Abstract
Interferon-stimulated gene 15 (ISG15) is a critical ubiquitin-like protein that can be conjugated to proteins via the ISGylation system to modify them posttranslationally. Furthermore, ISG15 can be detected as non-conjugated or free, intracellularly and/or extracellularly. Both conjugated and free ISG15 participate in different cancer types, including breast cancer. Here, we highlighted the findings on ISG15 and protein ISGylation, and their implications in the field of breast cancer research. ISG15 emerges as a central element in mammary tumors and may become a crucial protein in the strategies for detection, prognosis, and therapy of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a severe health problem. Worldwide, according to the International Agency for Research on Cancer (IARC), breast cancer is the first cause of women’s death by cancer and has the highest incidence among female cancer types. Breast cancer is defined as a collection of mammary tissue-associated neoplastic alterations [1,2,3]. More than 85% of breast cancers in women are not related to gene mutations, having multifactorial influences. At the histological level, almost 80% of breast cancers develop in the mammary ducts and are invasive (invasive ductal carcinoma). At the molecular level, mammary tumors are highly heterogeneous, leading to a deficiency of biomarkers for early detection, or prognostic biomarkers, as well as pharmacological targets of breast cancer [4, 5].
Interestingly, estrogen receptor alpha (ERα), progesterone receptor (PR), androgen receptor (AR), and human epidermal growth factor receptor 2 (HER2 or ERBB2) have been used as classical biomarkers for breast cancer. Among these, ERα is expressed in more than 70% of breast cancer cases, and the remaining 30% lack expression of this receptor; consequently, these cancer types are known as ERα+ and ERα− , respectively. ERα is a nuclear receptor, activated mainly by 17β–estradiol (E2), which acts as a pro-tumor transcriptional regulator in breast cancer cells. Thus, ERα is the therapeutic target for most cases of breast cancer. These treatments include the use of selective estrogen receptor degraders (SERDs), such as fulvestrant, and selective estrogen receptor modulators (SERMs), such as tamoxifen. In addition, aromatase inhibitors, such as letrozole, anastrozole, and exemestane, are used to inhibit the production of E2 hormone from androgens by blocking aromatase enzyme.
Breast cancer has been classified into four different subtypes based on the detection of ERα, PR, HER2, and Ki67 using immunohistochemical analysis: (1) luminal A-like (ERα+ , PR ≥ 20%, HER2− , Ki67 < 20%); (2) luminal B-like (ERα+ , PR < 20% and/or HER2+ and/or Ki67 ≥ 20%); (3) HER2-overexpressing (ERα− , PR− , HER2+); and (4) basal-like [ERα− , PR − , HER2− (triple-negative)]. The most common molecular subtype of invasive breast cancer is luminal A (~ 50% of breast cancers), which responds to endocrine therapy. The luminal B subtype (~ 20% of breast cancers) generally requires additional chemotherapy and has a worse prognosis than luminal A. The HER2-overexpressing subtype (~ 15% of all invasive breast cancers) is treated with anti-HER2-targeted therapy [6, 7]. Triple-negative breast cancer is aggressive and requires chemotherapy [8, 9].
Many research groups are trying to identify new biomarkers and pharmacological targets for the early identification and control of breast cancer. Here, we discuss ISG15/ISGylation as a potential factor in the context of breast cancer.
The basis of the ISG15 and ISGylation system
ISG15 protein (interferon-stimulated gene 15) is a protein of 156 aa and 15 kDa formed by two ubiquitin (UB)-like domains that are connected by a hinge sequence [10,11,12]. The ISG15 C-terminal domain has the sequence LRLRGG, which is also contained in UB, allowing its covalent interaction with target proteins’ lysine residues through the E1-activating enzyme, the E2-conjugating enzyme, and the E3-ligase enzyme. These three enzymes are known as the ISGylation system, in which the E1-activating enzyme catalyzes the formation of a thioester bond with C-terminal glycine from ISG15 in an ATP-dependent manner. Next, via trans-esterification, ISG15 is transferred to the E2-conjugating enzyme. Finally, ISG15 is transferred from the E2-conjugating enzyme by E3 ligase enzyme to specific proteins in lysine residues [13,14,15]. Some enzymes have been identified in the ISGylation system; for example, in humans, UBE1L E1-activating enzyme, UBCH8 E2-conjugating enzyme, and HERC5, HHARI, and EFP as E3-ligases enzymes [16,17,18,19,20,21,22,23,24]. Interestingly, HERC5 is the E3 ligase for ISG15 in humans, whereas mice express the E3 ligase HERC6 [25, 26]. Contrarily, USP18 (UBP43) is an enzyme able to remove ISG15 from ISGylated proteins, generating free ISG15 by a process known as de-ISGylation [27] (Fig. 1).
ISGylation represents a posttranslational modification that has not been thoroughly studied in comparison with other modifications, and consequently, few ISGylated target proteins have been reported. However, some of the protein targets of ISGylation are central to several cellular processes such as Filamin B, Parkin, BECN1, PCNA, β-catenin, and TP53. Interestingly, ISGylation has been detected in one, two, or multiple sites in lysine residues as monoISGylation. However, the functions of ISGylation are not entirely determined, despite being related to an increase or decrease in protein stability and the regulation of protein–protein interactions [20, 28,29,30,31,32].
It has been demonstrated that an integrin-like receptor, in natural killer (NK) cells, can recognize extracellular free ISG15 to promote the secretion of IFN-γ, suggesting that ISG15 may act as a cytokine and trigger a signaling pathway in some cellular contexts [33]. Indeed, there is still a need to further investigate the roles of ISG15 and ISGylation, but its potential involvement in several pathophysiological processes, including malignance in mammary tissues, can already be glimpsed.
Free ISG15 and protein ISGylation in breast cancer cells
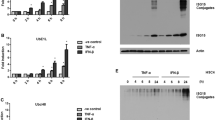
Free ISG15 levels and protein ISGylation are cell type-specific, and both seem to play a critical role in breast cancer cells. For instance, a study indicated that MCF-7 ERα+ breast cancer cells had the highest levels of free ISG15 protein in comparison with other cell types, and a specific profile of ISGylation marks [34]. More recently, free ISG15 and ISGylation were also detected in triple-negative breast cancer cells (ERα-, PR-, HER2-), showing numerous ISGylation marks and low levels of free ISG15 protein, a pattern contrary to that observed in MCF-7 ERα + breast cancer cells [34, 35]. Together, these data suggest that ISGylated proteins may be distinct between ERα+ and ERα− (like triple-negative) breast cancers. In addition, the differences between free ISG15 and ISGylation profiles may be associated with the ISGylation system expression (HERC5 and EFP E3 ligases expression) in these breast cancer types (Fig. 2).
Subcellular distribution of ISG15 and protein ISGylation in breast cancer cells
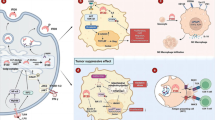
It has been suggested that ISG15 protein is distributed mainly in the cytoplasm, followed by the nucleus, mitochondria, plasma membrane, and cytoskeleton [34]. Experimentally, through immunofluorescence assays, it has been shown that ISG15 is localized in the cytoplasm and nucleus of breast cancer cells. Through cellular fractionation assays, a differential compartmentalization of free and conjugated ISG15 bands between the cytoplasmic and nuclear compartments has been shown. Free ISG15 protein is mainly distributed in the cytoplasm of MCF-7 and MDA-MB-231 breast cancer cells. However, ISGylated protein marks were mainly detected in the nucleus of MCF-7 cells, whereas ISGylation marks were detected mainly in the cytoplasm of MDA-MB-231 cells, suggesting that ISGylated proteins can be cytoplasmic and nuclear, having a specific ISGylation profile for ERα+ and ERα− breast cancer cells [35]. Thus, ISG15 may function in multiple ways as free or conjugated ISG15 in several intracellular compartments in addition to the extracellular actions of free ISG15 in breast cancer cells (Figs. 3, 4).
Association of ISG15 with the IFN-γ pathway in breast cancer
ISG15 is induced by interferons (IFNs) α and β, and recently, IFN-γ has appeared as one of the most important inductors of ISG15 in breast cancer contexts. The IFN-γ canonical pathway contains a specific heterotetrameric receptor complex (composed of IFNGR1 and IFNGR2 subunits) and the JAK-STAT1 system. IFN-γ binds to its receptor complex, and through the triggering of JAKs1/2 promotes the activation of STAT1 via its phosphorylation, followed by its homodimerization and its binding to the gamma interferon-activated site (GAS) on the regulatory sequences of IFN-γ target genes to regulate its expression [36].
Specifically, in breast cancer, IFN-γ seems to mediate apoptosis and cell cycle arrest. Moreover, IFN–γ autocrine signaling may be generated in breast cancer cells [37, 38]. An initial study showed that IFN–γ increases the mRNA levels of ISG15 in MCF-7 cells [39]. More recently, it was demonstrated that IFN-γ also increases the mRNA levels of ISG15 in MDA-MB-231 breast cancer cells [40]. Likewise, IFN-γ enhances free ISG15 protein levels and protein ISGylation marks, and this upregulation occurs in parallel with changes in the morphology of breast cancer cells [40, 41]. Furthermore, it has not been observed to affect the subcellular transport dynamic of free and conjugated ISG15 induced by IFN-γ. However, IFN-γ differentially increased ISGylation patterns between the nucleus and the cytoplasm of MCF-7 and MDA-MB-231 cells, whereas IFN-γ enhances the levels of intracellular free ISG15 in the cytoplasm of both cell types [34, 35].
The IFN–γ pathway increases sensitivity to tamoxifen and restores sensitivity to fulvestrant in breast cancer cell lines. For this reason, IFN-γ-induced genes, including ISG15, may also affect the response to endocrine therapy with fulvestrant or tamoxifen in these cells [38, 42, 43]. In addition, patient-derived ERα− mammary tumor xenografts (PDXs) in athymic mice can secrete IFN-γ. In this study, residual tumor cells of chemo-responder PDXs displayed strong overexpression of IFN-target genes after adjuvant chemotherapy. One of the IFN-inducible genes associated with chemotherapy response in ERα- breast cancer was ISG15. In this process, the upregulation of ISG15 correlated with an increase in STAT1 phosphorylation levels, as well as DNA damage and apoptosis [44].
It has been suggested that IFNs-responsive cells, expressing genes like ISG15, are sensitive to DNA damage and consequently are responsive to chemotherapy [44]. However, another study demonstrated that experimental resistance to DNA damage is associated with the expression of IFN-related DNA damage resistance signature (IRDS), which includes ISG15. The authors propose that chronic stimulation of the interferons pathway could lead to constitutive IRDS expression, promoting DNA damage resistance, cytotoxic signal failure, and pro-survival signals [45]. Thus, the IFN-γ pathway and its target genes seem to be involved in the sensitivity to DNA damage and in the resistance to DNA damage in the chronic activation condition of IFN-γ signaling.
On the other hand, NMIIA and IQGAP1 are two cytoplasmic proteins that have been identified as ISGylation targets in breast cancer cells, and IFN-γ increases their ISGylation [41, 46] NMIIA is a 230 kDa protein that associates with the filaments of actin, generating the actomyosin complex for cytoskeletal remodeling and cellular spreading, which is implicated in cell motility [47]. Both NMIIA and ISG15 proteins co-localize in the marginal spreading lamellar region and in the cytoplasm of MDA-MB-231 cells during the spreading of these cells induced by fibronectin (FN) substrate, suggesting that ISGylation of NMIIA is important in this process [41]. In addition, IQGAP1 is also associated with cytoskeletal remodeling [40, 48]. Thus, IFN-γ-induced cytoplasmic protein ISGylation in breast cancer may be relevant to the reorganization of the cytoskeleton of these cells [40, 41]. To date, few proteins have been identified as ISGylation targets in breast cancer induced by IFN-γ. Many other cytoplasmic and nuclear proteins modified by ISGylation remain to be elucidated. Together, these data suggest that the IFN-γ pathway could activate ISGylation of proteins related to cytoskeletal changes, and these changes are important for processes, such as invasion involved in cancer progression.
Association of ISG15 with other signaling pathways in breast cancer cells
Other signaling pathways can also modulate ISG15/ISGylation in breast cancer cells. It has been demonstrated that the reduction of Ki-Ras expression by interfering RNA in MDA-MB-231, ZR-75-1, and MCF-7 breast cancer cells leads to a decrease in the levels of free ISG15 and protein ISGylation, suggesting that oncogenic Ki-Ras pathways may regulate the expression of ISG15 and the ISGylation system [49]. In addition, a positive correlation between ISG15 and KSRI (kinase suppressor of Ras 1) expression has been reported in breast cancer [50]. Moreover, long-term estrogen exposure increases ISG15 expression in MCF-7 cells, and its expression is not affected by RAL (raloxifene, an anti-estrogen SERM) [51]. In triple-negative breast cancer, low levels of p53 and ARF (ADP-ribosylation factor) correlated with high levels of STAT1 and ISG15, leading to proliferation and tumorigenicity. This is important, because some malignant mammary tumors have a co-inactivation of p53 and ARF. Thus, the tumor suppressor pathways of ARF and p53 may be affected in breast cancers, facilitating the upregulation of STAT1–ISG15 signaling [52]. In addition, it has been reported that fibronectin (FN), an extracellular matrix protein, may induce ISG15 expression and protein ISGylation via integrin-dependent signaling in breast cancer cells [53]. Likewise, a connection between ISGylation, FN, and IFN-γ may exist in breast cancer cells, modulating changes in cell morphology and cell spreading [41] (Table 1).
Effects of ISG15 expression in vivo and in vitro studies in a breast cancer context
To evaluate the significance of ISG15 expression in breast cancer, cell lines derived from this cancer have been studied. The reduction of ISG15 expression using specific interfering RNA decreases the proliferation and migration of breast cancer cells. Using the same experimental strategy, the reduction of UBCH8 (E2-enzyme of the ISGylation system) decreases the levels of protein ISGylation, showing similar effects to the reduction of ISG15. Both the reduction of ISG15 expression and the reduction of UBCH8 expression reverse the epithelial–mesenchymal transition (EMT) of MDA-MB-231 breast cancer cells [49]. Furthermore, ISG15 affects the cytoskeleton and enhances cellular motility in ZR-75-1 breast cancer cells. ISG15 and UBCH8 expression disrupts F-actin architecture and the formation of focal adhesions, and enhances the migration of these cells [54]. Some studies have proposed that protein ISGylation confers oncological abilities to breast cancer cells [49, 54, 55]. In this context, ISGylation has been suggested as a mechanism for interfering with the ubiquitination pattern in breast cancer cell lines [54]. Therefore, ISG15 expression seems to have pro-tumor activity in in vitro studies.
Intriguingly, the expression of ISG15 in vivo using athymic mouse models seems to have a contrary effect to the results shown by in vitro studies. For example, xenotransplantation of ZR-75-1 and MDA-MB-231 breast cancer cells treated with interfering RNA for ISG15 into athymic mice promoted tumor development and decreased NK cell filtration in xenograft tumors in comparison with the controls. Hence, ISG15 expression seems to have anti-tumor activity for in vivo studies. The use of these animal models suggests that some elements of the in vivo tumor microenvironment may be critical to modulate or coordinate the actions of ISG15 in mammary tumor progression, which was not evidenced by in vitro studies. Most importantly, in the same study, recombinant purified free ISG15 was injected subcutaneously near the site of tumor implantation with MDA-MB-231 cells with interfering RNA control. Suppression of xenograft tumor growth in nude mice injected with free ISG15 was clearly shown, along with an increase in NK cell infiltration in tumor sections and an increase in major histocompatibility complex (MHC II) surface expression [56]. These data are critical, showing an antitumor function promoted by extracellular free ISG15. Therefore, a possible protumor action for protein ISGylation and anti-tumor for extracellular free ISG15 has been suggested [56] (Fig. 4).
ISG15 as a potential biomarker
Some studies have detected an upregulation of ISG15 expression and protein levels in breast cancer compared with normal breast tissue [34, 40, 57]. For instance, both the mRNA and protein levels of ISG15 were upregulated in breast carcinoma cells compared with normal mammary tissue, evidencing that ISG15 overexpression significantly correlated with unfavorable prognosis of breast cancer [57]. Furthermore, using Cancer Genome Atlas Program (TCGA, 450 patients) and Curtis (1700 patients) data sets from Oncomine, the mRNA levels for ISG15 were analyzed in tumors of patients diagnosed with invasive ductal breast cancer and compared with normal mammary tissue. ISG15 expression was upregulated in breast cancer, and the highest mRNA levels of this gene were detected in grade 3 tumors, suggesting that its expression may be associated with the progression of mammary tumors [40]. At the protein level, an analysis to detect ISG15 through immunohistochemistry (IHC) was performed using a breast cancer microarray that contained 16 cases of invasive breast cancer in duplicate, as well as adjacent normal tissue from the same patients. ISG15 protein was strongly detected in all cases of breast cancer in comparison with the adjacent normal mammary tissue [40]. All these data indicated that ISG15 might be useful as a potential biomarker for breast cancer.
It is also known that mammary tumors can invade tissues such as the lung, bone, liver, and brain. However, the role of ISG15 in distal metastasis from mammary tumors has not been completely defined. Nonetheless, a retrospective study reported that THBS1 (Thrombospondin 1), AP1M1 (Adaptor-Related Protein Complex 1 Subunit Mu 1), and ISG15 genes can be potential biomarkers for central nervous system metastasis from triple-negative tumors [58]. Similarly, through a meta-analysis of three microarray data sets, it was proposed that the overexpression of CD80 and ISG15 was a biomarker for the progression and metastasis of mammary tumors [59].
Furthermore, in vitro and in vivo studies demonstrated that the signaling triggered by LIPG (endothelial lipase) stimulates tumor initiation, as well as metastasis in triple-negative breast cancer and one of the mediators seems to be ISG15, since LIPG protein increases the expression of ISG15 in breast cancer cells. Moreover, DTX3L protein inhibits proteasome-mediated LIPG degradation, leading to LIPG accumulation, and consequently increases ISG15 expression, promoting metastasis of triple-negative tumors [60]. These studies also indicate that ISG15 may be useful as a metastasis biomarker in breast cancer, but more studies are needed to evaluate the functional role of ISG15 in cancer dissemination.
ISG15 as a therapeutic strategy for breast cancer
Thus far, it would be difficult to state predictive values of ISG15 gene expression or free ISG15/ISGylation for resistance to treatments, including chemotherapy and radiation, for breast cancer. However, some studies have analyzed the role of ISG15 expression in breast cancer treatments. For instance, one study determined that the sensitivity of breast cancer cells to camptothecin (a DNA topoisomerase inhibitor) is dependent on ISG15 expression. Camptothecin and its derivatives are used as second- or third-line treatments for breast cancer resistant to endocrine therapy [61]. In this study, it was observed that when cells express high levels of ISG15, they are more sensitive to this drug compared with control cells. However, the depletion of UBCH8 (E2-conjugating enzyme for ISG15) decreases the sensitivity to camptothecin, suggesting that alterations in the ISGylation system can affect the sensitivity of breast cancer cells to this drug [62] (Table 2).
Another case is ΔNp63 alpha (a variant of p63), which suppresses the transcriptional activity of p53 family members. ISGylation may act as a tumor suppressor, since ΔNp63 alpha is ISGylated to block its activity when the mammary epithelial cells are treated with doxorubicin. Hence, this modification may mediate some of the anticancer effects of doxorubicin [63]. More studies are required to determine how ISG15 expression and activity may affect the response to chemotherapy in breast cancer cells. In addition, ISG15 is being considered as a tumor-associated antigen for cancer immunotherapy [64].
Future challenges
In breast cancer, extracellular free ISG15 seems to have anti-tumor activity in vivo, whereas ISGylation has been associated in vitro with pro-tumor activity. Therefore, the regulation of ISG15 expression at all levels and the elements of ISGylation and de-ISGylation systems are critical, because the molecular mechanisms involved in this regulation could determine the proportion of free and conjugated ISG15 levels, leading to anti-tumor or pro-tumor effects in breast cancer cells. Furthermore, the microenvironment of breast tumors may affect the free ISG15/ISGylation activity; for example, extracellular matrix proteins such as FN can induce ISG15 expression in breast cancer cells [53]. In addition, ERα status appears to have an influence on the profile of free ISG15 and ISGylation marks by mechanisms not yet determined. Hence, a better understanding of free or conjugated ISG15 and its regulation is still required in mammary tumors classified as ERα– and ERα+. The analysis of patient samples shows that ISG15 may be a potential biomarker for breast cancer, but it is necessary to investigate whether ISG15 can be used as an indicator for the selection of the therapy and/or as a prognostic marker. Furthermore, the potential of ISGylation system elements and USP18 as biomarkers and target therapeutics should also be explored. ISGylation targets in breast cancer cells are pending identification, but many of them may be central in molecular pathways for promoting or controlling the development of mammary tumors. To date, the identification of IQGAP1 and NMIIA as ISGylated proteins indicates the implications of ISGylation and its regulatory factors (IFN-γ, integrins, FN) in changes of the cytoskeletal structure, cell morphology, and probably migration and invasion of these cells. The identification of new ISGylation targets in breast cancer would help to understand the role of ISG15/ISGylation pathways in this pathology. The potential of free ISG15 as a therapeutic molecule to control tumor development demands further studies to support this possible action and generate novel strategies for the treatment of this cancer. Thus, the ISGylation system and free ISG15 are emergent factors in the understanding of breast cancer and open new study lines to explore the basic molecular aspects, biological and functional transcendence, and medical implications in breast cancer.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53.
Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2018. https://gco.iarc.fr/today. Accessed 20 July 2020
Aleskandarany MA, Abduljabbar R, Ashankyty I, et al. Prognostic significance of androgen receptor expression in invasive breast cancer: transcriptomic and protein expression analysis. Breast Cancer Res Treat. 2016;159:215–27.
Turashvili G, Brogi E. Tumor heterogeneity in breast cancer. Front Med. 2017;4:227.
Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–23.
Tsang JYS, Tse GM. Molecular classification of breast cancer. Adv Anat Pathol. 2020;27:27–35.
Bertucci F, Chaffanet M, Birnbaum D. An ICGC major achievement in breast cancer: a comprehensive catalog of mutations and mutational signatures. Chin Clin Oncol. 2017;6:4.
Dai X, Xiang L, Li T, Bai Z. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J Cancer. 2016;7:1281–94.
Knight E Jr, Fahey D, Cordova B, et al. A 15-kDa interferon-induced protein is derived by COOH-terminal processing of a 17-kDa precursor. J Biol Chem. 1988;263:4520–2.
Potter JL, Narasimhan J, Mende-Mueller L, Haas AL. Precursor processing of pro-ISG15/UCRP, an interferon-beta-induced ubiquitin-like protein. J Biol Chem. 1999;274:25061–8.
Reich N, Evans B, Levy D, Fahey D, Knight E Jr, Darnell JE Jr. Interferon-induced transcription of a gene encoding a 15-kDa protein depends on an upstream enhancer element. Proc Natl Acad Sci USA. 1987;84:6394–8.
Loeb KR, Haas AL. The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J Biol Chem. 1992;267:7806–13.
Malakhov MP, Kim KI, Malakhova OA, Jacobs BS, Borden EC, Zhang DE. High-throughput immunoblotting. Ubiquitiin-like protein ISG15 modifies key regulators of signal transduction. J Biol Chem. 2003;278:16608–13.
Narasimhan J, Wang M, Fu Z, Klein JM, Haas AL, Kim JJ. Crystal structure of the interferon-induced ubiquitin-like protein ISG15. J Biol Chem. 2005;280:27356–65.
Dastur A, Beaudenon S, Kelley M, Krug RM, Huibregtse JM. Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells. J Biol Chem. 2006;281:4334–8.
Kim KI, Giannakopoulos NV, Virgin HW, Zhang DE. Interferon-inducible ubiquitin E2, Ubc8, is a conjugating enzyme for protein ISGylation. Mol Cell Biol. 2004;24:9592–600.
Malakhova OA, Yan M, Malakhov MP, et al. Protein ISGylation modulates the JAK-STAT signaling pathway. Genes Dev. 2003;17:455–60.
Park JH, Yang SW, Park JM, et al. Positive feedback regulation of p53 transactivity by DNA damage-induced ISG15 modification. Nat Commun. 2016;7:12513.
Park JM, Yang SW, Yu KR, et al. Modification of PCNA by ISG15 plays a crucial role in termination of error-prone translesion DNA synthesis. Mol Cell. 2014;54:626–38.
Wong JJ, Pung YF, Sze NS, Chin KC. HERC5 is an IFN-induced HECT-type E3 protein ligase that mediates type I IFN-induced ISGylation of protein targets. Proc Natl Acad Sci USA. 2006;103:10735–40.
Zhao C, Beaudenon SL, Kelley ML, et al. The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein. Proc Natl Acad Sci USA. 2004;101:7578–82.
Zou W, Wang J, Zhang DE. Negative regulation of ISG15 E3 ligase EFP through its autoISGylation. Biochem Biophys Res Commun. 2007;354:321–7.
Zou W, Zhang DE. The interferon-inducible ubiquitin-protein isopeptide ligase (E3) EFP also functions as an ISG15 E3 ligase. J Biol Chem. 2006;281:3989–94.
Oudshoorn D, van Boheemen S, Sanchez-Aparicio MT, Rajsbaum R, Garcia-Sastre A, Versteeg GA. HERC6 is the main E3 ligase for global ISG15 conjugation in mouse cells. PLoS ONE. 2012;7:e29870.
Takeuchi T, Inoue S, Yokosawa H. Identification and Herc5-mediated ISGylation of novel target proteins. Biochem Biophys Res Commun. 2006;348:473–7.
Basters A, Geurink PP, El Oualid F, et al. Molecular characterization of ubiquitin-specific protease 18 reveals substrate specificity for interferon-stimulated gene 15. FEBS J. 2014;281:1918–28.
Huang YF, Bulavin DV. Oncogene-mediated regulation of p53 ISGylation and functions. Oncotarget. 2014;5:5808–18.
Im E, Yoo L, Hyun M, Shin WH, Chung KC. Covalent ISG15 conjugation positively regulates the ubiquitin E3 ligase activity of parkin. Open Biol. 2016;6:160193.
Jeon YJ, Choi JS, Lee JY, et al. ISG15 modification of filamin B negatively regulates the type I interferon-induced JNK signalling pathway. EMBO Rep. 2009;10:374–80.
Lee JH, Bae JA, Lee JH, et al. Glycoprotein 90K, downregulated in advanced colorectal cancer tissues, interacts with CD9/CD82 and suppresses the Wnt/beta-catenin signal via ISGylation of beta-catenin. Gut. 2010;59:907–17.
Xu D, Zhang T, Xiao J, et al. Modification of BECN1 by ISG15 plays a crucial role in autophagy regulation by type I IFN/interferon. Autophagy. 2015;11:617–28.
Swaim CD, Scott AF, Canadeo LA, Huibregtse JM. Extracellular ISG15 signals cytokine secretion through the LFA-1 integrin receptor. Mol Cell. 2017;68(581–90):e5.
Tecalco Cruz AC, Mejia-Barreto K. Cell type-dependent regulation of free ISG15 levels and ISGylation. J Cell Commun Signal. 2017;11:127–35.
Tecalco-Cruz AC, Cruz-Ramos E. Protein ISGylation and free ISG15 levels are increased by interferon gamma in breast cancer cells. Biochem Biophys Res Commun. 2018;499:973–8.
Mojic M, Takeda K, Hayakawa Y. The dark side of IFN-gamma: its role in promoting cancer immunoevasion. Int J Mol Sci. 2017;19:89.
Gooch JL, Herrera RE, Yee D. The role of p21 in interferon gamma-mediated growth inhibition of human breast cancer cells. Cell Growth Differ. 2000;11:335–42.
Niu XL, Wang Y, Yao Z, et al. Autocrine interferon-gamma may affect malignant behavior and sensitivity to tamoxifen of MCF-7 via estrogen receptor beta subtype. Oncol Rep. 2015;34:3120–30.
Cui XF, Imaizumi T, Yoshida H, Borden EC, Satoh K. Retinoic acid-inducible gene-I is induced by interferon-gamma and regulates the expression of interferon-gamma stimulated gene 15 in MCF-7 cells. Biochem Cell Biol. 2004;82:401–5.
Tecalco-Cruz AC, Cortes-Gonzalez CC, Cruz-Ramos E, Ramirez Jarquin JO, Romero-Mandujano AK, Sosa-Garrocho M. Interplay between interferon-stimulated gene 15/ISGylation and interferon gamma signaling in breast cancer cells. Cell Signal. 2019;54:91–101.
Cruz-Ramos E, Macias-Silva M, Sandoval-Hernandez A, Tecalco-Cruz AC. Non-muscle myosin IIA is post-translationally modified by interferon-stimulated gene 15 in breast cancer cells. Int J Biochem Cell Biol. 2019;107:14–26.
Ning Y, Riggins RB, Mulla JE, Chung H, Zwart A, Clarke R. IFNgamma restores breast cancer sensitivity to fulvestrant by regulating STAT1, IFN regulatory factor 1, NF-kappaB, BCL2 family members, and signaling to caspase-dependent apoptosis. Mol Cancer Ther. 2010;9:1274–85.
Thakkar SG, Peereboom D, Olencki T, et al. Short communication: phase I clinical and gene modulatory evaluation of tamoxifen and IFN-alpha2b. J Interferon Cytokine Res. 2006;26:800–3.
Legrier ME, Bieche I, Gaston J, et al. Activation of IFN/STAT1 signalling predicts response to chemotherapy in oestrogen receptor-negative breast cancer. Br J Cancer. 2016;114:177–87.
Weichselbaum RR, Ishwaran H, Yoon T, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci USA. 2008;105:18490–5.
Giannakopoulos NV, Luo JK, Papov V, et al. Proteomic identification of proteins conjugated to ISG15 in mouse and human cells. Biochem Biophys Res Commun. 2005;336:496–506.
Dulyaninova NG, House RP, Betapudi V, Bresnick AR. Myosin-IIA heavy-chain phosphorylation regulates the motility of MDA-MB-231 carcinoma cells. Mol Biol Cell. 2007;18:3144–55.
Cerikan B, Schiebel E. DOCK6 inactivation highlights ISGylation as RHO-GTPase balancer. Cell Cycle. 2017;16:304–5.
Burks J, Reed RE, Desai SD. ISGylation governs the oncogenic function of Ki-Ras in breast cancer. Oncogene. 2014;33:794–803.
Zhang H, Angelopoulos N, Xu Y, et al. Proteomic profile of KSR1-regulated signalling in response to genotoxic agents in breast cancer. Breast Cancer Res Treat. 2015;151:555–68.
Englert NA, Spink BC, Spink DC. Persistent and non-persistent changes in gene expression result from long-term estrogen exposure of MCF-7 breast cancer cells. J Steroid Biochem Molr Biol. 2011;123:140–50.
Forys JT, Kuzmicki CE, Saporita AJ, Winkeler CL, Maggi LB Jr, Weber JD. ARF and p53 coordinate tumor suppression of an oncogenic IFN-beta-STAT1-ISG15 signaling axis. Cell Rep. 2014;7:514–26.
Hermann MR, Jakobson M, Colo GP, et al. Integrins synergise to induce expression of the MRTF-A-SRF target gene ISG15 for promoting cancer cell invasion. J Cell Sci. 2016;129:1391–403.
Desai SD, Reed RE, Burks J, et al. ISG15 disrupts cytoskeletal architecture and promotes motility in human breast cancer cells. Exp Biol Med. 2012;237:38–49.
Kiessling A, Hogrefe C, Erb S, et al. Expression, regulation and function of the ISGylation system in prostate cancer. Oncogene. 2009;28:2606–20.
Burks J, Reed RE, Desai SD. Free ISG15 triggers an antitumor immune response against breast cancer: a new perspective. Oncotarget. 2015;6:7221–31.
Bektas N, Noetzel E, Veeck J, et al. The ubiquitin-like molecule interferon-stimulated gene 15 (ISG15) is a potential prognostic marker in human breast cancer. Breast Cancer Res. 2008;10:R58.
Rojas LK, Trilla-Fuertes L, Gamez-Pozo A, et al. Proteomics characterisation of central nervous system metastasis biomarkers in triple negative breast cancer. Ecancermedicalscience. 2019;13:891.
Li Y, Bai W, Zhang L. The overexpression of CD80 and ISG15 are associated with the progression and metastasis of breast cancer by a meta-analysis integrating three microarray datasets. Pathol Oncol Res. 2020;26:443–52.
Lo PK, Yao Y, Lee JS, et al. LIPG signaling promotes tumor initiation and metastasis of human basal-like triple-negative breast cancer. eLife. 2018;7:e31334.
Tesauro C, Simonsen AK, Andersen MB, et al. Topoisomerase I activity and sensitivity to camptothecin in breast cancer-derived cells: a comparative study. BMC Cancer. 2019;19:1158.
Desai SD, Wood LM, Tsai YC, et al. ISG15 as a novel tumor biomarker for drug sensitivity. Mol Cancer Ther. 2008;7:1430–9.
Jeon YJ, Jo MG, Yoo HM, et al. Chemosensitivity is controlled by p63 modification with ubiquitin-like protein ISG15. J Clin Investig. 2012;122:2622–36.
Wood LM, Pan ZK, Seavey MM, Muthukumaran G, Paterson Y. The ubiquitin-like protein, ISG15, is a novel tumor-associated antigen for cancer immunotherapy. Cancer Immunol Immunother. 2012;61:689–700.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tecalco-Cruz, A.C., Ramírez-Jarquín, J.O. & Cruz-Ramos, E. Regulation and action of interferon-stimulated gene 15 in breast cancer cells. Human Cell 33, 954–962 (2020). https://doi.org/10.1007/s13577-020-00414-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00414-x