Abstract
Anterior gradient 2 (AGR2) was proved to modulate cancer progression. However, the role of AGR2 on endometrial cancer was not established. Here, we investigated the effects of AGR2 expression on endometrial cancer and explored the regulation mechanism. In the study, we found that AGR2 was overexpressed in tumor tissues of 30 endometrial cancer patients. A high level of AGR2 promoted endometrial cancer cells proliferation, migration and invasion. AGR2 induced the expression of lactate dehydrogenase A (LDHA), phosphoglycerate kinase 1 (PGK1), kallikrein 2 (HK2), and enolase 1-α (ENO1), glucose uptake and lactate production. AGR2 could bind to MUC1 and induce MUC1 and hypoxia-inducible factor 1α (HIF-1α). The inhibition effects of AGR2 knockdown on cells proliferation, migration and invasion ability were abolished by the overexpression of MUC1. Besides, the overexpression of MUC1 also reversed the inhibition effects of AGR2 knockdown on the expression of LDHA, HK2, PGK1 and ENO1, glucose uptake and lactate production. AGR2 knockdown inhibited tumor growth, the levels of Ki-67, MUC1, HIF-1α and glycolysis. In conclusion, AGR2 was overexpressed in endometrial cancer and AGR2-induced glucose metabolism facilitated the progression of endometrial carcinoma via the MUC1/HIF-1α pathway. AGR2 may be an effective therapeutic target for endometrial carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial carcinoma is a common malignant gynecologic tumor and the incidence of cancer is on the rise in recent years [1, 2]. As a result of the early-stage diagnosis, the prognosis of endometrial carcinoma was favorable, while cancer recurrence and metastasis occurred in 8% to 10% of endometrial carcinoma patients at an early stage [3]. Besides, the 5-year survival rate for endometrial carcinoma patients in advanced stages was less than 20% [4]. The mortality rate of endometrial carcinoma in the USA is second to that of ovarian cancer in 2013 [5]. At present, the major strategies for endometrial carcinoma treatment such as hysterectomy, chemotherapy and hormonal therapy have been effective for patients in early stage rather than patients in advanced stages [6]. Consequently, it is critical to explore the mechanisms of endometrial carcinoma occurrence and development at the molecular level and search novel strategies for endometrial carcinoma therapy.
Anterior gradient 2 (AGR2) is a member of the protein disulfide isomerase family (PDI) of endoplasmic reticulum-resident proteins [7]. In some cancers, the expression of AGR2 was elevated, indicating that AGR2 exerted vital roles in cancer regulation. AGR2 is nearly expressed in all tumor tissues in lung cancer, and a high level of AGR2 is associated with poor survival of patients [8]. AGR2 expression also increased in tumor tissues of gastric cancer patients and the aberrant expression of AGR2 was related to tumor size, lymph node metastasis, vessel invasion, and poor prognosis of gastric cancer patients [9]. Furthermore, AGR2 presented aberrant expression and functioned as a regulator in hormone-dependent tumors. For example, AGR2 was overexpressed in mucinous ovarian tumor tissues and a high expression of AGR2 promoted tumor growth and cell migration [10]. In breast cancer, the AGR2 expression was up-regulated and AGR2 was essential for breast cancer cell proliferation, invasion and migration induced by twist family bHLH transcription factor 1 (Twist1) [11]. Also, a differential expression of AGR2 was also found in endometrial carcinoma. A high level of AGR2 was present in metastatic lesions of endometrial carcinoma compared to primary tumors [12]. However, a study of AGR2 on cancer development and regulation of endometrial carcinoma has not been established. Thus, we investigated the effect of AGR2 expression on endometrial carcinoma and explored the molecular mechanism in the study.
Mucin 1 (MUC1) is a member of the transmembrane mucin family, and it can exert lubrication and barrier functions [13]. MUC1 was considered as an oncogene in many cancers [14, 15] and its high expression in cancers was also confirmed. MUC1 was overexpressed in breast cancers and up-regulation of MUC1 was associated with axillary node metastases of breast cancer [16]. In endometrial carcinoma, MUC1 expression was also increased [17]. MUC1 activated EGFR expression and regulated cell proliferation in endometrial carcinoma [18]. Also, MUC1 mediated glucose metabolism in pancreatic cancer through cross talk with hypoxia-inducible factor 1α (HIF-1α) [19]. In the study, the function of MUC1 on glucose metabolism in endometrial carcinoma was explored.
Materials and methods
Tissue collection
A total of 30 endometrial carcinoma patients diagnosed in The Fourth Affiliated Hospital of Xinjiang Medical University were enrolled in the current study. After patients’ written informed consents were obtained, tumor tissues and adjacent normal tissues of 30 endometrial carcinoma patients were collected. The protocol of the study was approved by the Ethics Committee of The Fourth Affiliated Hospital of Xinjiang Medical University (No. 2017065).
Cell culture
Endometrial carcinoma cell lines RL952, ISHIKAWA, HEC1A and HEC1B were obtained from American Type Culture Collection (ATCC). RL952 cells were cultured in McCoy’s 5A modified medium plus 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA) at 37 °C, 5% CO2 atmosphere. HEC1A cells were maintained in DMEM/F12 medium plus 10% FBS (Gibco, Carlsbad, CA, USA) at 37 °C, 5%CO2 atmosphere. ISHIKAWA and HEC1B cells were cultured using DMEM medium plus 10% FBS (Gibco, Carlsbad, CA, USA) at 37 °C, 5%CO2 atmosphere. To prevent cell contamination, 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco, Carlsbad, CA, USA) were supplemented to two kinds of media. In the indicated experiments, RL952 and HEC1A cells were treated with normoxic (20%) and hypoxic (1%) conditions.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated with RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA was then reverse transcribed into cDNA using RT2 First strand kit (Qiagen, Hilden, Germany) and qRT-PCR was performed with Mx3000P RT-PCR equipment using SYBR Green real-time PCR kit (TaKaRa, Kusatsu, Japan). The primer sequences were: AGR2, forward: 5′-CTTGTGGCCCTCTCCTACAC-3′, reverse: 5′-GTCCAGATGAGTTGGTCACCC-3′; lactate dehydrogenase A (LDHA), forward: 5′-TACAGTTGTTGGGGTTGGTG-3′, reverse: 5′-GCCAGAGACAATCTTTGGTGT-3′; phosphoglycerate kinase 1 (PGK1), forward: 5′-GCTGGACAAGCTGGACGTTA-3′, reverse: 5′-CTCTGGTTGTTTGTTATCTGGTTGT-3′; kallikrein 2 (HK2) forward, 5′-GGTTCCGCAAGGAGATGGAG-3′, reverse: 5′-TGGAGCCCATTGTCCGTTAC-3′; enolase 1-α (ENO1), forward: 5′-GGGAATCCCACTGTTGAGGT-3′, reverse: 5′-CGGAGCTCTAGGGCCTCATA-3′; MUC1, forward: 5′-GGGCACCCAGTCTCCTTTC-3′, reverse: 5′-CAACTGTTGCGGGTTTAGGG-3′; HIF-1α, forward: 5′-TGGACACAGGAATTGTACCAGA-3′; reverse: 5′-CACCTCCGACTCCTTTCCAC-3′. The PCR program was set to 95 °C for 1 min, 40 cycles of for 95 °C for 15 s, 60 °C for 15 s and at 72 °C for 30 s. The results were analyzed with the 2−ΔΔCt method using β-actin as a control gene.
Immunohistochemistry
Tumor tissues and adjacent normal tissues of endometrial carcinoma patients and tumor tissues of mice were fixed and embedded by formaldehyde and paraffin, respectively, and then tissues were cut into 5 µm sections. Endogenous peroxidase of sections was inhibited by 3% hydrogen peroxide and antigen retrieval was accomplished using citrate buffer (pH 6.0). Sections were incubated with anti-AGR2 antibody (1:200) (Abcam, Cambridge, UK), anti-Ki-67 antibody (1:200) (Abcam, Cambridge, UK), anti-MUC1 antibody (1:200) (Abcam, Cambridge, UK), and anti-HIF-1α antibody (1:200) (Abcam, Cambridge, UK) for 12 h at 4 °C. Sections were then treated by horseradish peroxidase-conjugated goat anti-rabbit IgG (Abcam, Cambridge, UK) for 1 h at 37 °C. The stained sections were observed by two pathologists under light microscopy (Olympus, Japan). The grading of immunohistochemistry slides was obtained by combining the percentages of stained cells and staining intensity (percentages multiplied by intensity) [20]. The percentages of stained cells were scored following the standard (0–3): 0, negative (< 10% positive cells); 1, weak (< 30% positive cells); 2, moderate (< 50% positive cells), 3, strong (≥ 50% positive cells) [20]. The staining intensity of endometrial carcinoma tumor cell was evaluated following the standard (0–3): 0, no coloring; 1, weak staining; 2, moderate staining (yellow); 3, strong staining (brown) [20].
Western blot
After indicated treatment, RL952 cells and HEC1A cells were lysed using RIPA buffer (Thermo Fisher, Waltham, MA, USA), and protein content in lysates was determined by bicinchoninic acid (BCA) kit (Beyotime, Beijing, China). Lysates were then separated using SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a PVDF membrane for protein detection. After the PVDF membranes were blocked by 4% non-fat milk for 1 h, the membranes were incubated with anti-AGR2 antibody (1:500) (Abcam, Cambridge, UK), anti-MUC1 antibody (1:500) (Abcam, Cambridge, UK), anti-HIF-1α antibody (1:500) (Abcam, Cambridge, UK) and anti-GAPDH (1:1000) (Abcam, Cambridge, UK) antibody and subsequently treated with HRP-conjugated secondary antibody (Abcam, Cambridge, UK). Protein bands were detected using the ECL detection kit (Thermo Fisher, Waltham, MA, USA). GAPDH was used as an internal control.
Cell transfection
Short hairpin RNA (shRNA) negative control (shNC), shRNA of AGR2 (shAGR2), pcDNA3.1 negative control (pcDNA3.1-NC), pcDNA3.1-AGR2, pcDNA3.1-MUC1, adenovirus shRNA negative control (Ad-shNC), and adenovirus shRNA of AGR2 (Ad-shAGR2) were obtained from RiboBio (RiboBio, Guangzhou, China). To perform cell transfection, RL952 cells and HEC1A cells were seeded into six-wells plates and cultured for 24 h at 37 °C, 5% CO2 atmosphere. RL952 cells and HEC1A cells were transfected into shNC, shAGR2, pcDNA3.1-NC, pcDNA3.1-AGR2, Ad-shNC and Ad-shAGR2 respectively, using Lipofectamine 3000 (Life Technologies, Carlsbad, CA, USA). After 48 h of transfection, RL952 cells and HEC1A cells were harvested for experiments.
MTT assay
To assess cell viability, RL952 cells and HEC1A cells were made into a cell suspension and inoculated into 96-well plates at a density of 5 × 103/well after transfection treatment. After cells were cultured for 3 days, the MTT solution (20 μL, 5 mg/mL) (Sigma, St. Louis, Missouri, USA) was added per well of RL952 cells and HEC1A cells. The 96-well plates were continued to be cultured at 37 °C, 5% CO2 atmosphere for 4 h. Dimethyl sulfoxide (DMSO, 150 μL) (Sigma, St. Louis, Missouri, USA) was then added to each well to dissolve formazan crystals and the absorbance of each well was determined using a microplate reader (Thermo Fisher, Waltham, MA, USA) at 490 nm.
Colony formation assay
After RL952 cells and HEC1A cells were transfected with shNC, shAGR2, pcDNA3.1-NC or pcDNA3.1-AGR2, RL952 cells and HEC1A cells were made into cell suspension and inoculated into 96-well plates at a density of 1 × 103/well. After all cells were cultured for 2 weeks, the colonies were fixed with 4% paraformaldehyde and then stained with 0.1% crystal violet. Finally, the colony (> 0.5 mm) numbers were counted.
Wound healing assay
After RL952 cells and HEC1A cells were transfected with shNC shAGR2, pcDNA3.1-NC or pcDNA3.1-AGR2, RL952 cells and HEC1A cells were inoculated into six-well plates and grown to approximately 90% confluence. The scratch wound was then generated using 10 μL pipette tip and cells were continued to be cultured at 37 °C and 5% CO2 atmosphere. The wound width was measured at 0 h and 24 h under a microscope (Olympus, Tokyo, Japan).
Transwell invasion assay
For invasion assays, Matrigel was added to the upper chamber of Transwell to coat the chamber. After RL952 cells and HEC1A cells were transfected with shNC shAGR2, pcDNA3.1-NC or pcDNA3.1-AGR2, RL952 cells, and HEC1A cells were made into cell suspension using serum-free medium and inoculated in the upper chamber. The medium containing 10% FBS was added to the lower chamber of Transwell. After the cells were cultured at 37 °C for 24 h, the invaded cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet for 15 min, respectively. The numbers of invaded RL952 and HEC1A cells were counted in five random fields under a light microscope (Olympus, Tokyo, Japan).
Glucose uptake and lactate secretion
After the RL952 and HEC1A cells were transfected with shNC, shAGR2, pcDNA3.1-NC or pcDNA3.1-AGR2 and treated by hypoxia (1%), the glucose uptake ability of RL952 and HEC1A cells was determined by measurement of the uptake of [3H] 2-deoxy-D-glucose ([3H] 2DG). RL952 and HEC1A cells were seeded into six-well plates and incubated with Earle’s/HEPES solution containing 5.5 mmol/L glucose for 2 h. The RL952 and HEC1A cells were then incubated with [3H] 2DG (5 μCi/mL). After incubation, RL952 and HEC1A cells were lysed using RIPA buffer and extracts were measured by a scintillation counter (Beckman Coutler, Fullerton, CA, USA).
After the RL952 and HEC1A cells were transfected with shNC shAGR2, pcDNA3.1-NC or pcDNA3.1-AGR2 and treated with normoxic (20%) or hypoxia (1%), RL952 and HEC1A cells were cultured in McCoy’s 5A modified medium or DMEM/F12 medium, respectively, to determine lactate secretion. Three days later, lactate secretion was measured with a lactate colorimetric assay kit (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer's instructions.
Immunoprecipitation (IP)
RL952 cells were harvested and lysed using lysis buffer supplemented with protease inhibitors (Thermo Fisher, Waltham, MA, USA). The protein content of lysates was determined by bicinchoninic acid (BCA) kit (Beyotime, Beijing, China) after lysates were clarified by centrifugation. The lysates were incubated with anti-MUC1 antibody, anti-AGR2 antibody and anti-IgG antibody mixed with protein A/G agarose beads (Santa Cruz Biotechnology), and then the mixture was incubated at 4 °C for 12 h. After completion of the immunoprecipitation reaction, the beads were collected via centrifugation and washed five times with lysis buffer. Finally, the immune complexes were analyzed using Western blot.
Xenograft tumor establishment
A total of 20 BALB/c nude mice aged 4–6 weeks were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. All animal experiments were approved by the Ethics Committee of The Fourth Affiliated Hospital of Xinjiang Medical University (No. 2017065). All mice were divided into two groups including Ad-shNC group and Ad-shAGR2. RL952 cells were stably transfected with Ad-shNC or Ad-shAGR2 using Lipofectamine 3000. Tumor xenografts were established by subcutaneously injecting 1 × 105 of stably transfected RL952 cells at the buttocks of mice (n = 10 in each group). Tumor size was determined every 7 days until 35 days. After all tumors were harvested, immunohistochemistry was performed to detect the expression of Ki-67, AGR2, MUC1, and HIF-1α, and qRT-PCR was conducted to determine the expression of LDHA, PGK1, HK2 and ENO1.
Statistical analysis
Statistical analysis was conducted by SPSS Statistics software 22.0 (Chicago, IL, USA. All data were represented as mean ± standard deviation (SD). The differences of groups were analyzed by Student’s t test or one-way ANOVA. P < 0.05 was represented as statistically significant.
Results
AGR2 is up-regulated in endometrial carcinoma
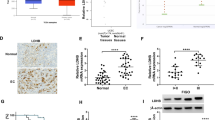
To discern the expression of AGR2 in endometrial carcinoma, we analyzed the mRNA expression of AGR2 in tumor tissues and adjacent normal tissues of 30 endometrial carcinoma patients. The detailed information on endometrial carcinoma patients is described in Table 1. We observed that the mRNA levels of AGR2 were significantly up-regulated in tumor tissues compared to adjacent normal tissues (P < 0.01, Fig. 1a). Immunohistochemistry additionally showed that AGR2-positive expression was higher in tumor tissues compared to adjacent normal tissues (Fig. 1b). The high expression of AGR2 in endometrial carcinoma was proved to correlate with poor overall survival of endometrial carcinoma patients by Kaplan–Meier survival analysis (P < 0.05, Fig. 1c). Also, the AGR2 expression also was associated with the Federation International of Gynecology and Obstetrics (FIGO) stage (P < 0.05) and lymph node metastasis (P < 0.01) (Table 1).
AGR2 was up-regulated in endometrial carcinoma. a The expression of AGR2 in tumor tissues and adjacent normal tissues of 30 patients was determined by qRT-PCR assay (N = 4). b Immunohistochemistry was performed to detect the AGR2 expression in endometrial carcinoma patients (N = 4). c The relationship between AGR2 expression and survival of endometrial carcinoma patients was analyzed by Kaplan–Meier survival analysis. **: P < 0.01
AGR2 promotes cell proliferation, migration and invasion in endometrial carcinoma
To explore the role of AGR2 in endometrial carcinoma, the expression of AGR2 in four endometrial carcinoma cells lines including RL952, ISHIKAWA, HEC1A and HEC1B was first determined. The AGR2 mRNA and protein levels were the highest in the RL952 cells and lowest in the HEC1A cells among the four endometrial carcinoma cell lines (all P < 0.01, Fig. 2a). Therefore, RL952 cells and HEC1A were selected to perform subsequent experiments. To understand the influence of AGR2 on endometrial carcinoma cell behaviors, the RL952 cells with down-regulated AGR2 and HEC1A cells that overexpressed AGR2 were generated by transfecting with shAGR2 or pcDNA 3.1-AGR2, which were confirmed by qRT-PCR assay and Western blot (all P < 0.01, Fig. 2b). For knockdown of AGR2, two shRNAs targeting AGR2 were transfected into RL952 cells, respectively, and the shAGR2 with higher knockdown efficiency was used for subsequent experiments. MTT assay proved that the knockdown of AGR2 in RL952 cells significantly inhibited cell viability (P < 0.05), while AGR2 overexpression in HEC1A cells significantly increased cell viability (P < 0.01, Fig. 2c). To further explore the influence of AGR2 on cell behaviors, we performed colony formation assay, wound healing assay and Transwell invasion assays. AGR2 knockdown resulted in inhibition of colony formation ability in RL952 cells (P < 0.01) and overexpression of AGR2 promoted colony formation in HEC1A cells (P < 0.01) (Fig. 2d). Wound healing assay showed that the cell migration ability was inhibited after knockdown of AGR2 (P < 0.01) and overexpression of AGR2 promoted cell migration ability (P < 0.01) (Fig. 2e). Also, knockdown of AGR2 inhibited cell invasion ability in RL952 cells and overexpression of AGR2 promoted cell invasion ability (P < 0.01) (Fig. 2f). These results show that AGR2 promotes cells proliferation, migration and invasion in endometrial carcinoma.
AGR2 promoted cell proliferation, migration and invasion in endometrial carcinoma. a The mRNA and protein levels of AGR2 in RL952, ISHIKAWA, HEC1A and HEC1B cells were determined by qRT-PCR and Western blot, respectively (N = 4). b The mRNA and protein levels of AGR2 in RL952 cells transfected with shNC or shAGR2 and HEC1A transfected with pcDNA3.1-NC or pcDNA3.1-AGR2 were determined by qRT-PCR and Western blot, respectively (N = 4). c The MTT assay was used to detect cell viability of RL952 cells transfected with shNC or shAGR2 and HEC1A transfected with pcDNA3.1-NC or pcDNA3.1-AGR2 (N = 4). d Colony formation assay was used to detect colony formation ability of RL952 cells transfected with shNC or shAGR2 and HEC1A transfected with pcDNA3.1-NC or pcDNA3.1-AGR2 (N = 4). e Wound healing assay was used to assess the cell migration ability of RL952 cells transfected with shNC or shAGR2 and HEC1A transfected with pcDNA3.1-NC or pcDNA3.1-AGR2 (N = 4). f The invasion ability of RL952 cells transfected with shNC or shAGR2 and HEC1A transfected with pcDNA3.1-NC or pcDNA3.1-AGR2 was determined by Transwell invasion assay (N = 4). *P < 0.05; **P < 0.01
AGR2 promotes glycolysis in endometrial carcinoma
To further detect the role of AGR2 in glycometabolism, the protein and mRNA expression levels of glycolysis-related genes including LDHA, PGK1, HK2 and ENO1 were investigated. The protein and mRNA levels of LDHA, PGK1, HK2 and ENO1 in hypoxic condition were all significantly higher than that in the normoxic condition in RL952 and HEC1A cells (all P < 0.01). In hypoxic condition, the mRNA levels of LDHA, PGK1, HK2 and ENO1 in RL952 cells with AGR2 knockdown were also significantly higher than that in normoxic condition (all P < 0.01), while the levels of LDHA, PGK1, HK2 and ENO1 in RL952 cells with AGR2 knockdown in hypoxia condition were lower than that in RL952 cells transfected with shNC (Fig. 3a, b). Contrarily, in hypoxic condition, the protein and mRNA levels of LDHA, PGK1, HK2 and ENO1 of HEC1A cells with AGR2 overexpression were also significantly higher than that in normoxic condition (all P < 0.01), while the levels of LDHA, PGK1, HK2 and ENO1 in HEC1A cells with AGR2 overexpression in hypoxia condition were higher than that in HEC1A cells transfected to pcDNA3.1-NC (Fig. 3a, b). In hypoxia condition, glucose uptake and lactate production were inhibited by knockdown of AGR2 in RL952 cells compared to RL952 cells transfected with shNC (all P < 0.01), while AGR2 overexpression in HEC1A cells significantly promoted glucose uptake and lactate production compared to HEC1A cells transfected with pcDNA3.1-NC (all P < 0.01) (Fig. 3c, d). These results show that AGR2 promotes glycolysis process in endometrial carcinoma.
AGR2 promoted glycolysis in endometrial carcinoma. a The protein levels of LDHA, PGK1, HK2 and ENO1 in normoxic and hypoxic conditions of RL952 cells transfected with shNC or shAGR2 and HEC1A transfected with pcDNA3.1-NC or pcDNA3.1-AGR2 were detected by Western blot (N = 4). b The mRNA levels of LDHA, PGK1, HK2 and ENO1 in normoxic and hypoxic conditions of RL952 cells transfected with shNC or shAGR2 and HEC1A transfected with pcDNA3.1-NC or pcDNA3.1-AGR2 were detected by qRT-PCR assay (N = 4). c Glucose uptake ability was determined by measurement of the uptake of [3H] 2DG of RL952 cells transfected with shNC or shAGR2 and HEC1A transfected with pcDNA3.1-NC or pcDNA3.1-AGR2 in normoxic and hypoxic conditions (N = 4). d Lactate secretion was determined for RL952 cells transfected with shNC or shAGR2 and HEC1A transfected with pcDNA3.1-NC or pcDNA3.1-AGR2 in normoxic and hypoxic conditions (N = 4). *P < 0.05; **P < 0.01
AGR2 induces glucose metabolism via the MUC1/HIF-1α pathway
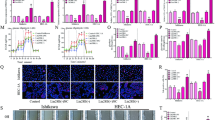
MUC1, a member of the transmembrane mucin family, has been proved to regulate oncogenesis and development of some cancers including endometrial carcinoma and induce glycolysis via HIF-1α modulation in cancers [21]. Thus, we speculated that AGR2, the oncogene and glycolysis regulator in endometrial carcinoma, might interact with MUC1. The immunoprecipitation assay was performed in RL952 cells and results showed that AGR2 could bind to MUC1 (Fig. 4a). Also, the levels of MUC1 and HIF-1α were significantly decreased after knockdown of AGR2 in RL952 cells treated with hypoxic condition, while overexpression of AGR2 induced the expression of MUC1 and HIF-1α in HEC1A cells treated by hypoxic condition (all P < 0.01, Fig. 4b). Overexpression of MUC1 reversed the inhibition effect of AGR2 knockdown on MUC1 and HIF-1α expression (all P < 0.01, Fig. 4c). Besides, the inhibition effects of AGR2 knockdown on the cell viability (P < 0.01), cell colony formation ability (P < 0.01), cell migration ability (P < 0.01) and cell invasion ability (P < 0.01) of RL952 were also rescued by overexpression of MUC1 (Fig. 4d–g). Also, the inhibition effect of AGR2 knockdown on the mRNA and protein expression levels of LDHA, HK2, PGK1 and ENO1 was also reversed by overexpression of MUC1 (all P < 0.01, Fig. 4h, i). Similarly, knockdown of AGR2 decreased the glucose uptake and lactate section, which was reversed by overexpression of MUC1 (all P < 0.01, Fig. 4e, f). These results indicate that AGR2 induces glucose metabolism via MUC1/HIF-1α pathway.
AGR2 induces glucose metabolism via the MUC1/HIF-1α pathway. a Immunoprecipitation assay was conducted to determine the relationship between AGR2 and MUC1 (N = 4). b Protein levels of MUC1 and HIF-1α after treatment with hypoxic conditions in RL952 cells transfected with shNC or shAGR2 and HEC1A transfected with pcDNA3.1-NC or pcDNA3.1-AGR2 were detected using Western blot (N = 4). c Protein levels of MUC1 and HIF-1α in RL952 cells transfected with shAGR2 or pcDNA3.1-MUC1 were detected by Western blot in hypoxic conditions (N = 4). d The cell viability of RL952 transfected with shAGR2 or pcDNA3.1-MUC1 was determined by MTT assay (N = 4). e The colony formation ability of RL952 cells transfected with shAGR2 or pcDNA3.1-MUC1 was detected by colony formation assay (N = 4). f The migration ability of RL952 cells transfected with shAGR2 or pcDNA3.1-MUC1 was determined by wound healing assay (N = 4). g Transwell invasion assay was used to determine the invasion ability of RL952 cells transfected with shAGR2 or pcDNA3.1-MUC1 (N = 4). h The mRNA levels LDHA, PGK1, HK2 and ENO1 in RL952 cells which were transfected with shAGR2 or pcDNA3.1-MUC1 and treated with hypoxic conditions were determined by qRT-PCR (N = 4). i The protein levels LDHA, PGK1, HK2 and ENO1 in RL952 cells which were transfected with shAGR2 or pcDNA3.1-MUC1 and treated with hypoxic conditions were determined by Western blot (N = 4). j Glucose uptake ability was determined by measurement of the uptake of [3H] 2DG in RL952 cells transfected with shAGR2 or pcDNA3.1-MUC1 and treated with hypoxic conditions (N = 4). k Lactate secretion was determined in RL952 cells transfected with shAGR2 or pcDNA3.1-MUC1 and treated with hypoxic conditions (N = 4). **P < 0.01
AGR2 knockdown inhibits tumor growth
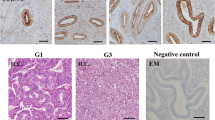
To further investigate the role of AGR2 in tumor growth in endometrial carcinoma, we established xenograft tumors using RL952 cells stably transfected with Ad-shAGR2. Results showed that knockdown of AGR2 significantly inhibited tumor growth (P < 0.01, Fig. 5a). The expression of Ki-67 which was a protein associated with cell proliferation in tumor tissues was decreased after knockdown of AGR2 (Fig. 5b). In xenograft tumors, knockdown of AGR2 also inhibited the expression of AGR2, MUC1 and HIF-1α. Also, the expression levels of glucose metabolism-related genes including LDHA, HK2, PGK1 and ENO1 were significantly decreased after knockdown of AGR2 (all P < 0.01, Fig. 5c). These results proved that AGR2 knockdown inhibits tumor growth and glucose metabolism in vivo.
Knockdown of AGR2 inhibits tumor growth. a Xenograft tumors were established in mice using RL952 cells stably transfected with Ad-shAGR2 or Ad-shNC and tumor volume of mice at 7, 14, 21, 28 and 35 days was detected (N = 4). b The levels of Ki-67, AGR2, MUC1 and HIF-1α in xenograft tumor tissues were detected by immunohistochemistry (N = 4). c The mRNA levels of LDHA, HK2, PGK1, and ENO1 in xenograft tumor tissues were explored using qRT-PCR (N = 4). **P < 0.01
Discussion
As a common malignant gynecologic tumor, endometrial carcinoma usually occurs in postmenopausal women [22]. The survival and prognosis are poor for endometrial carcinoma in advanced stages [3, 4]. Therefore, it is urgent to delineate the regulation mechanisms of endometrial carcinoma development. AGR2 expression is elevated in cancers and AGR2 functions as a regulator for tumor progression in mucinous ovarian cancer and breast cancer [10, 11]. Thus, we surmised that AGR2 might regulate cancer progression in endometrial carcinoma. To investigate the role of AGR2 in endometrial carcinoma, we first detected AGR2 expression. AGR2 expression was up-regulated in tumor tissues. The result was in agreement with lung cancer [8], gastric cancer [9], salivary adenoid cystic carcinoma [23], nasopharyngeal carcinoma [24] and so on. In the study performed by Kamal et al., AGR2 was up-regulated in endometrial carcinoma with low grade compared to postmenopausal endometrium, and high level of AGR2 expression was also found in metastatic lesions compared to primary tumors [12]. In addition, they also found that the level of AGR2 in low-grade endometrial carcinoma was higher than that of high-grade endometrial carcinoma [12]. In the present study, we found that AGR2 was associated with FIGO stage lymph node metastasis. Furthermore, the AGR2 expression level was also related to overall survival. Endometrial carcinoma patients with high expression of AGR2 presented poor survival. The result was contrary to the study performed by Kamal et al. [12], in which they found that high levels of AGR2 were related to better overall survival in all endometrial carcinoma except for endometrial carcinoma with high expression of estrogen alpha (ERα).
AGR2 has been proposed to act as an oncogene in cancers [25, 26]. The aberrant expression of AGR2 in endometrial carcinoma implied its vital role in endometrial carcinoma. Here, we reported that high expression of AGR2 promoted cell proliferation, migration and invasion ability in endometrial carcinoma, and down-regulation of AGR2 inhibited cell proliferation, migration, invasion and tumor growth. The effects of AGR2 in the regulation of cancer cell behaviors in endometrial carcinoma were similar to previous studies [10, 25, 27].
In cancer cells, glucose metabolism pattern was usually altered to glycolysis instead of the oxidative phosphorylation pathway [28]. Glycolysis led to acidification of the tumor environment, which was a benefit for generating more aggressive and invasive phenotype [28]. In the study, we found that a high level of AGR2 induced the expression of glycolysis-related genes including LDHA, PGK1, HK2 and ENO1. In xenograft tumors, down-regulation of AGR2 inhibited the expression of glycolysis-related genes. Glucose uptake and lactate production were also promoted by overexpression of AGR2 in endometrial carcinoma cells. On the whole, a high level of AGR2 could promote the glycolysis process in endometrial carcinoma. Thus, we inferred that AGR2 might promote cell proliferation, migration and invasion ability through inducing glycolysis in endometrial carcinoma. AGR2-induced glucose metabolism might facilitate the progression of endometrial carcinoma.
MUC1 was considered an oncogene in cancers [29, 30]. AGR2 was proved to modulate MUC1 expression in pancreatic intraepithelial neoplasia [31]. Moreover, MUC1 was regarded as a novel metabolic regulator [32]. The study performed by Kosugi et al. confirmed that MUC1 participated in glycolysis in cancer cells [33]. Knockdown of MUC1 inhibited glycolysis [33]. In addition, MUC1 could activate HIF-1α and then mediate glucose metabolism [19]. Therefore, we conjectured AGR2 might regulate cancer progression and glycolysis via the MUC1/HIF-1α pathway. According to the results in the present study, AGR2 interacted with MUC1 and induced the expression of MUC1 and HIF-1α. In xenograft tumors, knockdown of AGR2 inhibited the expression of MUC1 and HIF-1α in vivo. The inhibition of glycolysis caused by knockdown of AGR2 was reversed by overexpression of MUC1. Furthermore, the inhibition effects of AGR2 knockdown on cell proliferation, migration and invasion ability were abolished by overexpression of MUC1. In other words, the MUC1/HIF-1α pathway mediated the regulation of AGR2 on glycolysis. Therefore, we concluded that AGR2 might promote cells proliferation, migration, invasion and tumor growth through inducing glycolysis in endometrial carcinoma via the MUC1/HIF-1α pathway. AGR2-induced glucose metabolism might facilitate the progression of endometrial carcinoma via the MUC1/HIF-1α pathway. AGR2 may be an effective target for endometrial carcinoma therapy.
Conclusion
AGR2 was overexpressed in endometrial carcinoma. AGR2-induced glucose metabolism might facilitate the progression of endometrial carcinoma via the MUC1/HIF-1α pathway. AGR2 may be an effective therapeutic target for endometrial carcinoma.
References
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet (Lond Engl). 2016;387(10023):1094–108. https://doi.org/10.1016/s0140-6736(15)00130-0.
Al-Maghrabi J, Abdelrahman A, Al-Maghrabi B, Buhmeida A, Abuzenadah A, Al-Qahtani M, et al. Loss of p27 expression in endometrial carcinoma patients with recurrent tumor is significantly associated with poor survival. Eur J Gynaecol Oncol. 2018;39(1):119–23.
Abdulfatah E, Ahmed Q, Alosh B, Bandyopadhyay S, Bluth MH, Ali-Fehmi R. Gynecologic cancers: molecular updates 2018. Clin Lab Med. 2018;38(2):421–38. https://doi.org/10.1016/j.cll.2018.02.007.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. https://doi.org/10.3322/caac.21387.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. https://doi.org/10.3322/caac.21166.
Vale CL, Tierney J, Bull SJ, Symonds PR. Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database Syst Rev. 2012;8:CD003915. https://doi.org/10.1002/14651858.CD003915.pub4.
Persson S, Rosenquist M, Knoblach B, Khosravi-Far R, Sommarin M, Michalak M. Diversity of the protein disulfide isomerase family: identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol Phylogenet Evol. 2005;36(3):734–40. https://doi.org/10.1016/j.ympev.2005.04.002.
Alavi M, Mah V, Maresh EL, Bagryanova L, Horvath S, Chia D, et al. High expression of AGR2 in lung cancer is predictive of poor survival. BMC Cancer. 2015;15:655. https://doi.org/10.1186/s12885-015-1658-2.
Zhang J, Jin Y, Xu S, Zheng J, Zhang QI, Wang Y, et al. AGR2 is associated with gastric cancer progression and poor survival. Oncol Lett. 2016;11(3):2075–83. https://doi.org/10.3892/ol.2016.4160.
Kyoungsook P, Jin CY, Hyekyung S, Kwangsoo K, Junsoo P, Mijoung O, et al. AGR2, a mucinous ovarian cancer marker, promotes cell proliferation and migration. Exp Mol Med. 2011;43(2):91–100.
Jung SY, Yun J, Kim SJ, Kang S, Kim DY, Kim YJ, et al. Basic helix-loop-helix transcription factor Twist1 is a novel regulator of anterior gradient protein 2 homolog (AGR2) in breast cancer. Biochem Biophys Res Commun. 2019;516(1):149–56. https://doi.org/10.1016/j.bbrc.2019.05.191.
Kamal A, Valentijn A, Barraclough R, Rudland P, Rahmatalla N, Martin-Hirsch P, et al. High AGR2 protein is a feature of low grade endometrial cancer cells. Oncotarget. 2018;9(59):31459–72. https://doi.org/10.18632/oncotarget.25838.
Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70(1):431–57.
Ren J, Raina D, Chen W, Li G, Huang L, Kufe D. MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res Mcr. 2006;4(11):873.
Singh PK, Yunfei W, Swanson BJ, Kandavel S, Andrius K, Cerny RL, et al. Platelet-derived growth factor receptor beta-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res. 2007;67(11):5201–10.
McGuckin MA, Walsh MD, Hohn BG, Ward BG, Wright RG. Prognostic significance of MUC1 epithelial mucin expression in breast cancer. Hum Pathol. 1995;26(4):432–9. https://doi.org/10.1016/0046-8177(95)90146-9.
Hebbar V, Damera G, Sachdev GP. Differential expression of MUC genes in endometrial and cervical tissues and tumors. BMC Cancer. 2005;5(1):124.
Engel BJ, Bowser JL, Broaddus RR, Carson DD. MUC1 stimulates EGFR expression and function in endometrial cancer. Oncotarget. 2016;7(22):32796–809.
Shukla SK, Purohit V, Mehla K, Gunda V, Chaika NV, Vernucci E, et al. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic cancer. Cancer Cell. 2017;32(1):71–87.e7.
Huang J, Liu L, Feng M, An S, Zhou M, Li Z, et al. Effect of CoCl2 on fracture repair in a rat model of bone fracture. Mol Med Rep. 2015;12(4):5951–6. https://doi.org/10.3892/mmr.2015.4122.
Chaika NV, Gebregiworgis T, Lewallen ME, Purohit V, Radhakrishnan P, Liu X, et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci USA. 2012;109(34):13787–92. https://doi.org/10.1073/pnas.1203339109.
Prat J. Pathology of cancers of the female genital tract. Int J Gynaecol Obst. 2015;131(Suppl 2):S132–S145145. https://doi.org/10.1016/j.ijgo.2015.06.010.
Ma SR, Mao L, Deng WW, Li YC, Bu LL, Yu GT, et al. AGR2 promotes the proliferation, migration and regulates epithelial-mesenchymal transition in salivary adenoid cystic carcinoma. Am J Transl Res. 2017;9(2):507–19.
Li Y, Wang W, Liu Z, Jiang Y, Lu J, Xie H, et al. AGR2 diagnostic value in nasopharyngeal carcinoma prognosis. Clin Chim Acta Int J Clin Chem. 2018;484:323–7. https://doi.org/10.1016/j.cca.2017.12.023.
Fritzsche FR, Dahl E, Dankof A, Burkhardt M, Pahl S, Petersen I et al (2007) Expression of AGR2 in non small cell lung cancer. Histol Histopathol
Milewski D, Balli D, Ustiyan V, Le T, Dienemann H, Warth A, et al. FOXM1 activates AGR2 and causes progression of lung adenomas into invasive mucinous adenocarcinomas. PLoS Genet. 2017;13(12):e1007097.
Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008;68(2):492–7.
Annibaldi A, Widmann C. Glucose metabolism in cancer cells. Curr Opin Clin Nutr Metab Care. 2010;13(4):466–70.
Kufe DW. Functional targeting of the MUC1 oncogene in human cancers. Cancer Biol Ther. 2009;8(13):1197–203.
Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22(38):6107.
Norris AM, Gore A, Balboni A, Young A, Longnecker DS, Korc M. AGR2 is a SMAD4-suppressible gene that modulates MUC1 levels and promotes the initiation and progression of pancreatic intraepithelial neoplasia. Oncogene. 2013;32(33):3867–76. https://doi.org/10.1038/onc.2012.394.
Mehla K, Singh PK. MUC1: a novel metabolic master regulator. Biochim Biophys Acta (BBA) Rev Cancer 2014;1845(2):126–135
Kosugi M, Ahmad R, Alam M, Uchida Y, Kufe D. MUC1-C oncoprotein regulates glycolysis and pyruvate kinase M2 activity in cancer cells. PLoS One. 2011;6(11):e28234.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
WG conceived and designed the experiments, BE and XW analyzed and interpreted the results of the experiments, and YL and LW performed the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests, and all authors would confirm its accuracy.
Ethics approval and consent to participate
The protocol of the study was approved by the Ethics Committee of The Fourth Affiliated Hospital of Xinjiang Medical University (No. 2017065), and all the patients signed written informed consent.
All animal experiments were approved by the Ethics Committee of The Fourth Affiliated Hospital of Xinjiang Medical University (No. 2017065).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Patient consent for publication
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gong, W., Ekmu, B., Wang, X. et al. AGR2-induced glucose metabolism facilitated the progression of endometrial carcinoma via enhancing the MUC1/HIF-1α pathway. Human Cell 33, 790–800 (2020). https://doi.org/10.1007/s13577-020-00356-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00356-4