR-21-5p promotes proliferation, invasion and migration of ovarian cancer cells
As miR-21-5p was incorporated in the exosomes from ovarian cancer cells, the role of exosomal miR-21-5p in the regulation of proliferation and apoptosis of ovarian cancer cells was then assessed. Exosomes were extracted from A2780 and SKOV-3 cells transfected with miR-21-5p mimic, inhibitor, or the controls, and the cells were further treated with exosomes. Edu assay showed that the cell proliferation was promoted by miR-21-5p mimic while reduced by miR-21-5p inhibitor in the cells (P < 0.01) (Fig. 3a). MiR-21-5p mimic could increase but miR-21-5p inhibitor could decrease the migration and invasion of SKOV-3 and A2780 cells (Fig. 3b). These results indicated that exosomal miR-21-5p could promote proliferation, invasion, and migration of ovarian cancer cells.
Exosomal miR-21-5p promotes proliferation, invasion and migration of ovarian cancer cells. (a and b) The exosomes were extracted from the SKOV-3 and A2780 cells treated with miR-21-5p mimic, miR-21-5p inhibitor, or the corresponding controls, and the SKOV-3 and A2780 cells were further treated with the exosomes. a The cell proliferation was analyzed by Edu assays in the cells. b The cell migration and invasion were examined by transwell assays in the cells. The experiments were independently repeated for at least three times. Data are presented as mean ± SD. Statistic significant differences were indicated: *P < 0.05, **P < 0.01
Exosomal miR-21-5p inhibits apoptosis of ovarian cancer cells
Next, the role of exosomal miR-21-5p in modulating apoptosis in ovarian cancer cells was assessed. Cell apoptosis was not affected by miR-21-5p mimic but was enhanced by transfection of miR-21-5p inhibitor in SKOV-3 and A2780 cells (P < 0.001) (Fig. 4a). Similarly, miR-21-5p inhibitor could enhance the expression levels of pro-apoptotic cleaved caspase3 (c-caspase3) and Bax, while reduce the expression levels of anti-apoptotic Bcl2 in the cells (P < 0.01) (Fig. 4b). These results indicated that exosomal miR-21-5p could inhibit apoptosis of ovarian cancer cells.
Exosomal miR-21-5p inhibits apoptosis of ovarian cancer cells. a and b The exosomes were extracted from the SKOV-3 and A2780 cells treated with miR-21-5p mimic, miR-21-5p inhibitor, or the corresponding controls, and the SKOV-3 and A2780 cells were further treated with the exosomes. a The cell apoptosis was measure by flow cytometry analysis in the cells. b The expression of caspase3, CD81, cleaved caspase3 (c-caspase3), Bax, Bcl2, and β-actin was assessed by Western blot analysis in the cells. The results of Western blot analysis were quantified by ImageJ software. The experiments were independently repeated for at least three times. Data are presented as mean ± SD. Statistic significant differences were indicated: *P < 0.05, **P < 0.01, ***P < 0.01
Exosomal miR-21-5p targets CDK6 in ovarian cancer cells
Bioinformatic analysis using Targetscan (http://www.targetscan.org/vert_72/) identified the miR-21-5p-targeted site in the 3′-UTR of CDK6 (Fig. 5a). Notably, the transfection with miR-21-5p mimic inhibited luciferase activities of the wild type CDK6 but did not affect the mutant CDK6 with the miR-21-5p-binding site mutated in the cells (P < 0.01) (Fig. 5b). Exosomes were extracted from A2780 and SKOV-3 cells transfected with miR-21-5p mimic, inhibitor, or the controls, and A2780 and SKOV-3 cells were further treated with the exosomes. RNA pulldown assay identified the interaction of miR-21-5p with CDK6 in the cells (P < 0.001) (Fig. 5c). The expression of CDK6 was inhibited by miR-21-5p mimic but enhanced by miR-21-5p inhibitor in the cells (P < 0.05) (Fig. 5d). Similarly, miR-21-5p mimic could decrease, but miR-21-5p inhibitor could increase the expression levels of CDK6 protein in the cells (P < 0.05) (Fig. 5e), indicating that exosomal miR-21-5p targeted CDK6 in the ovarian cancer cells.
Exosomal miR-21-5p targets CDK6 in the ovarian cancer cells. a The interaction of miR-21-5p and CDK6 3′-UTR was identified by bioinformatic analysis using Targetscan (http://www.targetscan.org/vert_72/). b SKOV-3 and A2780 cells were treated with miR-21-5p mimic or the control mimic. The luciferase activities of wild type CDK6 (CDK6WT) and CDK6 with the miR-21-5p-binding site mutant (CDK6 MUT) were determined by luciferase reporter gene assays in the cells. c The interaction of miR-21-5p and CDK6 was analyzed by RNA pulldown assays in the cells. (D and E) The exosomes were extracted from SKOV-3 and A2780 cells treated with miR-21-5p mimic, miR-21-5p inhibitor, or the corresponding controls, and the SKOV-3 and A2780 cells were further treated with the exosomes. d The expression of CDK6 mRNA was measured by qPCR assays in the cells. e The expression of CDK6 protein was tested by Western blot analysis in the cells. The results of Western blot analysis were quantified by ImageJ software. The experiments were independently repeated for at least three times. Data are presented as mean ± SD. Statistic significant differences were indicated: *P < 0.05, **P < 0.01
Exosomal miR-21-5p contributes to the tumor growth of ovarian cancer in vivo
Next, the impact of exosomal miR-21-5p on the development of ovarian cancer was assessed in vivo. Tumorigenicity analysis was performed in nude mice injected with A2780 cells, which were treated with the exosomes extracted from A2780 cells transfected with miR-21-5p mimic, miR-21-5p, or controls. It showed that miR-21-5p mimic significantly enhanced, while miR-21-5p inhibitor remarkably attenuated tumor growth of A2780 cells in vivo, as demonstrated by tumor volume (P < 0.05) (Fig. 6a), tumor size (Fig. 6b), and tumor weight (P < 0.01) (Fig. 6c). Besides, the expression levels of CDK6 were decreased by miR-21-5p mimic while increased by miR-21-5p inhibitor in tumor tissues of the mice (P < 0.05) (Fig. 6d). Moreover, the expression of miR-21-5p was validated in the system (P < 0.01) (Fig. 6e). These results suggested that exosomal miR-21-5p could promote tumor growth of ovarian cancer in vivo.
Exosomal miR-21-5p contributes to the tumor growth of ovarian cancer in vivo. a–d The effect of exosomal miR-21-5p on tumor growth of ovarian cancer cells in vivo was analyzed by nude mice tumorigenicity assay. The A2780 cells were treated with the exosomes extracted from the A2780 cells treated with miR-21-5p mimic, miR-21-5p inhibitor, or the corresponding controls. And the A2780 cells were subcutaneously injected in the mice. a The average tumor volume was calculated and shown. b Representative images of dissected tumors from nude mice were presented. c The average tumor weight was calculated and shown. d The protein expression levels of CDK6 and β-actin were examined by Western blot analysis in the tumor tissues. The results of Western blot analysis were quantified by ImageJ software. e The expression of miR-21-5p was tested by qPCR assays in the tumor tissues. The experiments were independently repeated for at least three times. Data are presented as mean ± SD. Statistic significant differences were indicated: *P < 0.05, **P < 0.01
Discussion
The mechanisms of the progression of ovarian cancer remain elusive, limiting the development of biomarkers for diagnosis, prognosis, and therapy of ovarian cancer patients [32]. During the past decades, exosome-derived ncRNAs have demonstrated crucial roles in the modulation of ovarian cancer. In this study, we identified that exosomal miR-21-5p contributed to ovarian cancer progression by regulating CDK6.
It has been shown that aberrantly expressed miRNAs participate in the development of ovarian cancer. For example, miR-552 is elevated in ovarian cancer and enhances progression of ovarian cancer through regulating the PTEN signaling [30]. MiR-126-3p is downregulated in ovarian cancer tissues and represses ovarian cancer invasion or proliferation by regulating PLXNB2 [33]. The expression levels of miR-338-3p are decreased in cisplatin-resisted models, promoting ovarian cancer cell sensitivity to cisplatin by down-regulating WNT2B [34]. MiR-21-5p, a well-studied miRNA, is abnormally expressed in multiple cancer models and contributes to the modulation of cancer development. For instance, miR-21-5p was remarkably upregulated in NSCLC tissues and contributes to the progression of NSCLC by targeting SMAD7 [35]. The role of miR-21-5p has been identified in several other gynecologic malignancies. It has been reported that miR-21-5p increases breast cancer progression by inhibiting mitogen-activated protein kinase10 (MAPK10) [36]. MiR-21-5p promotes the paclitaxel resistance and progression in drug-resistant breast cancer cells by regulating PDCD4 [37]. MiR-21-5p contributes to epithelial to mesenchymal transition in endometrial cancer by modulating SRY box 17 [38]. These studies demonstrated the critical role of miR-21-5p in different cancer models. Furthermore, miR-21-5p is abnormally expressed in ovarian cancer [19]. MiR-21 is involved in the regulation of ovarian cancer malignant progression (PMID: 25579119; 27166999). Exosomes-derived miR-21 functions as an epigenetic modulator in melanoma development (PMID: 32751207) [20,21,22]. In this study, we identified that the expression levels of miR-21-5p were elevated in ovarian cancer tissues, plasma exosomes of ovarian cancer patients, and exosomes from ovarian cancer cells. These findings indicated that abnormally expressed miR-21-5p in the exosomes may be involved in the progression of ovarian cancer in the clinical context, serving as a potential biomarker of ovarian cancer.
The exosome-transferred miRNAs play crucial roles in the pathogenesis of ovarian cancer. It has been reported that macrophages-obtained exosomes transfer miR-223 to ovarian cancer cells to exert a chemoresistant function [39]. Exosomal miR-99a-5p is un-regulated in sera of ovarian cancer patients and increases invasion of cancer cells through enhancing the expression of vitronectin and fibronectin in peritoneal mesothelial cells [40]. Exosome-derived miR-10a produced from amniotic fluid stem cells protects ovarian follicles against chemotherapy [41]. Plasma exosomal miRNA-34a serves as a potential marker of ovarian cancer [42]. Meanwhile, it has been reported that exosome miR-21-5p originated from gastric cancer cells increases metastasis by regulating the transition of mesothelial to mesenchymal [43]. Exosomal miR-21-5p is involved in Ovatodiolide/STAT3/β-Catenin-mediated oncogenic transformation of normal gingival fibroblasts and oral cancer malignancy [44]. In the present study, we revealed that exosomal miR-21-5p could enhance migration, invasion, and proliferation, and attenuate apoptosis in ovarian cancer cells. Exosomal miR-21-5p promoted tumor growth of ovarian cancer in vivo. These data present a novel function of exosomal miR-21-5p in ovarian cancer progression, providing valuable evidence for the fundamental role of exosomal miRNAs in the development of ovarian cancer.
It has been identified that CDK6 functions as an essential regulator in the development of ovarian cancer. Inhibition of CDK6 benefits the development of therapy in MYC driven ovarian cancer [45]. CDK6 is abnormally expressed in ovarian cancer cells and associated with cell cycle arrest [46]. CDK6 is involved in Alpinetin-attenuated migration and proliferation of ovarian cancer cells by inhibiting the STAT3 signaling [47]. Meanwhile, serval miRNAs can target CDK6 in ovarian cancer. MiR-211 represses the cell-cycle progression and proliferation of epithelial ovarian cancer through regulating CDK6 [48]. MiR-506 promotes senescence and represses proliferation by directly regulating the CDK4/6-FOXM1 signaling in ovarian cancer [49]. MiR-145 promotes ovarian cancer cell sensitivity to paclitaxel by modulating CDK6 [50]. Furthermore, it has been reported that CDK6 is involved in the exosomal PCAT-1/miR-182/miR-217 signaling-mediated Kras-associated chemoresistance and immunosuppression of cancer progression [28]. Our findings further demonstrated that CDK6 was targeted by exosomal miR-21-5p in ovarian cancer cells. These data identified new downstream target CDK6 of miR-21-5p, uncovering the correlation of CDK6 with miR-21-5p in the modulation of ovarian cancer. It was reported that miR-21 can be activated by the JNK-1/Jun pathway in cancer development (PMID: 24865582) [20,21,22], which is an up-stream regulation mechanism of miR-21-5p. We identified a downstream mechanism involving CDK6 of miR-21-5p, enriching the modulation network of miR-21-5p in cancer progression.
In conclusion, we discovered that exosomal miR-21-5p contributed to ovarian cancer progression by regulating CDK6. Our findings provide new insights into the mechanism by which exosomal miR-21-5p contributes to the development of ovarian cancer, enhancing understanding of the correlation of exosomal miRNA with ovarian cancer. Exosomal miR-21-5p may serve as a potential target for ovarian cancer therapy and treatment targeting exosomal miR-21-5p may be useful for ovarian cancer therapy.
References
Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14.
Gao MQ, Choi YP, Kang S, Youn JH, Cho NH. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29:2672–80.
Lalremmawia H, Tiwary BK. Identification of molecular biomarkers for ovarian cancer using computational approaches. Carcinogenesis. 2019;40:742–8.
van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–9.
Delort L, Kwiatkowski F, Chalabi N, Satih S, Bignon YJ, Bernard-Gallon DJ. Central adiposity as a major risk factor of ovarian cancer. Anticancer Res. 2009;29:5229–34.
Bull CJ, Yarmolinsky J, Wade KH. Commentary: Mendelian randomization analysis identifies circulating vitamin D as a causal risk factor for ovarian cancer. Int J Epidemiol. 2016;45:1631–3.
Kazerouni N, Greene MH, Lacey JV Jr, Mink PJ, Schairer C. Family history of breast cancer as a risk factor for ovarian cancer in a prospective study. Cancer. 2006;107:1075–83.
Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202–7.
Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–22.
Sun T, Kalionis B, Lv G, Xia S, Gao W. Role of exosomal noncoding RNAs in lung carcinogenesis. Biomed Res Int. 2015;2015:125807.
Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24.
Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35.
Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–20.
He L, Zhu W, Chen Q, et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9:8206–20.
Wang L, Zhao F, Xiao Z, Yao L. Exosomal microRNA-205 is involved in proliferation, migration, invasion, and apoptosis of ovarian cancer cells via regulating VEGFA. Cancer Cell Int. 2019;19:281.
Chen C, Liu X, Chen C, Chen Q, Dong Y, Hou B. Clinical significance of let-7a-5p and miR-21-5p in patients with breast cancer. Ann Clin Lab Sci. 2019;49:302–8.
Chen JC, Hsieh YY, Lo HL, Li A, Chou CJ, Yang PM. In vitro and in silico mechanistic insights into miR-21–5p-mediated topoisomerase drug resistance in human colorectal cancer cells. Biomolecules. 2019;9(9):467.
Wang P, Chen D, Ma H, Li Y. LncRNA MEG3 enhances cisplatin sensitivity in non-small cell lung cancer by regulating miR-21-5p/SOX7 axis. Onco Targets Ther. 2017;10:5137–49.
Alharbi M, Sharma S, Guanzon D, et al. miRNa signature in small extracellular vesicles and their association with platinum resistance and cancer recurrence in ovarian cancer. Nanomedicine. 2020;28:102207.
Pink RC, Samuel P, Massa D, Caley DP, Brooks SA, Carter DR. The passenger strand, miR-21-3p, plays a role in mediating cisplatin resistance in ovarian cancer cells. Gynecol Oncol. 2015;137:143–51.
Baez-Vega PM, Echevarria Vargas IM, Valiyeva F, Encarnacion-Rosado J, Roman A, Flores J, et al. Targeting miR-21-3p inhibits proliferation and invasion of ovarian cancer cells. Oncotarget. 2016;7:36321–37.
Melnik BC, John SM, Carrera-Bastos P, Schmitz G. MicroRNA-21-enriched exosomes as epigenetic regulators in melanomagenesis and melanoma progression: the impact of western lifestyle factors. Cancers (Basel). 2020;12(8):2111.
Kollmann K, Heller G, Schneckenleithner C, et al. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell. 2013;24:167–81.
Scheicher R, Hoelbl-Kovacic A, Bellutti F, et al. CDK6 as a key regulator of hematopoietic and leukemic stem cell activation. Blood. 2015;125:90–101.
Tigan AS, Bellutti F, Kollmann K, Tebb G, Sexl V. CDK6-a review of the past and a glimpse into the future: from cell-cycle control to transcriptional regulation. Oncogene. 2016;35:3083–91.
Handschick K, Beuerlein K, Jurida L, et al. Cyclin-dependent kinase 6 is a chromatin-bound cofactor for NF-kappaB-dependent gene expression. Mol Cell. 2014;53:193–208.
Uras IZ, Walter GJ, Scheicher R, et al. Palbociclib treatment of FLT3-ITD+ AML cells uncovers a kinase-dependent transcriptional regulation of FLT3 and PIM1 by CDK6. Blood. 2016;127:2890–902.
Domvri K, Petanidis S, Anestakis D, et al. Exosomal lncRNA PCAT-1 promotes Kras-associated chemoresistance via immunosuppressive miR-182/miR-217 signaling and p27/CDK6 regulation. Oncotarget. 2020;11:2847–62.
Pan R, Zhou H. Exosomal transfer of lncRNA H19 promotes erlotinib resistance in non-small cell lung cancer via miR-615-3p/ATG7 axis. Cancer Manag Res. 2020;12:4283–97.
Zhao W, Han T, Li B, Ma Q, Yang P, Li H. miR-552 promotes ovarian cancer progression by regulating PTEN pathway. J Ovarian Res. 2019;12:121.
Zou YT, Gao JY, Wang HL, Wang Y, Wang H, Li PL. Downregulation of microRNA-630 inhibits cell proliferation and invasion and enhances chemosensitivity in human ovarian carcinoma. Genet Mol Res. 2015;14:8766–77.
Kroeger PT Jr, Drapkin R. Pathogenesis and heterogeneity of ovarian cancer. Curr Opin Obstet Gynecol. 2017;29:26–34.
Xiang G, Cheng Y. MiR-126-3p inhibits ovarian cancer proliferation and invasion via targeting PLXNB2. Reprod Biol. 2018;18:218–24.
Niu Q, Liu Z, Gao J, Wang Q. MiR-338-3p enhances ovarian cancer cell sensitivity to cisplatin by downregulating WNT2B. Yonsei Med J. 2019;60:1146–56.
Li X, Wu X. MiR-21-5p promotes the progression of non-small-cell lung cancer by regulating the expression of SMAD7. Onco Targets Ther. 2018;11:8445–54.
Xie Y, Liu Y, Fan X, et al. MicroRNA-21 promotes progression of breast cancer via inhibition of mitogen-activated protein kinase10 (MAPK10). 2019. Biosci Rep. https://doi.org/10.1042/BSR20181000.
Tao L, Wu YQ, Zhang SP. MiR-21-5p enhances the progression and paclitaxel resistance in drug-resistant breast cancer cell lines by targeting PDCD4. Neoplasma. 2019;66:746–55.
Wang C, Li Q, He Y. MicroRNA215p promotes epithelial to mesenchymal transition by targeting SRYbox 17 in endometrial cancer. Oncol Rep. 2020;43:1897–905.
Zhu X, Shen H, Yin X, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019;38:81.
Yoshimura A, Sawada K, Nakamura K, et al. Exosomal miR-99a-5p is elevated in sera of ovarian cancer patients and promotes cancer cell invasion by increasing fibronectin and vitronectin expression in neighboring peritoneal mesothelial cells. BMC Cancer. 2018;18:1065.
Xiao GY, Cheng CC, Chiang YS, Cheng WT, Liu IH, Wu SC. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep. 2016;6:23120.
Maeda K, Sasaki H, Ueda S, et al. Serum exosomal microRNA-34a as a potential biomarker in epithelial ovarian cancer. J Ovarian Res. 2020;13:47.
Li Q, Li B, Li Q, et al. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018;9:854.
Chen JH, Wu ATH, Bamodu OA, et al. Ovatodiolide suppresses oral cancer malignancy by down-regulating exosomal Mir-21/STAT3/beta-Catenin cargo and preventing oncogenic transformation of normal gingival fibroblasts. Cancers (Basel). 2019;12(1):56.
Konecny GE. Combining PARP and CDK4/6 inhibitors in MYC driven ovarian cancer. EBioMedicine. 2019;43:9–10.
Yuan JM, Shi XJ, Sun P, et al. Downregulation of cell cycle-related proteins in ovarian cancer line and cell cycle arrest induced by microRNA. Int J Clin Exp Med. 2015;8:18476–81.
Zhao X, Guo X, Shen J, Hua D. Alpinetin inhibits proliferation and migration of ovarian cancer cells via suppression of STAT3 signaling. Mol Med Rep. 2018;18:4030–6.
Xia B, Yang S, Liu T, Lou G. miR-211 suppresses epithelial ovarian cancer proliferation and cell-cycle progression by targeting Cyclin D1 and CDK6. Mol Cancer. 2015;14:57.
Liu G, Sun Y, Ji P, et al. MiR-506 suppresses proliferation and induces senescence by directly targeting the CDK4/6-FOXM1 axis in ovarian cancer. J Pathol. 2014;233:308–18.
Zhu X, Li Y, Xie C, et al. miR-145 sensitizes ovarian cancer cells to paclitaxel by targeting Sp1 and Cdk6. Int J Cancer. 2014;135:1286–96.
Funding
This work was supported by 2018 Nanjing Health Science and Technology Development Project (Grant No. YKK18158) and 2019 Natural Science Research Project of Anhui Educational Committee (Grant No. KJ2019A0384).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
The clinical samples used in this study under the written approval of the patients and healthy cases. This study conformed to the experimental guidelines of the World Medical Association and the Ethics Committee of The First Affiliated Hospital of Bengbu Medical College. Animal care and method procedure were authorized by the Animal Ethics Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.












Rights and permissions
About this article
Cite this article
Cao, J., Zhang, Y., Mu, J. et al. Exosomal miR-21-5p contributes to ovarian cancer progression by regulating CDK6. Human Cell 34, 1185–1196 (2021). https://doi.org/10.1007/s13577-021-00522-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00522-2