Abstract
Myxofibrosarcoma (MFS) is among the most aggressive and complex sarcoma types that require novel therapeutic approaches for improved clinical outcomes. MFS displays highly complex karyotypes, and frequent alterations in p53 signaling and cell cycle checkpoint genes as well as loss-of-function mutations in NF1 and PTEN have been reported. The effects of radiotherapy and chemotherapy on MFS are limited, and complete surgical resection is the only curative treatment. Thus, the development of novel therapeutic strategies for MFS has long been long desired for MFS. Patient-derived cell lines are an essential tool for basic and translational research in oncology. However, public cell banks provide only a limited number of MFS cell lines. In this study, we aimed to develop a novel patient-derived MFS cell line, which was established from the primary tumor tissue of a 71-year-old male patient with MFS and was named NCC-MFS2-C1. A single-nucleotide polymorphism assay revealed that NCC-MFS2-C1 cells exhibited gain and loss of genetic loci. NCC-MFS2-C1 cells were maintained as a monolayer culture for over 24 passages for 10 months. The cells exhibited spindle-like morphology, continuous growth, and capacity for spheroid formation and invasion. Screening of 213 anticancer agents revealed that bortezomib, gemcitabine, romidepsin, and topotecan at low concentrations inhibited the proliferation of NCC-MFS2-C1 cells. In conclusion, we established a novel MFS cell line, NCC-MFS2-C1, which can be used for studying the molecular mechanisms underlying tumor development and for the in vitro screening of anti-cancer drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myxofibrosarcoma (MFS) is one of the most aggressive soft tissue sarcomas, accounting for approximately 5% of soft tissue sarcoma diagnoses. MFS commonly occurs in the elderly population, particularly in the limbs [1]. It displays highly complex karyotypes; frequent alterations in p53 signaling (51%) and cell cycle checkpoint genes (43%) [2] as well as loss-of-function mutations in NF1 (5/35) and PTEN (1/35) [3] have been reported. However, their functional significance in MFS progression and their clinical value are not yet understood. The effects of radiotherapy and chemotherapy on MFS are limited, and complete surgical resection is the only curative treatment [4, 5]. However, local post-operative recurrence is observed in 50–60% of MFS cases, even when wide-ranging local excision is performed with negative surgical margins [1, 6, 7]. Recurrent tumors have metastatic potential, and approximately 30% of recurrent MFS progresses to higher grade tumors with poor prognosis [1, 7,8,9,10]. Therefore, the development of novel therapeutic strategies for MFS has been long desired for improved clinical outcomes.
Patient-derived cancer cell lines are indispensable tools for pre-clinical studies, and their use in research has promoted fundamental discoveries in oncology. Recently, large cell line panels have been used to identify therapeutic targets in combination with predictive biomarkers in multiple cancer types [11,12,13,14,15,16,17,18,19]. Such studies pave the way to comprehensive understanding of cancer biology and novel therapeutics. However, when it comes to MFS, besides the clinical need for novel therapies, a limited number of MFS cell lines has been reported previously [20,21,22,23,24,25,26], and only two cell lines, both named NMFH-1, are available from public cell banks (JCRB; JCRB1507, RCB; RCB2346 [20]). A paucity of adequate pre-clinical models may result in a lack of effective systematic treatments. Although MFS was first formally described in 1977 [8], effective chemotherapy has not been established yet. Thus, the establishment of additional cell lines for MFS is urgently needed.
In this study, we established a novel patient-derived cell line from the tumor tissue of a patient with MFS, designated as NCC-MFS2-C1, and investigated its karyotype and culture characteristics.
Materials and methods
Patient background
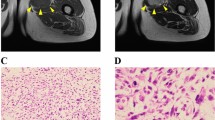
A 71-year-old male patient was referred to the Tochigi Cancer Center Hospital, Tochigi, Japan due to a growing tumor in his right lower thigh. Magnetic resonance imaging (MRI) revealed a tumor mass in the right lower thigh, with a tail-like multidirectional extensive spread along the fascial plane, which is typical of MFS (Fig. 1a; Gd-enhanced MRI coronal view). MRI showed low intensity at T1 (Fig. 1b) and high intensity at T2 (Fig. 1c); Gd-enhanced MRI showed a contrast effect along the fascia. In part, tumor infiltration in the skeletal muscle across the fascia was observed in the deep direction. (Fig. 1d). Distant metastasis was not detected by computed tomography. An open biopsy sample was pathologically diagnosed as MFS (Fig. 1e). Wide excision and split-thickness skin grafting was performed, and the excised tissue was used for pathological diagnosis and establishment of the cell line in this study. The pathological diagnosis of the excised tumor was MFS; myxoid stroma and curvilinear blood vessels with condensation of cells around the vessels were detected (Fig. 1e). This study was approved by the ethics committee of the Tochigi Cancer Center and the National Cancer Center, and written informed consent was obtained from the patient. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Clinical imaging data. MRI showed a tumor mass in the right lower thigh, with a tail-like multidirectional extensive spread along the fascial plane, which is typical for MFS (a; Gd-enhanced MRI coronal view). MRI also showed low intensity at T1 (b), high intensity at T2 (c), and a contrast effect along the fascia in Gd-enhanced MRI. In part, tumor infiltration of the skeletal muscle across the fascia was observed in the deep direction. d HE showed elliptical to spindle-shaped tumor cells growing patternless or in a storiform pattern. Some cells were conspicuous with large nucleoli. Tumor stroma showed capillary hyperplasia and formation of mucoid stroma (e)
Cell line establishment
Tumor tissue obtained at the time of surgery was sent from Tochigi Cancer Center (Tochigi, Japan) to the National Cancer Center (Tokyo, Japan) for cell line establishment. As per our previous report [27], the tumor tissue was mechanically dissected into small pieces and treated with collagenase type II at a concentration of 1 mg/mL (Worthington Biochemical Corporation, Lakewood, NJ, USA). The cells were plated on a collagen-coated culture dish (Fujifilm Co. Ltd., Tokyo, Japan) and maintained in Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with GlutaMAX (Thermo), 5% heat-inactivated fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA), 10 µM Y-27632 (Selleck Chemicals: ROCK inhibitor), 10 ng/mL basic fibroblast growth factor (Sigma, St Louis, MO, USA), 5 ng/mL epidermal growth factor (Sigma), 5 µg/mL insulin (Sigma), 0.4 µg/mL hydrocortisone (Sigma), 100 μg/mL penicillin, and 100 µg/mL streptomycin (Nacalai Tesque, Kyoto, Japan). When the culture was sub-confluent, the cells were washed with PBS (Nacalai), detached using Accutase (Nacalai), and transferred to another tissue culture plate. The cell cultures were incubated at 37 ℃ in a humidified atmosphere with 5% CO2.
Short tandem repeat analysis for cell line validation
The short tandem repeats (STRs) of the original tumor tissue and the established cell line were examined for cell line authentication [28] as described in our previous report [27]. Briefly, genomic DNA was extracted using the AllPrep DNA/RNA mini kit (Qiagen, Venlo, Netherland), and DNA concentration was measured using NanoDrop 8000 (Thermo Fisher Scientific). STR multiplex assays were performed using GenePrint 10 (Promega, Madison, WI, USA) and a 3500xL Genetic Analyzer (Applied Biosystems, Waltham, MA, USA). The STR data were analyzed using GeneMapper 5 (Applied Biosystems). The obtained STR profiles were examined against the reference STRs of cell lines available in public cell banks using the standard match threshold of 80% [29].
Evaluation of mycoplasma contamination
To assess mycoplasma contamination, mycoplasma-specific DNA fragments in the culture medium of the established cell line were examined by PCR [30] as described in our previous report [27]. Briefly, DNA in the culture medium was amplified using the e-Myco Mycoplasma PCR Detection Kit (Intron Biotechnology, Gyeonggi-do, Korea), and PCR products were analyzed using agarose gel electrophoresis.
Characterization of cell growth
Cell proliferation was assessed as described previously [27]. In brief, cells (5 × 104) were plated in 24-well culture plates, and the number of cells in each well was counted at multiple time points. The doubling time was calculated based on the growth curve. All experiments were performed in triplicate. The cell viability was monitored using the CCK-8 reagent (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) for constructing growth curves.
Spheroid formation assay
The spheroid formation ability of the cells was evaluated as described previously [27]. In brief, 1 × 104 cells were seeded into round bottom, 96-well, low attachment plates (Ultra Low Culture Dish, Thermo Fisher Scientific) and grown for 3 days. The presence of spheroids was confirmed under a microscope (Keyence, Osaka, Japan).
Invasion assay
Invasion ability was assessed using BD Biocoat Matrigel Invasion Chambers (BD Biosciences, Bedford, MA) according to our previous report [27]. Cells at a concentration of 1 × 105 or 2 × 105 were plated in serum-free medium in the upper chamber, and the lower chamber was filled with DMEM/F12 containing 10% FBS. After 24 or 48 h, the cells on the bottom surface, which were stained using Diff-Quik stain (Sysmex, Kobe, Japan), were counted under a microscope.
Single-nucleotide polymorphism array
SNP array genotyping was performed to identify copy number alterations using an Infinium OmniExpressExome-8 v. 1.4 BeadChip (Illumina, San Diego, CA) following the manufacturer’s instructions. Genomic DNA was extracted from the cells and from tumor tissues and was amplified. Amplified DNA was reacted with the array slides in an iScan system (Illumina). Log R ratios and B allele frequencies were calculated using Genome Studio 2011.1 with cnvPartition v3.2.0 (Illumina) and KaryoStudio Data Analysis Software v. 1.0 (Illumina). The human genome data, hg19 version, was used for annotation mapping. The whole-genome (except chromosome X and Y) log10 ratio (tumor/reference) value was smoothed, and abnormal copy number regions were detected using a circular binary segmentation algorithm [31, 32] with the R package ‘DNAcopy’ from Bioconductor [33]. Amplifications were defined as regions for which the copy number was > 3. Deletions were defined as regions for which less than one copy was present in the tumor cells. Among the identified genes with copy number alterations, we searched for “cancer related genes” using “Cancer Gene Census” in the Catalogue Of Somatic Mutations In Cancer (COSMIC) database (GRCh 37 v91) [34].
Screening of anti-cancer drugs for cell growth inhibition
Cells were seeded at a density of 5 × 103 cells/well in DMEM containing 10% FBS in a 96-well culture plate and were grown overnight at 37 ℃ with 5% CO2. Overall, 213 agents (Selleck Chemicals, Houston, TX, USA) (Supplementary Table 1) were added into the plate using the Bravo automated liquid handling platform (Agilent Technologies, Santa Clara, CA, USA). After 72 h of treatment, viable cells were quantified using the CCK-8 assay. The experiment was performed in duplicate.
Results
Establishment and authentication of NCC-MFS2-C1 cell line
We established a novel cell line from the primary tumor tissue of a patient with MFS and designated it as NCC-MFS2-C1. NCC-MFS2-C1 cells were maintained as monolayer cultures for more than 24 passages in 10 months. By comparing STR profiles, the identity of the NCC-MFS2-C1 cell line was confirmed (Table 1). The STR pattern of NCC-MFS2-C1 cells was identical to that of the original tumor and was distinct from that of any other cell lines deposited in major public cell banks according to the Cellosaurus search results [35]. We concluded that NCC-MFS2-C1 was a novel MFS cell line. The NCC-MFS2-C1 cells were negative for mycoplasma contamination (data not shown).
Characteristics of NCC-MFS2-C1 cells
The results of SNP analysis showed that the NCC-MFS2-C1 cells had gains and losses in almost entire parts of chromosomes. The copy number variants mostly involved partial amplification of chromosomal arms (4pq) and partial deletion of chromosomal arms (6pq, 13pq, and 14q) (Fig. 2). Loss or amplification of cancer related genes was not identified. NCC-MFS2-C1 cells exhibited spindle-shape morphology (Fig. 3a, b). They showed constant growth with a doubling time of 13 h (Fig. 3c), and an ability to form spheroids on low-attachment substrates (Fig. 3d). The invasive capacity of NCC-MFS2-C1 cells was monitored using a Transwell invasion assay. As shown in Fig. 3e, the number of invaded cells was parallel with the number of seeded cells and the incubation time. NCC-MFS2-C1 cells showed higher invasive capability than MG63 osteosarcoma cells, which are often used for sarcoma research.
Copy number profiling of NCC-MFS2-C1 cells by a single-nucleotide polymorphism (SNP) array. SNP genotyping copy number profiles for NCC-MFS2-C1 cells. The x-axis indicates the chromosome number. Chromosomes 1–22 are demonstrated from left to right with green and black colors. Chromosomal segments are shown in red dots. The y-axis indicates the copy number value
Phenotypic characterization of NCC-MFS2-C1 cells. a, b Representative phase contrast micrograph. c Growth curve of NCC-MFS2-C1 cells. Each point represents the mean ± standard deviation (n = 3). d Spheroid formation examined on low-attachment substrates. e Invasion ability was observed in NCC-MFS2-C1 cells
Sensitivity of the cell line to anti-cancer drugs
The sensitivity of NCC-MFS2-C1 cells to clinically available anti-cancer drugs was assessed. For the first screening, the cells were treated with 213 anti-cancer drugs at a universal concentration of 10 µM (Supplementary Table 2). Among them, we examined the half maximal inhibitory concentration (IC50) values for agents that showed high inhibitory effects or those used for treatments with sarcomas (Supplementary Fig. 3). We then found that four agents, namely bortezomib, gemcitabine, romidepsin, and topotecan, had IC50 values less than 100 nM (Fig. 4).
Discussion
Patient-derived cell lines are an essential tool for cancer research, as they provide valuable information on the mechanisms of carcinogenesis and development of novel therapies. The utility of cell line collections for clinical applications has been demonstrated in previous publications [11,12,13,14,15,16,17,18,19]. Considering the heterogeneity of MFS, a panel of MFS cell lines with various characteristics from multiple patients should be used in the study of MFS. Although MFS cell lines, namely CNIO BG [36], IM-MFS-1 [37], MUG-Myx1 [22], NMFH-1 (JCRB), NMFH-1 (RCB) [20], and NMFH2 [25], have been reported, they were not annotated with detailed clinical information. We had previously reported a novel MFS cell line, NCC-MFS1-C1 [26]; here, we report our second MFS cell line, NCC-MFS2-C1. This cell line was derived from the primary tumor of a patient with MFS and was annotated with the detailed clinical and pathological data of the donor patient.
We characterized NCC-MFS2-C1 cells and found that the cells demonstrated constant growth, spheroid formation, and invasion. These characteristics make NCC-MFS2-C1 a suitable cell line for in vitro pre-clinical study. Further, we examined the anti-proliferative effects of 213 agents on NCC-MFS2-C1 cells. We also examined these anti-proliferative effects in our previous study on the NCC-MFS1-C1 cell line [26]. We found that bortezomib and romidepsin had low IC50 values in both NCC-MFS1-C1 and NCC-MFS2-C1. Bortezomib is a proteasome inhibitor, and romidepsin is a histone deacetylase inhibitor. In a previous phase II study on advanced sarcoma, bortezomib was found to show minimal activity [38]. However, that trial did not include patients with MFS. Romidepsin has been examined in metastatic or unresectable soft tissue sarcomas in phase II trials; the treatments were not completed in most of the cases, largely because of disease progression, and MFS was not included in this trial either (ClinicalTrials.gov Identifier: NCT00112463). Thus, clinical trials focusing on MFS are required to clarify the antitumor effects of bortezomib and romidepsin. The high throughput screening for anti-cancer drugs is the experiment that only cell line can make it, and we can have some ideas for further studies. However, the responses to treatments with anti-cancer drugs in the tissue culture cells are not always reproduced in the patients, and the results should be validated by the other methods. Patient-derived xenograft may be a suitable model for the further validation study of drug responses.
The cell lines have a versatile utility for cancer research, and the cell lines are especially useful for the high throughput anti-cancer drug screening. However, there are several experiments that we did not examine using NCC-MFS2-C2. In that sense, this study has some important limitations that should be addressed by future studies. First, although MFS tumor tissues include several different tumor cell populations, we did not select and clone a single cell population, and NCC-MFS2-C1 may include multiple tumor cell lineages. Heterogenous cell populations may retain the complexity of the original tumor and can be useful for the study of tumor heterogeneity. However, cells change their characteristics when they are repeatedly propagated and maintained for a long period of time. As NCC-MFS2-C1 showed the short population doubling time, aberrant genomic features, and considerable capability of invasion, the majority of cells should be tumor cells. However, non-tumor mesenchymal cells may be included in NCC-MFS2-C1, and it is not impossible that non-tumor mesenchymal cells dominate the tumor cells after extensive passaging or under certain tissue culture conditions. Thus, we need to be careful when we use NCC-MFS2-C1. Second, during the process of cell line establishment, specific tumor cell populations may be selectively propagated, and such tumor cells do not always represent the overall tumor characteristics. For example, the population-doubling time of NCC-MFS2-C1 cells was 13 h, whereas MFS is an aggressive but a slowly growing tumor and most commonly arises as a slowly enlarging mass. Thus, NCC-MFS2-C1 may reflect aggressive tumor cell populations in tumor tissues. When we use NCC-MFS2-C1, we need to consider such characteristics of the cells; otherwise, it will be difficult to understand the results of experiments. Third, a single cell line from a single case cannot provide any conclusive results, and experiments using a single cell line should be validated by additional cell lines. The response or resistance to anti-cancer agents used in this study should also be validated in other MFS cell lines; thus, we need to establish more cell lines in the future. Fourth, NCC-MFS2-C1 are monolayer-cultured cells, and they do not hold three-dimensional architectures. Xenografts using NCC-MFS2-C1 will be necessary to examine the interaction of tumor cells with microenvironments, which include stromal cells and the extracellular matrix. The invasion potential of NCC-MFS2-C1 was demonstrated by the Matrigel invasion assay. However, the other methods to evaluate the metastatic potentials may provide the different results. Those experiments include the xenotransplantation to tail vein of mise, as well as the collagen invasion assay.
In conclusion, NCC-MFS2-C1 cells exhibited continuous proliferation and capacity for spheroid formation and invasion. A genomic study demonstrated the presence of genomic alterations in NCC-MFS2-C1 cells. We expect that the NCC-MFS2-C1 cell line will be useful in shedding light on the mechanism underlying disease progression and in screening novel therapeutic agents. Considering the diversity of the disease, more functionally characterized and clinically annotated cell lines are needed. Continuous efforts for cell line establishment are required for rare cancers, for which few cell lines are available.
References
Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F. Soft tissue and bone tumours. 4th ed. Geneva: WHO Press; 2020.
Ogura K, Hosoda F, Arai Y, et al. Integrated genetic and epigenetic analysis of myxofibrosarcoma. Nat Commun. 2018;9:2765.
Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–21.
Haglund KE, Raut CP, Nascimento AF, Wang Q, George S, Baldini EH. Recurrence patterns and survival for patients with intermediate- and high-grade myxofibrosarcoma. Int J Radiat Oncol Biol Phys. 2012;82:361–7.
Sanfilippo R, Miceli R, Grosso F, et al. Myxofibrosarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 2011;18:720–5.
Mentzel T, Calonje E, Wadden C, et al. Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 1996;20:391–405.
Merck C, Angervall L, Kindblom LG, Oden A. Myxofibrosarcoma. A malignant soft tissue tumor of fibroblastic-histiocytic origin. A clinicopathologic and prognostic study of 110 cases using multivariate analysis. Acta pathologica, microbiologica, et immunologica Scandinavica Supplement. 1983;282:1–40.
Weiss SW, Enzinger FM. Myxoid variant of malignant fibrous histiocytoma. Cancer. 1977;39:1672–85.
Willems SM, Debiec-Rychter M, Szuhai K, Hogendoorn PC, Sciot R. Local recurrence of myxofibrosarcoma is associated with increase in tumour grade and cytogenetic aberrations, suggesting a multistep tumour progression model. Mod Pathol. 2006;19:407–16.
Lin CN, Chou SC, Li CF, et al. Prognostic factors of myxofibrosarcomas: implications of margin status, tumor necrosis, and mitotic rate on survival. J Surg Oncol. 2006;93:294–303.
Barretina J, Caponigro G, Stransky N, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7.
Garnett MJ, Edelman EJ, Heidorn SJ, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–5.
Basu A, Bodycombe NE, Cheah JH, et al. An interactive resource to identify cancer genetic and lineage dependencies targeted by small molecules. Cell. 2013;154:1151–61.
Seashore-Ludlow B, Rees MG, Cheah JH, et al. Harnessing connectivity in a large-scale small-molecule sensitivity dataset. Cancer Discov. 2015;5:1210–23.
Rees MG, Seashore-Ludlow B, Cheah JH, et al. Correlating chemical sensitivity and basal gene expression reveals mechanism of action. Nat Chem Biol. 2016;12:109–16.
Haverty PM, Lin E, Tan J, et al. Reproducible pharmacogenomic profiling of cancer cell line panels. Nature. 2016;533:333–7.
Iorio F, Knijnenburg TA, Vis DJ, et al. A Landscape of Pharmacogenomic Interactions in Cancer. Cell. 2016;166:740–54.
Behan FM, Iorio F, Picco G, et al. Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature. 2019;568:511–6.
Townsend EC, Murakami MA, Christodoulou A, et al. The public repository of xenografts enables discovery and randomized phase II-like trials in mice. Cancer Cell. 2016;29:574–86.
Kawashima H, Ogose A, Gu W, et al. Establishment and characterization of a novel myxofibrosarcoma cell line. Cancer Genet Cytogenet. 2005;161:28–35.
Huang HY, Wu WR, Wang YH, et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res. 2013;19:2861–72.
Lohberger B, Stuendl N, Wolf E, Liegl-Atzwanger B, Leithner A, Rinner B. The novel myxofibrosarcoma cell line MUG-Myx1 expresses a tumourigenic stem-like cell population with high aldehyde dehydrogenase 1 activity. BMC Cancer. 2013;13:563.
Salawu A, Fernando M, Hughes D, et al. Establishment and molecular characterisation of seven novel soft-tissue sarcoma cell lines. Br J Cancer. 2016;115:1058–68.
Lohberger B, Stuendl N, Leithner A, et al. Establishment of a novel cellular model for myxofibrosarcoma heterogeneity. Sci Rep. 2017;7:44700.
Ariizumi T, Ogose A, Kawashima H, Hotta T, Umezu H, Endo N. Multinucleation followed by an acytokinetic cell division in myxofibrosarcoma with giant cell proliferation. J Exp Clin Cancer Res. 2009;28:44.
Kito F, Oyama R, Sakumoto M, et al. Establishment and characterization of a novel cell line, NCC-MFS1-C1, derived from a patient with myxofibrosarcoma. Hum Cell. 2019;32:214–22.
Yoshimatsu Y, Noguchi R, Tsuchiya R, et al. Establishment and characterization of NCC-CDS2-C1: a novel patient-derived cell line of CIC-DUX4 sarcoma. Hum Cell. 2020;33:427–36.
Workgroup ATCCSDO. Cell line misidentification: the beginning of the end. Nat Rev Cancer. 2010;10:441–8.
Capes-Davis A, Reid YA, Kline MC, et al. Match criteria for human cell line authentication: where do we draw the line? Int J Cancer. 2013;132:2510–9.
Capes-Davis A, Dirks W, MacLeod R, Uphoff C. Quality Matters: cell lines and their use in research. GIT Lab J Eur. 2014;17:12–3.
Olshen AB, Venkatraman ES, Lucito R, Wigler M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics. 2004;5:557–72.
Venkatraman ES, Olshen AB. A faster circular binary segmentation algorithm for the analysis of array CGH data. Bioinformatics. 2007;23:657–63.
Willenbrock H, Fridlyand J. A comparison study: applying segmentation to array CGH data for downstream analyses. Bioinformatics. 2005;21:4084–91.
Forbes SA, Tang G, Bindal N, et al. COSMIC (the Catalogue of Somatic Mutations in Cancer): a resource to investigate acquired mutations in human cancer. Nucleic Acids Res. 2010;38:D652–D657657.
Hattori E, Oyama R, Kondo T. Systematic review of the current status of human sarcoma cell lines. Cells. 2019;8:157.
Moneo V, Serelde BG, Fominaya J, et al. Extreme sensitivity to Yondelis (Trabectedin, ET-743) in low passaged sarcoma cell lines correlates with mutated p53. J Cell Biochem. 2007;100:339–48.
Miserocchi G, De Vita A, Mercatali L, et al. Characterization and drug sensitivity of a new high-grade myxofibrosarcoma cell line. Cells. 2018;7:186.
Maki RG, Kraft AS, Scheu K, et al. A multicenter Phase II study of bortezomib in recurrent or metastatic sarcomas. Cancer. 2005;103:1431–8.
Acknowledgements
We appreciate the technical support by Miss Yu Kuwata (Division of Rare Cancer Research, National Cancer Center). We would also like to thank the Tochigi Cancer Center Operating Room Nurse Team and the Secretary of the Medical Office for their assistance in processing and transporting the samples. The Fundamental Innovative Oncology Core at the National Cancer Center provided support for the SNParray experiment. We would like to thank Editage (www.editage.jp) for English editing and constructive comments on the manuscript.
Funding
This research was supported by the Japan Agency for Medical Research and Development grant 20ck0106537h0001, “Study to Overcome the Limits of Cancer Genome-based Medicine Using Patient-derived “Rare Cancer” Model”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the ethics committee of the Tochigi Cancer Center and the National Cancer Center, and written informed consent was obtained from the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13577_2020_420_MOESM3_ESM.tif
Supplementary Figure 3 Growth curves with agents that showed relatively higher inhibitory effects on NCC-MFS2-C1 cell proliferation (TIF 689 kb)
13577_2020_420_MOESM6_ESM.xlsx
Supplementary Table 3 IC50 values of four agents that showed high inhibitory effects on NCC-MFS2-C1 cell proliferation (XLSX 11 kb)
Rights and permissions
About this article
Cite this article
Noguchi, R., Yoshimatsu, Y., Ono, T. et al. Establishment and characterization of NCC-MFS2-C1: a novel patient-derived cancer cell line of myxofibrosarcoma. Human Cell 34, 246–253 (2021). https://doi.org/10.1007/s13577-020-00420-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00420-z