Abstract
Dedifferentiated liposarcoma (DDLPS) is one of the four subtypes of liposarcomas; it is characterized by the amplification of the 12q13-15 region, which includes MDM2 and CDK4 genes. DDLPS has an extremely high local recurrence rate and is refractory to chemotherapy and radiation, which leads to poor prognosis. Therefore, a novel therapeutic strategy should be urgently established for improving the prognosis of DDLPS. Although patient-derived cell lines are important tools for basic research, there are no DDLPS cell lines available from public cell banks. Here, we report the establishment of a novel DDLPS cell line. Using the surgically resected tumor tissue from a patient with DDLPS, we established a cell line and named it NCC-DDLPS1-C1. The NCC-DDLPS1-C1 cells contained 12q13-15, 1p32, and 1q23 amplicons and highly expressed MDM2 and CDK4 proteins. NCC-DDLPS-C1 cells exhibited constant growth, spheroid formation, aggressive invasion, and tumorigenesis in mice. By screening a drug library, we identified that the proteasome inhibitor, bortezomib, had inhibitory effects on the proliferation of NCC-DDLPS1-C1 cells. We concluded that the NCC-DDLPS1-C1 cell line may serve as a useful tool for basic and pre-clinical studies of DDLPS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dedifferentiated liposarcoma (DDLPS) is one of the four subtypes of liposarcoma. Each subtype of a liposarcoma (atypical lipomatous tumor/well-differentiated liposarcoma, dedifferentiated liposarcoma, myxoid liposarcoma, and pleomorphic liposarcoma) has different clinical behaviors and genetic characteristics [1, 2]. DDLPS was first reported by Evans [3]; it is defined by the association of an atypical lipomatous tumor/well-differentiated liposarcoma (WDLPS) component with a non-lipogenic sarcoma of variable histological grade [1, 2]. DDLPS is also characterized by the amplification of the 12q13-15 region, which includes MDM2 and CDK4 genes [4,5,6,7,8,9,10,11,12]. Besides the amplification of 12q13-15, the gain of 1p32, 1q21-23, and 6q23-24 is occasionally recognized in DDLPS, which also relates with tumor transformation and progression [4,5,6,7,8, 11, 12]. Recently, several studies reported that poorly differentiated sarcomas with MDM2 amplification, diagnosed earlier as malignant fibrous histiocytomas or undifferentiated pleomorphic sarcomas, are actually DDLPS, considering their clinical behaviors and molecular biology [13,14,15,16]. These studies suggested that sarcomas should be diagnosed by their unique genomic profiling if they lack well-differentiated components. And the latest edition of WHO classification of soft tissue and bone tumors mentioned that a well-differentiated component may not be identifiable [1]. DDLPS often occurs in the retroperitoneum or deep inside the extremity muscles of patients aged > 40 years and rarely in children [1, 2, 17]. While WDLPS is defined as an intermediate tumor with rare metastasis, DDLPS is associated with a 15–20% metastatic rate and a nearly 50% recurrence rate [2, 17]. Specifically, DDLPS arising from the retroperitoneum has an extremely higher recurrence rate (80–90%), which leads to poor prognosis, than DDLPS arising from extremities [17,18,19]. Despite over 40 years having passed since DDLPS was first reported in 1979, there is still not enough evidence of the effectiveness of chemotherapy and radiation for the treatment of DDLPS [17, 20]. As in the case of other sarcomas, adriamycin, docetaxel, or gemcitabine-based therapy might be used for DDLPS, but the response rates are low and such treatments rarely prolong survival [17, 20]. Recent clinical trials have reported the efficacy of new drugs (e.g., tyrosine kinase inhibitor, MDM2 inhibitor, CDK4 inhibitor, and immune checkpoint inhibitor) [17]. However, considering its rarity, basic and clinical research have not been sufficient, and further studies are needed.
Patient-derived cancer cell lines are an important tool for basic and pre-clinical studies. Although long-term culturing is associated with alterations in the genetic profiles of cell lines [21,22,23], those maintained for a short period often retain the original genetic and phenotype characteristics, which aid in examining the effects of novel drugs and evaluating the molecular mechanisms of cancer progression [24,25,26,27,28]. Therefore, there is a need to continuously establish new cell lines. Using the cell line database, Cellosaurus [29], we found that there were cell lines annotated as “liposarcoma.” However, there were fewer cell lines annotated as “dedifferentiated liposarcoma;” we identified 23 DDLPS cell lines previously reported, but there were no DDLPS cell lines publicly available from cell banks (Supplementary Table 1). Further, because DDLPS is clinically diverse and requires more therapeutic options, there is a need to establish more patient-derived DDLPS cell lines.
Here, we report a novel DDLPS cell line, NCC-DDLPS1-C1, established from a surgically resected tumor tissue of a patient with DDLPS. We characterized NCC-DDLPS1-C1 cells and examined the antiproliferative effects of anti-cancer agents on this cell line.
Materials and methods
Patient history
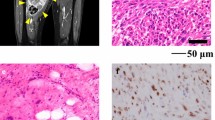
The patient donor was a 57-year-old man with pleomorphic spindle cell sarcoma, most consistent with dedifferentiated liposarcoma. He visited the hospital with a major mass on his left lower abdomen. A computed tomography (CT) and a magnetic resonance imaging (MRI) detected a retroperitoneal tumor (Fig. 1a–d). A malignant tumor was suggested, and he was referred to the National Cancer Center Hospital (Tokyo, Japan). Subsequently, needle biopsy was done, which suggested dedifferentiated liposarcoma. Although there was no evidence of metastatic lesions on CT and MRI scans, the tumor was too large and involved the surrounding organs. Therefore, the patient first received neoadjuvant chemotherapy (AI; doxorubicin and ifosfamide). After three courses of chemotherapy, wide resection was performed. A part of the resected tumor was used to establish the cell line described in this study. Histological evaluation of the resected specimen demonstrated an appearance typical of dedifferentiated liposarcoma (Fig. 1e–g); the spindle cells showed pleomorphic nuclei, prominent nucleoli, and frequent mitotic activity including atypical forms. MDM2/CDK4 immunohistochemistry showed positive nuclear labeling, although cytoplasm is often co-stained for CDK4. However, there was no evidence of well-differentiated components. These findings led to the diagnosis of DDLPS. The resection margin was positive microscopically, and the patient received postoperative chemotherapy (AI). However, 3 months after the operation, peritoneum dissemination was detected on CT. The patient received further chemotherapy (eribulin); however, the disease progressed rapidly. Finally, the patient died 4 months after the operation. The ethical committee of National Cancer Center approved the use of clinical materials for this study, and written informed consent was obtained from the donor patient in this study.
Clinical and pathological data. a Computed tomography showing a mass (83 × 70 × 70 mm) in the patient’s left retroperitoneal, with b mild enhancement by iodine contrast. c Image showing isointense lesion in a T2-weighted image, with d marked enhancement by gadolinium. e H&E showing fascicular proliferation of pleomorphic spindle cells. The tumor cells are diffusely positive for f MDM2 and g CDK4
Histological analysis
Histology examination was performed on 4 μm-thick sections from a representative paraffinized tumor sample. The sections were deparaffinized and stained with hematoxylin and eosin (H&E).
Immunohistochemistry
Immunohistochemistry was also performed with the deparaffinized tumor sample. The sections were exposed to 3% hydrogen peroxide for 15 min to block endogenous peroxidase activity and then washed in deionized water for 2–3 min. Preparations were pretreated with heat-induced epitope retrieval. The primary antibodies were MDM2 (IF2, 1:100; Zymed Laboratories, San Francisco, CA, USA), and CDK4 (DCS-31, 1:200; Biosource International, Camarillo, CA, USA). The slides were incubated for 1 h at room temperature, and subsequently labeled with peroxidase (EnVision system, Dako, Santa Clara, CA, USA).
Cell culture procedure
The surgically resected tumor tissue was used for establishing the cell line as previously described [30]. Fetal bovine serum was obtained from Gibco (Gibco, Grand Island, NY, USA). The cells were maintained for more than 3 months under tissue culture conditions and passaged more than 20 times.
Authentication and quality control of the established cell line
The established cell line was authenticated by examining short tandem repeats (STRs) in 10 loci by using the GenePrint 10 system (Promega, Madison, WI, USA) according to the manufacturer’s instructions and the procedure described in our previous study [30]. Mycoplasma contamination was examined using the DNA in the tissue culture medium of the cell line as previously reported [30].
Single-nucleotide polymorphism (SNP) array
SNP array genotyping was conducted with Infinium OmniExpressExome-8 v1.4 BeadChip (Illumina, Sn Diego, CA, USA) following the manufacturer’s instructions and the procedure described in our previous study [30]. Copy number analysis was performed using ASCAT (allele-specific copy number analysis of tumor) (version 2.1) considering non-neoplastic cell infiltration and tumor aneuploidy, and this resulted in integral allele-specific copy number profiles for the tumor cells.
Western blotting
The NCC-DDLPS1-C1 cell line, WDLPS cell lines (93T449 and 94T778; ATCC, Manassas, VA, USA), and normal human foreskin fibroblast cell line (HFF) were used for western blotting. The HFF cell line was kindly provided by Dr. Kiyono (Division of Carcinogenesis and Prevention, National Cancer Center Research Institute, Tokyo, Japan). Total proteins were extracted from each cell line using a lysis buffer (50-mM Tris–HCl at pH 7.5, 250-mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate, 20% glycerol, 10-μM zinc chloride, 2-mM dithiothreitol, 80-mM β-glycerophosphate, 50-mM sodium fluoride, 1-mM sodium orthovanadate, and a complete protease inhibitor tablet [Roche, Basel, Switzerland]). Protein concentrations were determined using the DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA). Protein lysates were electrophoresed on 12.5% SDS–polyacrylamide gels (e-PAGEL; ATTO corporation, Tokyo, Japan), and transferred to Immun-Blot PVDF membranes (Bio-Rad Laboratories, Inc., CA, USA). After incubation with 5% skim milk (BD Biosciences, Franklin Lakes, NJ, USA) in TBST (10-mM Tris, pH 8.0, 150-mM NaCl, 0.5% Tween 20) for 1 h, the membrane was first incubated with antibodies against MDM2 (1:100; Calbiochem, San Diego, CA, USA), CDK4 (1:1000; Santacruz, Dallas, Texas, USA) or β-actin (1:5000: abcam, Cambridge, UK) at 4 °C for 12 h. Membranes were washed 3 times for 10 min and then incubated with a 1:5000 dilution of horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies (GE Healthcare, Chicago, IL, USA) for 1 h. After the reaction, the membranes were washed 3 times and treated with Western Lightning Plus-ECL (PerkinElmer, Waltham, MA, USA). Subsequently, the images were taken using Amershan Imager 600 (GE Healthcare). Relative expression levels of each protein were compensated by β-actin.
Cell proliferation assay
The tissue-cultured cells were seeded at 2 × 104 cells/well in 24-well culture plates at day 0, and the cell number was counted at multiple time points as previously described [30]. The doubling time was calculated based on the growth curve.
Spheroid formation assay
Spheroid formation was examined as previously described [30]. Briefly, cells (1 × 105) were seeded into round-bottom 96-well ultra-low-attachment plates (96-well Clear Flat Bottom Ultra Low Attachment Microplate; Corning, Inc., Corning, NY, USA) in DMEM/F12 containing 10% FBS. The cells were maintained in a humidified atmosphere of 5% CO2 at 37 °C for 3 days, and the formation of spherical shaped colonies was confirmed microscopically (Keyence, Osaka, Japan). The colonies were prepared for paraffin sections using iPGell (Genostaff, Tokyo, Japan) according to the manufacturer’s instruction. Cell blocks were fixed with 10% formalin neutral buffer solution and embedded in paraffin. Four-micrometer-thick paraffin sections were prepared and H&E stained. All assays were performed in triplicate.
Transwell cell invasion assay
Cell invasion assays were performed with BD BioCoat Matrigel Invasion Chambers (BD Biosciences) according to the manufacturer’s instructions and the procedure described in our previous study [30] using NCC-DDLPS1-C1 and the osteosarcoma cell line, MG63 (JCRB Cell Bank, Ibaragi, Osaka, Japan).
Tumorigenesis in nude mice
All surgical procedures and care administered to the animals were in accordance with the institutional guidelines. A 100-μL volume of cells in a 1:1 mixture of Matrigel (BD Biosciences) was subcutaneously injected into female BALB/c nude mice (CLEA Japan, Inc., Tokyo, Japan) (1 × 106 cells). Subsequently, the tumor size was measured weekly. The tumor volume (mm3) was calculated as L × W2 × 0.52, where L is the longest diameter and W is the shortest diameter. After 2 weeks, the tumors were surgically resected, and the specimens were stained with H&E. Three tumor samples, except one outlier, were used to describe the graph of tumor growth curve. All animal experiments were performed in accordance with the guidelines for Animal Experiments of the National Cancer Center and approved by the Institutional Committee for Ethics of Animal Experimentation.
Screening for the antiproliferative effects of anti-cancer reagents
Screening for antiproliferative effects of anti-cancer reagents was performed according to the manufacturer’s instructions and the procedure that we previously reported [30]. We use 195 anti-cancer compounds, including FDA-approved drugs (Selleck Chemicals, Houston, TX, USA), and a list of the anti-cancer agents has been provided in Supplementary Table 2.
Dose–response experiments were also performed to validate available hits in the pilot screening according to the methods as previously reported [30]. IC50, the sample concentration required to inhibit cell growth by 50% in comparison with the growth of the control cell, was determined from the dose–response curves.
Results
Authentication of the established cell line
The NCC-DDLPS1-C1 cell line was authenticated by analyzing the STR status of 10 microsatellites in the NCC-DDLPS1-C1 cells and the corresponding original tumor tissue. STR allele patterns were completely identical between the NCC-DDLPS1-C1 cells and the corresponding original tumor tissue (Table 1, Supplementary Figure 1). The STR patterns of NCC-DDLPS1-C1 cells did not match those of any cell lines in the public cell banks examined using a function of the cell line database, Cellosaurus [29]. Thus, we concluded that NCC-DDLPS1-C1 was a novel cell line. We did not detect the DNA sequence unique to mycoplasma in the tissue culture medium of the NCC-DDLPS1-C1 cells (data not shown).
Characterization of the cell line
SNP array analysis revealed multiple allelic duplications in NCC-DDLPS1-C1 cells (amplified: 1p32, 1q23 and 12q13-15; Fig. 2, Supplementary Table 3). The amplification is characteristic of DDLPS, and the 12q13-15 region includes MDM2 and CDK4 genes. In western blotting, we used the WDLPS cell lines, which is known to highly express MDM2 and CDK4 proteins, as positive control, and the HFF cell line as negative control. We observed the overexpression of MDM2 and CDK4 proteins in NCC-DDLPS1-C1 cells by western blotting (Fig. 3a–c). These findings demonstrate that NCC-DDLPS1-C1 cell lines maintain the unique characteristics of DDLPS and could be utilized for pre-clinical studies.
Study of the single nucleotide polymorphism (SNP) array. Allele-specific copy number analysis revealed DNA copy number variations. SNP genotyping copy number profiles for NCC-DDLPS1-C1 cells showing logR and B allele frequency (BAF). The plot represents log R and BAF identified with the ASCAT algorithm
NCC-DDLPS1-C1 cells were composed of a mixed population of pleomorphic spindle and small round cells in 2D culture conditions (Fig. 4a, b). The proliferation of NCC-DDLPS1-C1 cells was monitored using a growth curve based on the number of cells. The population doubling time during the logarithmic growth phase was approximately 50 h (Fig. 4c). We confirmed that NCC-DDLPS1-C1 cells formed spheroids when they were seeded on a low-attachment microplate. The H&E stained spheroid section showed pleomorphic epithelioid cells with rhabdoid features (Fig. 4d). We also found that NCC-DDLPS1-C1 cells exhibited a more aggressive invasion ability than MG63 cells. The invasion ability of NCC-DDLPS1-C1 cells depended on the number of seeded cells and the time after seeding (Fig. 4e).
Characterization of NCC-DDLPS1-C1 cells. a, b NCC-DDLPS1-C1 cells showing pleomorphic spindle appearance under 2D culture conditions. c Growth curve of NCC-DDLPS1-C1 cells. The y-axis indicates the relative cell proliferation of NCC-DDLPS1-C1 cells, and the x-axis represents the day after seeding. d The H&E stained spheroid section showing pleomorphic epithelioid cells with rhabdoid features. e The invasion ability of NCC-DDLPS1-C1 cells in comparison to that of MG63 osteosarcoma cells
Tumorigenesis in nude mice
NCC-DDLPS1-C1 cells transplanted into BALB/c nude mice were able to undergo tumorigenesis under the described condition (Fig. 5a). The tumor consisted of dense proliferation of epithelioid to short spindle cells with nuclear atypia (Fig. 5b). The tumor size and volume consistently increased after the transplantation (Fig. 5c).
Tumorigenesis in nude mice. a NCC-DDLPS1-C1 cells transplanted into BALB/c nude mice have the capability of tumorigenesis. b The tumor consisting of dense proliferation of epithelioid to short spindle cells with nuclear atypia. c The graph showing estimated tumor volume. Bars represent the mean standard error
Sensitivity to anti-cancer drugs
The response of NCC-DDLPS1-C1 cells to antiproliferative effects was examined using a library of 195 anti-cancer agents (Supplementary Table 2). The cells were treated with the anti-cancer agents at a fixed concentration of 10 µM; the proliferation-suppressive effects are shown in Supplementary Table 4. Among the 195 anti-cancer agents examined, 4 agents that showed remarkable antiproliferative effects on NCC-DDLPS1-C1 cells were further examined to calculate their IC50 values. The IC50 values of these anti-cancer agents are listed in Table 2, and the growth curve based on which the IC50 values were calculated are demonstrated in Fig. 6.
Growth curves for IC50 value calculation of the investigated anti-cancer compounds. a NCC-DDLPS1-C1 cells treated with 195 anti-cancer agents at a concentration of 10 µM for 72 h. b–e Viability of the cells treated with 4 anti-cancer agents at different concentrations. The name of each anti-cancer agent is shown under the graph
Discussion
DDLPS frequently relapses and has poor prognosis, and there is only limited evidence of an effective response to chemotherapy until now [17, 20]. In particular, DDLPS arising from the retroperitoneum is extremely difficult to be completely resected because of its anatomical position [17,18,19]. To improve the prognosis of patients with DDLPS, establishing novel treatment strategies is urgently required. Patient-derived cell lines maintained for a short period retain their original characteristics. Therefore, these cell lines are indispensable tools to research the biology of tumors, which may lead to novel treatment methods. However, for DDLPS, only a few cell lines are available because of its rarity, and there are no means to obtain the cell lines from public cell banks. For those reasons, we established a novel DDLPS cell line, NCC-DDLPS1-C1, and demonstrated its utility.
The NCC-DDLPS1-C1 cell line was established from the retroperitoneal tumor. The site of origin of the tumor and patient’s age were both typical for DDLPS [1, 2, 17]. Further, with the overexpression of MDM2 and CDK4 proteins, the tumor was considered as DDLPS. However, there was no finding of well-differentiated components in the surgically resected specimen. Therefore, the tumor was diagnosed as pleomorphic spindle cell sarcoma, which is most consistent with DDLPS. In the retroperitoneum, it is difficult to distinguish between the well-differentiated components and normal fat, which often results in inadequate resection of the tumor. Therefore, the anatomical location of the tumor may have prevented us from finding the well-differentiated components. Recently, because the molecular biology of DDLPS has been gradually revealed, it is often diagnosed as DDLPS by its characteristic gene amplification of 12q13-15 along with the overexpression of MDM2 and CDK4 proteins, if there are no well-differentiated components [1, 13,14,15,16]. In this case, the original tumor had the characteristics of DDLPS, and the NCC-DDLPS1-C1 cell line also preserved the genetic features. We, therefore, concluded that this cell line could be used as a DDLPS cell line.
Besides the amplification of 12q13-15, NCC-DDLPS1-C1 cells presented the amplification of 1p32 and 1q23, which was often observed in DDLPS patients (24%–60%) [4,5,6,7,8, 11]. This feature also supported the fact that NCC-DDLPS1-C1 cells retain DDLPS characteristics. Hirata et al. reported that the amplification of 1p32 is associated with poor prognosis of DDLPS patients [4]. In this case, the patient unfortunately had rapid progression of DDLPS, and therefore, NCC-DDLPS1-C1 cells would be eligible for studying highly malignant DDLPS.
NCC-DDLPS1-C1 cells exhibited the typical morphology of spindle cell sarcoma, showing rapid proliferation and aggressive invasion ability, which might reflect the high malignancy and poor prognosis of DDLPS in the donor patient. NCC-DDLPS1-C1 cells had the ability to form spheroids, which is useful for understanding the behavior of DDLPS in a 3D environment. The further characterization of xenografted tumor cells may be useful for the research, which requires the cell line xenografts. In addition, NCC-DDLPS1-C1 cells were adapted to nude mice under the described condition and showed similar morphology to the original tumor. Therefore, we consider that NCC-DDLPS1-C1 cells as useful both in vitro and in vivo experiments. However, the cell lines do not recapitulate all features of tumors in human body, and the complemental use of other models such as patient-derived xenografts may be required for research.
DDLPS is known to be refractory to chemotherapy, and NCC-DDLPS1-C1 cells also demonstrated resistance to most of the 195 anti-cancer compounds. In contrast, bortezomib exhibited a remarkable effect at the nanomolar level. Bortezomib is a first-in-class proteasome inhibitor and is usually used for multiple myeloma [31]. The effectiveness of the combination therapy of bortezomib and other anti-cancer drugs for osteosarcoma, gastrointestinal stromal tumor, and various sarcoma types was reported previously [32, 33]. Bortezomib inhibits the 26S proteasome, and exerts its anti-tumor effects through multiple mechanisms involving NF-κβ, cell cycle proteins, apoptosis-regulatory proteins, and endoplasmic reticulum stress [31,32,33]. In DDLPS, the amplification of cell cycle protein, CDK4, is related to its oncogenesis, and this fact might reflect the effectiveness of bortezomib. Furthermore, Perez et al. reported the efficacy of bortezomib in sarcomas with high expression levels of the MAP17 protein, including liposarcomas [34]. MAP17 protein is a small non-glycosylated membrane protein and is reported to activate the Notch pathway [35], which may also relate to the cellular toxicity of bortezomib on DDLPS. DDLPS has a lot of genetic aberrations and diversity, therefore further investigation will be required to reveal the pharmacological mechanisms on DDLPS.
The clinical features of DDLPS are highly diverse in the view of its genomic background [4], and the experimental findings obtained from only a limited number of patient-derived cell lines might be inadequate. Therefore, more cell lines from a larger number of patients are required for basic and pre-clinical studies. Because DDLPS is extremely rare and the means of obtaining DDLPS cell lines are limited, patient-derived cancer cell lines should be shared within the research community.
We conclude that NCC-DDLPS1-C1 may serve as a useful patient-derived sarcoma cell line and contribute to reveal further molecular backgrounds of DDLPS.
References
The WHO Classification of Tumors Editorial Board. WHO classification of tumours of soft tissue and bone. 5th ed. Lyon: IARC; 2020.
Dei Tos AP. Liposarcomas: diagnostic pitfalls and new insights. Histopathology. 2014;64:38–52.
Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3:507–23.
Hirata M, Asano N, Katayama K, et al. Integrated exome and RNA sequencing of dedifferentiated liposarcoma. Nat Commun. 2019;10:5683.
Kanojia D, Nagata Y, Garg M, et al. Genomic landscape of liposarcoma. Oncotarget. 2015;6:42429–44.
Asano N, Yoshida A, Mitani S, et al. Frequent amplification of receptor tyrosine kinase genes in well differentiated/dedifferentiated liposarcoma. Oncotarget. 2017;8:12941–52.
Tap WD, Eilber FC, Ginther C, et al. Evaluation of well-differentiated/de-differentiated liposarcomas by high-resolution oligonucleotide array-based comparative genomic hybridization. Genes Chromosom Cancer. 2011;50:95–112.
Louis-Brennetot C, Coindre JM, Ferreira C, Perot G, Terrier P, Aurias A. The CDKN2A/CDKN2B/CDK4/CCND1 pathway is pivotal in well-differentiated and dedifferentiated liposarcoma oncogenesis: an analysis of 104 tumors. Genes Chromosom Cancer. 2011;50:896–907.
Italiano A, Bianchini L, Gjernes E, et al. Clinical and biological significance of CDK4 amplification in well-differentiated and dedifferentiated liposarcomas. Clin Cancer Res. 2009;15:5696–703.
Italiano A, Bianchini L, Keslair F, et al. HMGA2 is the partner of MDM2 in well-differentiated and dedifferentiated liposarcomas whereas CDK4 belongs to a distinct inconsistent amplicon. Int J Cancer. 2008;122:2233–41.
Snyder EL, Sandstrom DJ, Law K, et al. c-Jun amplification and overexpression are oncogenic in liposarcoma but not always sufficient to inhibit the adipocytic differentiation programme. J Pathol. 2009;218:292–300.
Mariani O, Brennetot C, Coindre JM, et al. JUN oncogene amplification and overexpression block adipocytic differentiation in highly aggressive sarcomas. Cancer Cell. 2007;11:361–74.
Coindre JM, Mariani O, Chibon F, et al. Most malignant fibrous histiocytomas developed in the retroperitoneum are dedifferentiated liposarcomas: a review of 25 cases initially diagnosed as malignant fibrous histiocytoma. Mod Pathol. 2003;16:256–62.
Coindre JM, Hostein I, Maire G, et al. Inflammatory malignant fibrous histiocytomas and dedifferentiated liposarcomas: histological review, genomic profile, and MDM2 and CDK4 status favour a single entity. J Pathol. 2004;203:822–30.
Chibon F, Mariani O, Derré J, et al. A subgroup of malignant fibrous histiocytomas is associated with genetic changes similar to those of well-differentiated liposarcomas. Cancer Genet Cytogenet. 2002;139:24–9.
Le Guellec S, Chibon F, Ouali M, et al. Are peripheral purely undifferentiated pleomorphic sarcomas with MDM2 amplification dedifferentiated liposarcomas? Am J Surg Pathol. 2014;38:293–304.
Gahvari Z, Parkes A. Dedifferentiated liposarcoma: systemic therapy options. Curr Treat Options Oncol. 2020;21:15.
Gootee J, Aurit S, Curtin C, Silberstein P. Primary anatomical site, adjuvant therapy, and other prognostic variables for dedifferentiated liposarcoma. J Cancer Res Clin Oncol. 2019;145:181–92.
Keung EZ, Hornick JL, Bertagnolli MM, Baldini EH, Raut CP. Predictors of outcomes in patients with primary retroperitoneal dedifferentiated liposarcoma undergoing surgery. J Am Coll Surg. 2014;218:206–17.
Crago AM, Singer S. Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr Opin Oncol. 2011;23:373–8.
Ben-David U, Siranosian B, Ha G, et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature. 2018;560:325–30.
Gisselsson D, Lichtenzstejn D, Kachko P, Karlsson J, Manor E, Mai S. Clonal evolution through genetic bottlenecks and telomere attrition: Potential threats to in vitro data reproducibility. Genes Chromosom Cancer. 2019;58:452–61.
Saito S, Morita K, Kohara A, et al. Use of BAC array CGH for evaluation of chromosomal stability of clinically used human mesenchymal stem cells and of cancer cell lines. Hum Cell. 2011;24:2–8.
Ben-David U, Beroukhim R, Golub TR. Genomic evolution of cancer models: perils and opportunities. Nat Rev Cancer. 2019;19:97–109.
Hattori E, Oyama R, Kondo T. Systematic review of the current status of human sarcoma cell lines. Cells. 2019;8:157.
Barretina J, Caponigro G, Stransky N, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7.
Crystal AS, Shaw AT, Sequist LV, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346:1480–6.
Teicher BA, Polley E, Kunkel M, et al. Sarcoma cell line screen of oncology drugs and investigational agents identifies patterns associated with gene and microRNA expression. Mol Cancer Ther. 2015;14:2452–62.
Bairoch A. The cellosaurus, a cell-line knowledge resource. J Biomol Tech. 2018;29:25–38.
Yoshimatsu Y, Noguchi R, Tsuchiya R, et al. Establishment and characterization of NCC-CDS2-C1: a novel patient-derived cell line of CIC-DUX4 sarcoma. Hum Cell. 2020;33:427–36.
Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017;36:561–84.
Deming DA, Ninan J, Bailey HH, et al. A Phase I study of intermittently dosed vorinostat in combination with bortezomib in patients with advanced solid tumors. Investig New Drugs. 2014;32:323–9.
Xian M, Cao H, Cao J, et al. Bortezomib sensitizes human osteosarcoma cells to adriamycin-induced apoptosis through ROS-dependent activation of p-eIF2alpha/ATF4/CHOP axis. Int J Cancer. 2017;141:1029–41.
Perez M, Peinado-Serrano J, Garcia-Heredia JM, et al. Efficacy of bortezomib in sarcomas with high levels of MAP17 (PDZK1IP1). Oncotarget. 2016;7:67033–46.
Garcia-Heredia JM, Lucena-Cacace A, Verdugo-Sivianes EM, Perez M, Carnero A. The cargo protein MAP17 (PDZK1IP1) regulates the cancer stem cell pool activating the Notch pathway by abducting NUMB. Clin Cancer Res. 2017;23:3871–83.
Acknowledgements
We thank Drs. F Nakatani, E Kobayashi, M Nakagawa, T Komatsubara, M Saito, C Sato (Department of Musculoskeletal Oncology), and Drs. T Shibayama, and H Tanaka (Department of Diagnostic Pathology), National Cancer Center Hospital, for sampling tumor tissue specimens from surgically resected materials. We thank Dr. T Kiyono (Division of Carcinogenesis and Prevention) for kindness in providing the HFF cell line. We also appreciate the technical assistance provided by Mr. K Tanoue, T Ono, and Ms. Y Kuwata (Division of Rare Cancer Research). We appreciate the technical support provided by Ms Y Shiotani, Mr. N Uchiya, and Dr. T Imai (Central Animal Division, National Cancer Center Research Institute). We would also like to thank Editage (https://www.editage.jp) for their help with English language editing and their constructive comments on the manuscript. This research was technically assisted by the Fundamental Innovative Oncology Core in the National Cancer Center.
Funding
This research was supported by the Japan Agency for Medical Research and Development (Grant number 20ck0106537h0001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Written informed consent for publication was provided by the patient.
Ethics approval
The ethical committee of the National Cancer Center approved the use of clinical materials for this study with the approval number 2004-050. The study using animal models was approved by the ethics committee of the National Cancer Center Research Institute (T13-016).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsuchiya, R., Yoshimatsu, Y., Noguchi, R. et al. Establishment and characterization of NCC-DDLPS1-C1: a novel patient-derived cell line of dedifferentiated liposarcoma. Human Cell 34, 260–270 (2021). https://doi.org/10.1007/s13577-020-00436-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00436-5